
Concepts and techniques: how to determine and identify the appropriate target segment in anatomical pulmonary segmentectomy?
Introduction
After the demonstration of the superiority of lobectomy over sublobar lung resection for lung cancer in 1995 by the Lung Cancer Study Group (1), there has been much discussion regarding the indications for anatomical segmentectomy for lung cancer. Notably, 25 years have passed since the study was conducted (1), and there are multiple limitations in the report in the light of current standards in 2019. Limitations include inhomogeneity of sublobar resections with wedge resection and segmentectomy both included, lack of routine preoperative computed tomography (CT) scans that detect earlier disease, and lack of lymph node dissection, which would have caused underestimation of the pathological stages. These factors could explain the poor outcomes following sublobar resection reported in the literature.
Although we await the final outcome of prospective randomized clinical trials (2,3), multiple studies suggest the validity of anatomical segmentectomy for early-stage lung cancer, especially for tumors with radiological ground-glass opacity (GGO) (4-6). As such, pulmonary anatomical segmentectomy has recently attracted increased interest because of increased detection of early-stage lung cancer with GGO and because of an increase in older and/or complicated patients. Indeed, the first international conference for sublobar lung resection for lung cancer was held in Paris, France in January 2018. Metastatic lung tumors located relatively deep in the pulmonary parenchyma may offer increased opportunity to perform anatomical segmentectomy.
Multiple methods have been proposed to identify intersegmental planes during pulmonary segmentectomy. There are advantages and disadvantages with each method, and rapid technological progress is changing the strategies. Furthermore, intersegmental planes in pulmonary segmentectomy should not be determined simply by the segmental anatomy. For example, when a tumor is located in segment 9 (S9) of the right lower lobe, there is no consensus regarding whether to perform isolated S9 segmentectomy, S8+9 segmentectomy, S9+10 segmentectomy, or basal (S7+8+9+10) segmentectomy, even if anatomical segmentectomy is a choice. Anatomical segmentectomy is a complex procedure both technically and conceptually. Surgeons must consider multiple factors such as surgical margins, venous drainage of the tumor, and the lung anatomy to be left behind.
The purpose of this review was first, to discuss the oncological and surgical principles in selecting target segments, and then to provide an overview of different methods of identifying intersegmental planes whereby surgeons can select an appropriate plane depending on the purpose of the surgery, selected surgical approach, available instruments and facilities, and preference.
Indications and approaches for anatomical segmentectomy
Multiple studies have reported oncological outcomes following anatomical segmentectomy that are equivalent to lobectomy for early-stage lung cancer, especially for peripherally-located tumors <2 cm in diameter with the radiological GGO (4-6). In the American College of Chest Physicians guidelines, sublobar resection with negative margins is suggested over lobectomy for patients with a clinical stage I predominantly GGO lesion ≤2 cm in diameter with recommendation grade 2C (7). Also, for patients with clinical stage I non-small cell lung cancer who may tolerate operative intervention but not lobar resection, because of decreased pulmonary function or comorbid disease, sublobar resection is recommended over nonsurgical therapy (grade 1B) (7).
Despite these recommendations, it is important to recognize multiple limitations in previous reports discussing anatomical segmentectomy and the controversy surrounding the procedure (8). First, many previous reports did not include details identifying ‘intentionally selected’ versus ‘compromised’ patients. Indeed, previous studies have shown significantly different overall- and disease-free survival when analyzing these two groups of patients separately (9). Second, although there are established factors favoring sublobar resection, tumor diameter <2 cm, peripheral location, GGO on imaging, favorable adenocarcinoma histopathological subtypes, and margins >2 cm were not recorded in the database (8). Third, no intraoperative details were provided, specifically regarding frozen section analysis of mediastinal and hilar lymph nodes or margin assessment, which would mandate that surgeons convert segmentectomy to lobectomy (10).
