
Role of wedge resection in bronchial carcinoid (BC) tumors: SEER database analysis
Introduction
Bronchial carcinoid (BC) tumors are histologically classified into typical and atypical carcinoids (TC and AC). While there is wide agreement that the more biologically aggressive AC tumors should be treated by anatomic resection, there are now several case series and small, single-institutional, retrospective studies that have questioned the role of WR in the management of BC tumors. This draws on the recent studies of sublobar and wedge resections (WRs) in early stage NSCLC (1). However, due to the small number of patients included in those studies, a clinically meaningful conclusion couldn’t be obtained (1,2). Moreover, it is unlikely that a randomized, controlled trial with an adequate sample size could be conducted because of the rarity of the disease. We therefore sought to explore the oncological outcomes of in a large cohort of patients undergoing WR for BC tumors by comparing them to patients who underwent Lob/Seg in the Surveillance Epidemiology End Results (SEER) database.
Methods
Data sources
The SEER database was queried for patients with BC tumors treated with surgical resection (WR or Lob/Seg). We used the Public Use data version collected from the SEER 18 registries between 1973 and 2013. We limited our analysis to patients presenting with a single tumor, as the survival of patients with multiple primary tumors could not be ascribed to a single anatomic cancer site.
Study population and inclusion criteria
We used the International Classification of Diseases for Oncology, 3rd edition histological codes (ICD-0-3) to identify patients with typical (8240/3) and atypical carcinoid tumors (8249/3) (3,4). Patients who underwent WR were compared to those who underwent lobectomy or segmentectomy (Lob/Seg). Patients with multiple primaries and those who underwent pneumonectomy or have an unspecified surgical procedure were excluded. Moreover, patients diagnosed with BC on autopsy/death certificate, and those lacking survival data were excluded.
Study variables, survival and follow-up data
Data on gender, age, race, year of diagnosis, histology, grade, stage with its TNM breakdown, tumor size, surgical procedure, survival months, and vital status (alive or dead) at the last follow-up visit and cause of death were retrieved from the SEER database.
Overall survival (OS) was defined as the time from the date of diagnosis until the date of death from any cause, while cancer specific survival (CSS) was defined as the time from the date of diagnosis until the date of death from lung cancer.
Median follow up of the entire cohort was 70 months [interquartile range (IQR), 33–116 months]. Median follow-up in typical, and atypical carcinoid was 72 (IQR, 34–118), and 49 (IQR, 22–79) months, respectively.
Study objectives
The primary objective of the current study was to compare CSS and OS in patients with BC tumors treated with WR or Lob/Seg, both in the entire cohort and in the propensity matched groups. Survival probabilities were estimated using the Kaplan-Meier method and differences in survival between the study groups were compared using the Log-rank test.
The secondary objectives were to compare different clinicopathological parameters in Lob/Seg vs. WR groups and to compare different clinicopathological parameters and CSS in different BC histological subtypes (typical vs. atypical).
Statistical methods
Continuous variables were presented as median (IQR) and were compared using the Mann Whitney U test while categorical variables were reported as numbers (percentages) and were compared using Pearson’s Chi (χ2) test.
Factors predicting CSS were explored by multivariable Cox-regression analysis and presented as hazard ratio (HR) and 95% confidence interval (95% CI). A propensity score matched analysis (1:1, controlling for age, race, gender, year of diagnosis, histology and stage) was done to compare CSS differences in balanced cohorts of patients undergoing WR or Lob/Seg. Matching was done by using the nearest neighbor methods with no replacement, caliper =0.20). For all statistical analyses, P value <0.05 was considered statistically significant. Statistical analyses were performed by using the IBM SPSS software (IBM Corp. Released 2013. IBM SPSS Statistics for Windows, Version 22.0; IBM Corp., Armonk, NY, USA), the PS-matching package version 3.03 and the SPSS statistics R essentials were used for statistical analyses (5,6).
Results
Demographic and clinicopathological data
A total of 22,350 patients with BC tumors were identified during the study period (1.86% of all non-small cell lung cancer patients). A total of 4,450 cases (3,511 Lob/Seg, 939 WR) met our inclusion criteria. Median age was 59.0 years (IQR =49.0–68.0 years), 3,006 were females (67.6%) and median tumor size was 2 cm (1.5–3 cm).