The benefit of segmentectomy in preserving pulmonary function better than lobectomy is still under debate. Generally, for patients with clinical stage I non-small cell lung cancer, a minimally invasive approach such as video-assisted thoracic surgery (VATS) is preferred over thoracotomy for anatomical pulmonary resection (7). However, although not randomized, a recent prospective study demonstrated that the benefit of VATS segmentectomy in preserving pulmonary function compared with VATS lobectomy may be limited, and the functional loss per resected segment may be doubled in segmentectomy compared with lobectomy (11). It is true that the benefit of segmentectomy over lobectomy regarding functional aspects is sometimes obvious. For example, a patient undergoing previous lobectomy may not tolerate additional lobectomy, but could tolerate segmentectomy. However, the significance of the findings in the recent prospective report (11) is that the advantage may not be as obvious as we assumed. Moreover, as we discuss later, there are potential oncological disadvantages and potential complications associated with segmentectomy. Therefore, careful patient evaluation and selection is mandatory. In addition, despite the favored minimally invasive approach of VATS (7), it is important to recognize that both visualization of the field and surgeons’ ability to palpate the lesion are limited compared with conventional thoracotomy. Introduction of the uniportal VATS approach has further limited surgeons’ ability in this regard, despite reported success by expert centers (12,13).
Considering all factors, anatomical segmentectomy is likely to be a valuable procedure for appropriately-selected patients. However, surgeons must recognize the limitations in the literature as well as the technical pitfalls. In the following sections, we focus on how to determine and identify appropriate target segment(s), which is one of the most important technical issues in anatomical segmentectomy.
Plan and determine the extent of resection
Although there is no consensus, thoracic surgeons generally consider the following factors when determining the extent of resection by anatomical segmentectomy: extension of the tumor and resulting resection margins, nature of the tumor (e.g., pure GGO vs. solid lung cancer), pulmonary functional reserve, and anatomical characteristics.
Resection margins
Evidence shows a higher incidence of locoregional recurrence and inferior patient survival with insufficient surgical margins. Although consensus is lacking regarding the sufficiency of resection margins, a resection margin/tumor diameter ratio >1 is a commonly-applied criteria for sufficiency. When the tumor diameter is >2 cm, a >2 cm margin is also frequently used. Indeed, during sublobar resection of solid tumors in compromised patients, application of this criteria has been recommended in the American College of Chest Physicians guidelines with recommendation grade 1C (7). Moreover, there is no standardized measurement method for resection margins; evaluation options include: preoperative CT images, macroscopically on the gross specimen, or microscopic/pathological examination. Most commonly, surgeons use CT imaging in preoperative planning, intraoperative macroscopic evaluation, and postoperative microscopic examination, which might necessitate considering additional resection. Given that lung size decreases during deflation and surgical margins planned on CT images may become smaller in the surgical field, it is safe to plan somewhat larger resection margins when using preoperative CT images. Currently, it is almost standard to use three-dimensional (3D) imaging based on high-resolution CT, preoperatively. Calculation or simulation of resection margins using 3D images plays an important role in determining the extent of anatomical segmentectomy.
To secure resection margins, “extended segmentectomy” (14) is an important consideration. In extended segmentectomy, the resection line can be extended beyond conventional anatomical segments, either by cutting into adjacent segments in a non-anatomical manner (i.e., by adding only peripheral lung resection without additional higher resection) or by resecting an adjacent subsegment or with a sub-subsegment after additional hilar resection (Figure 1A,B). To determine the extent of resection in anatomical segmentectomy, it is also important to consider the patient’s particular anatomy. By considering smaller anatomical units such as subsegments, resection of combined subsegments could also be an option (15) (Figure 1C).
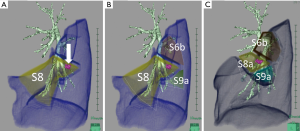
The nomenclature is sometimes misleading when determining appropriate resection units (16). For example, the right upper lobe is commonly divided into three segments: apical (S1), posterior (S2), and anterior (S3) as shown in Figure 2A. However, regarding Figure 2B, how is the apical segment (S1) defined? We usually apply the term, S1 to the area supplied by two bronchi that would be labelled “B1a” and “B1b”. However, once the bronchial anatomy is observed directly, ignoring pre-existing knowledge, we realize that the right upper lobe in Figure 2B is primarily divided into two anatomical units, which could be named “S2 + S1a” and “S3 + S1b”; however, these names are applied only because we artificially apply the text-book anatomy to the patient. When a tumor is located in the area supplied by B1a, and anatomical segmentectomy is an option, simply resecting S1 may not be anatomically valid, and S2 + S1a is anatomically simple and might be more oncologically appropriate. Resecting “S1” in such a case is practically a combined subsegmentectomy of S1a + S1b, which may be an important option to secure surgical margins, given the less invasive nature of the disease.

In general, unlike standardized lobectomy, far greater flexibility is required to determine the extent of resection in anatomical segmentectomy. Many factors must be considered including the patient’s particular anatomy and oncological validity. A practical approach is to break down the patient’s bronchial tree into smaller units than segments (i.e., subsegments or sub-subsegments) regardless of the nomenclature, ideally using a 3D-imaging workstation, and to determine the extent of resection as a combination of these smaller anatomical units.
Oncological validity
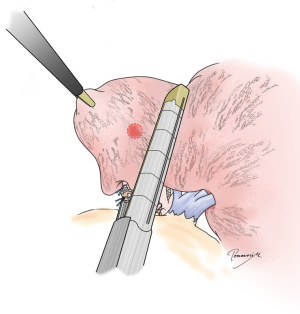
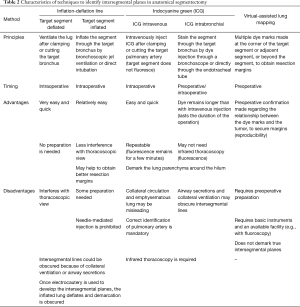
In addition to securing resection margins, other factors must be considered regarding the oncological validity of anatomical segmentectomy, including the nature of the disease, location of the disease, and drainage of pulmonary veins and/or lymph. Figure 3 shows four different lung tumors located in the right lower lobe. Given that their location is exactly the same, should we perform the same segmentectomy for all? Once again, there is no consensus, and a surgeon’s preference strongly affects the decision. However, an important question before each surgery is whether we would select wedge resection if the lesion was located more peripherally. In our center, wedge resection is usually selected for peripheral pure small GGO lesions <2 cm in diameter and for metastatic lung tumors. When such a lesion is located centrally, and wedge resection appears challenging, we select hilar management similar to segmentectomy to obtain sufficient resection margins, but it would not be important to meticulously resect the whole segment. Rather, hilar dissection helps lift the lesion to obtain sufficient resection margins using a stapler (Figure 4). This is a hybrid of wedge resection and anatomical segmentectomy. Although some intrapulmonary vasculature or small bronchi could be stapled peripherally, we consider this is an oncologically valid operation.
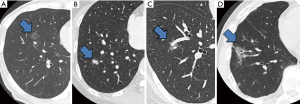
Conversely, for primary lung cancer with a solid component, surgical oncological validity must be more carefully examined. Lymphatic invasion is always a potential concern for such lesions, and resection planning should consider lymphatic drainage. In general, lymphatic drainage follows the bronchi and pulmonary veins. Thus, when a pulmonary vein associated with the lesion is located in the intersegmental plane, we consider this vein should also be resected. However, this surgical/oncological strategy could be problematic if intersegmental plane resection is performed without using staplers (e.g., by electrocautery), making the intersegmental vein a landmark to develop the intersegmental plane (Figure 5).