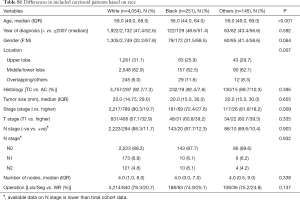
WR was utilized more frequently in older patients, females, lower lobe tumors, typical carcinoid and early stage disease (Table 1).
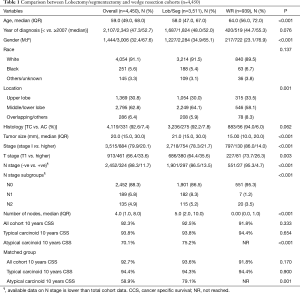
Full table
Typical vs. atypical histology
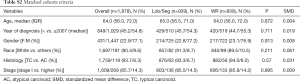
Among our cohort, the vast majority of patients with BC tumors had TC histology [n=4,119 (92.6%)] compared to [n=331 (7.4%)] for AC tumors. Compared to patients with AC tumors, patients with TC tumors were younger (59.0 vs. 61.0 years, P=0.001), had earlier stage disease (stage I; 81.5% vs. 59.9%, P<0.001), and had more node negative disease (90.7% vs. 71.0%, P<0.001). Differences in other demographics and clinicopathological variables are summarized in Table 2.
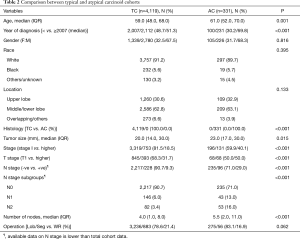
Full table
Survival data
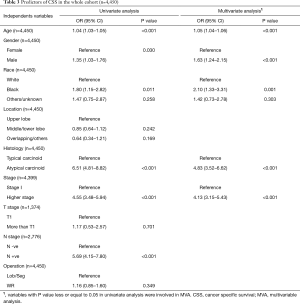
Ten years OS and CSS for the entire cohort were 79.8% and 92.4% respectively (Figure 1). On multivariable analysis (MVA) of the entire cohort, older age (HR =1.05, 95% CI: 1.04–1.06), male gender (HR =1.63, 95% CI: 1.24–2.15), black race (HR =2.10, 95% CI: 1.33–3.31), AC histology (HR =4.83, 95% CI: 3.52–6.62) and advanced stage (HR =4.13, 95% CI: 3.15–5.43) were associated with poor CSS. While the type of surgical procedure was not associated CSS (WR, HR =1.16, 95% CI: 0.85–1.60) (Tables 3, S1).
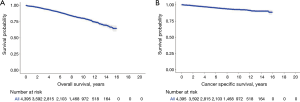
Full table
Full table
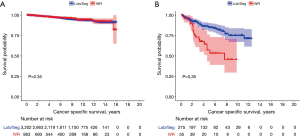
There were no differences in the 10 years CSS between WR and Lob/Seg in the entire whole cohort (92.5% vs. 91.8%, P=0.333), and in patients with TC histology (93.8% vs. 94.4%, P=0.654). However, Lob/Seg was associated with better CSS in patients with AC tumors (P<0.001) (Table 1, Figure 2). Similarly, among patients presenting with T1a tumors (≤2 cm), there was no difference in ten years CSS between WR and Lob/Seg in the entire cohort (91.7% vs. 91.6%, P=0.719), and in patients presenting with TC histology (92.8% vs. 93.6%, P=0.945). However, Lob/Seg was associated with better CSS in patients with T1a AC tumors (P=0.002).
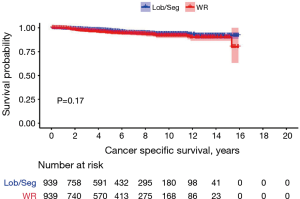
In propensity-score matched cohorts (n= 939 patients in each group), there was no difference between WR and Lob/Seg in 10 years CSS neither in the entire cohort (93.6% vs. 91.8%, P=0.170) nor in patients presenting with TC histology (94.3% vs. 94.4%, P=0.900). However, in patients presenting with AC histology, Lob/Seg was associated with a higher 10 years CSS rate (P=0.001) (Tables 1,S2, Figure 3).
Full table
Discussion
In the current study, we found no differences in CSS between typical carcinoid patients treated by either WR or anatomical resection, both in the multivariate model and in the propensity-matched analysis.
Our group and others have previously reported that a WR might be associated with comparable oncologic outcomes, compared to an anatomic resection in carefully staged cT1N0 NSCLC patients (1). Similarly, a subgroup analysis of the American College of Surgical Oncology Group (ACOSOG) Z4032 trial found that WR and segmentectomy had comparable local recurrence rates, disease fee and overall survival rates (7). Several single- and multi-institutional retrospective studies have reported a favorable outcome with the use of sublobar resection for BC tumors (8-11).
The vast majority of thoracic surgeons recommend performing formal anatomical resection for patients presenting with ACs as they have an aggressive biological behavior and dismal prognosis. However, for the slowly growing, indolent, typical carcinoid tumors, the interest to perform a limited resection continues to grow (12,13). Filosso and his group reported a survival advantage with the use of anatomic resection (lobectomy or segmentectomy) over WR for patients with Stage 1 typical carcinoids. However, their study assessed OS rather than cancer specific or disease free survival (14). Some might point to the worse OS for the wedge group as an evidence for an inferior approach. However, the comparable CSS noted in the current study may imply that the inferior OS of WR in Filosso study was due to higher comorbidities and older age.
Some thoracic surgeons may argue that conservative therapy is misplaced even in TC tumors that are associated with low incidence of mediastinal nodal disease and even a rare distant metastatic potential (15). In the current series, WR was associated with lower number of resected node sand nodal upstaging compared to Lob/Seg. Despite that, there was no difference in CSS between wedge and anatomic resection in patients with TCs.
Our institutional practice is to perform preoperative needle biopsy and intraoperative frozen section examination to try to differentiate between TC and AC. The decision to perform a completion lobectomy for patients treated with WR who turn out to have a postoperative pathologic diagnosis of AC is complex and should take into consideration patient’s age and comorbidity, and should only be performed after a detailed discussion with the patients and their families. For patients whose tumors are only identified post operatively as atypical and have undergone a WR and are N0 we would generally recommend close follow up and no additional surgery.
In the current study, we found that Black gender was associated with a more advanced disease and poor survival compared to Whites and other races. Similar findings were reported before in lung and esophageal cancer (16,17).
The principal finding of the current study is that in patients with typical carcinoid tumors, a WR appears to be a good oncologic alternative to an anatomic resection.
In the absence of prospective randomized controlled trials comparing WR and anatomical resection for BC, the large number of patients in the SEER database and the propensity score–matched analyses performed in the current study may provide useful guidance, as it diminishes potential imbalances associated with other retrospective, observational studies (18).
Two large randomized controlled trials that are comparing lobectomy and sublobar resection for early-stage NSCLC have recently completed their targeted accrual [the Japanese Clinical Oncology Group trial in Japan (JCOG0802) and the Alliance trial (CALGB140503) in North America]. While, the preliminary analyses of the CALGB140503 trial’s data suggest comparable short-term postoperative morbidity and mortality in patients undergoing lobectomy and sublobar resection (19). Data on long-term oncological outcomes, that are expected to be available in the next few years, will shed the light on the oncological adequacy of sublobar resection (wedge and Segmentectomy) in the management of early stage NSCLC.
The study’s limitations include its retrospective nature and the lack of detailed perioperative data such as smoking history, comorbidities, extent of lymph node dissection, tumor centrality and patterns of local/distant failure. While it would have been of interest to explore whether the use of minimally invasive approaches [uni-, multi-portal video-assisted thoracoscopic surgery (VATS) and robotic surgery] affect the oncologic outcomes in patients with BC, unfortunately, the surgical approach is not included in the SEER database (20-22).
Conclusions
The current study found that a WR may offer equivalent CSS to anatomic resection in highly selected patients with small, early-stage, TC disease. While, the number of resected lymph nodes and the rate of nodal upstaging was higher in the anatomic resection group, this was not reflected in a worse CSS in patients undergoing WR.
Acknowledgements
None.
Footnote
Conflicts of Interest: Presented at the 54th Annual Society of Thoracic Surgery Meeting, January 30th, 2018 in Fort Lauderdale, FL.
References
- Altorki NK, Kamel MK, Narula N, et al. Anatomical Segmentectomy and Wedge Resections Are Associated with Comparable Outcomes for Patients with Small cT1N0 Non-Small Cell Lung Cancer. J Thorac Oncol 2016;11:1984-92. [Crossref] [PubMed]
- Afoke J, Tan C, Hunt I, et al. Is sublobar resection equivalent to lobectomy for surgical management of peripheral carcinoid? Interact Cardiovasc Thorac Surg 2013;16:858-63. [Crossref] [PubMed]
- Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol 2015;10:1243-60. [Crossref] [PubMed]
- Fritz A, Percy C, Jack A, Shanmugaratnam K, Sobin L, Parkin DM, et al. International classification of diseases for oncology. [Internet]. World Health Organization; 2000 [cited 2017 Apr 14]. Available online: https://www.cabdirect.org/cabdirect/abstract/20013032699
- Hansen BB, Klopfer SO. Optimal full matching and related designs via network flows. J Comput Graph Stat 2006;15:609-27. [Crossref]
- Ho DE, Imai K, King G, et al. Matching as nonparametric preprocessing for reducing model dependence in parametric causal inference. Polit Anal 2007;15:199-236. [Crossref]
- Fernando HC, Landreneau RJ, Mandrekar SJ, et al. Impact of Brachytherapy on Local Recurrence Rates After Sublobar Resection: Results From ACOSOG Z4032 (Alliance), a Phase III Randomized Trial for High-Risk Operable Non-Small-Cell Lung Cancer. J Clin Oncol 2014;32:2456-62. [Crossref] [PubMed]
- Hage R, de la Rivière AB, Seldenrijk CA, et al. Update in pulmonary carcinoid tumors: a review article. Ann Surg Oncol 2003;10:697-704. [Crossref] [PubMed]
- Mezzetti M, Raveglia F, Panigalli T, et al. Assessment of outcomes in typical and atypical carcinoids according to latest WHO classification. Ann Thorac Surg 2003;76:1838-42. [Crossref] [PubMed]
- Detterbeck FC. Clinical presentation and evaluation of neuroendocrine tumors of the lung. Thorac Surg Clin 2014;24:267-76. [Crossref] [PubMed]
- Thomas CF, Tazelaar HD, Jett JR. Typical and atypical pulmonary carcinoids: outcome in patients presenting with regional lymph node involvement. Chest 2001;119:1143-50. [Crossref] [PubMed]
- Davini F, Gonfiotti A, Comin C, et al. Typical and atypical carcinoid tumours: 20-year experience with 89 patients. J Cardiovasc Surg (Torino) 2009;50:807. [PubMed]
- Lim E, Yap YK, De Stavola BL, et al. The impact of stage and cell type on the prognosis of pulmonary neuroendocrine tumors. J Thorac Cardiovasc Surg 2005;130:969-72. [Crossref] [PubMed]
- Filosso PL, Guerrera F, Falco NR, et al. Anatomical resections are superior to wedge resections for overall survival in patients with Stage 1 typical carcinoids. Eur J Cardiothorac Surg 2019;55:273-79. [Crossref] [PubMed]
- Stamatis G, Freitag L, Greschuchna D. Limited and radical resection for tracheal and bronchopulmonary carcinoid tumour. Report on 227 cases. Eur J Cardiothorac Surg 1990;4:527-32; discussion 533. [Crossref] [PubMed]
- Rahouma M, Harrison S, Kamel M, et al. Consequences of Refusing Surgery for Esophageal Cancer: A National Cancer Database Analysis. Ann Thorac Surg 2018;106:1476-83. [Crossref] [PubMed]
- Richards TB, Henley SJ, Puckett MC, et al. Lung cancer survival in the United States by race and stage (2001-2009): Findings from the CONCORD-2 study. Cancer 2017;123 Suppl 24:5079-99. [Crossref] [PubMed]
- D’Agostino RB. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med 1998;17:2265-81. [Crossref] [PubMed]
- Altorki NK, Wang X, Wigle D, et al. Perioperative mortality and morbidity after sublobar versus lobar resection for early-stage non-small-cell lung cancer: post-hoc analysis of an international, randomised, phase 3 trial (CALGB/Alliance 140503). Lancet Respir Med 2018;6:915-24. [Crossref] [PubMed]
- Paul S, Isaacs AJ, Treasure T, et al. Long term survival with thoracoscopic versus open lobectomy: propensity matched comparative analysis using SEER-Medicare database. BMJ 2014;349:g5575. [Crossref] [PubMed]
- Abouarab AA, Rahouma M, Kamel M, et al. Single versus multi-incisional video-assisted thoracic surgery: a systematic review and meta-analysis. J Laparoendosc Adv Surg Tech A 2018;28:174-85. [Crossref] [PubMed]
- Ghaly G, Kamel M, Nasar A, et al. Video-assisted thoracoscopic surgery is a safe and effective alternative to thoracotomy for anatomical segmentectomy in patients with clinical stage I non-small cell lung cancer. Ann Thorac Surg 2016;101:465-72. [Crossref] [PubMed]