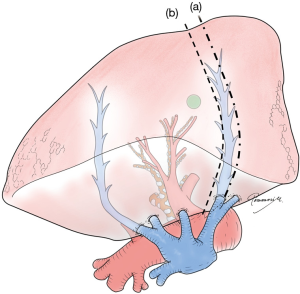
Anatomy of the remaining lung
Distortion
When we consider the extent of anatomical segmentectomy, we also consider the balance with pulmonary functional reserve. In principle, the more we remove, the less pulmonary function is reserved; however, in reality, there are occasions when the preserved lung does not function but also does cause problems, for anatomical reasons. For example, left upper trisegmentectomy (S1+2+S3) often results in partial or total atelectasis of the lingular segment secondary to kinking of the bronchus. To prevent this complication, avoiding extensive dissection of the interlobar fissure and/or fixation of the lung tissue after dissection should be considered (i.e., avoiding complete dissection of the anterior fissure and/or anchoring the lingular segment to S8 toward the end of surgery in left upper trisegmentectomy).
Venous drainage and congestion
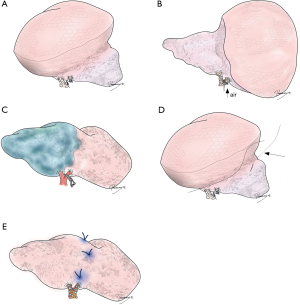
Of particular concern is leaving part of the lung extremely congested by limiting venous drainage from the area or segment of the lung following segmentectomy. Anatomically and technically, this can happen easily in right S7 with concurrent right S10 segmentectomy (17). Congestion of other segments is often experienced. For example, in left upper culminectomy (left S1+2+S3), conservation of V3 between the upper and lingular segments is sometimes necessary to ensure correct drainage of the lingular segment. Importantly, venous congestion should be considered carefully with venous resection to maintain oncological validity, as discussed earlier. For example, when a potentially invasive lung cancer is located in S9, and the intersegmental pulmonary vein between S8 and S9 is considered a drainage vein for the tumor (Figure 2), resection of the intersegmental pulmonary vein may leave a severely congested S8. In such a case, other drainage veins from S8 should be carefully examined on CT or 3D images (i.e., veins between S8 and S6, S7/10, or middle/lingular lobe). If these other veins are unreliable, S8+9 segmentectomy may be a better choice.
Arterial blood supply and infarction
In segmentectomy, surgeons tend to believe in collateral blood supply from surrounding segments. However, remaining lung tissue can indeed suffer from reduced blood supply and ischemia (18). Similar to congestion, it is also dangerous to rely on a single tiny pulmonary artery for blood supply. For example, when S8a is resected, leaving a very small A8b for subsegmentectomy of S8a, it is important to recognize the risk of infarction in S8b. Although this complication seems rare, it likely happens more easily when the remaining segment is anatomically isolated. With S8a resection, the well-developed major fissure and more protruded S8b with less contact area with adjacent segments such as S9 are important factors to be considered. Anatomical isolation of the supplying pulmonary artery (e.g., A8b) by dissection is also likely to contribute to kinking the artery and resulting ischemia. Interestingly, intraoperative indocyanine green (ICG) angiography may play a role in avoiding such ischemic complications by visualizing unexpectedly devascularized segments or areas (19).
With these considerations, in addition to considering the balance between oncological validity and preserving pulmonary function, it is most important for surgeons to simulate and imagine the postoperative anatomy, lung shape, blood supply, and venous drainage of the lung left behind, to prevent complications (Table 1). In other words, anatomical simplicity of resection is also important, depending on the surgeon’s experience. Even if a complex segmentectomy appears to be an option in 3D-image simulation, the procedure might be too complicated and risky in reality.

Full table
Segmentation by 3D imaging and virtual reality
Each patient’s particular anatomy guides the surgeon in selecting the target segment(s) to perform oncologically-valid surgery as well as to avoid compromising viability in the remaining segments. As discussed, there are multiple advantages in planning and simulating anatomical segmentectomy using 3D-imaging technology, and several programs and workstations are commercially available. Each program and workstation have different properties, but all share the ability to detect detailed arterial, venous, and bronchial anatomy (20). In addition, many recent workstations can plan segmentation and resection margins based on the particular patient’s segmentation (21). Some workstations can also indicate optimal resection margins, which would greatly help surgeons to determine the appropriate target segment(s) (22). However, it is important to carefully interpret the 3D model and to question the computer-generated plan; small vessel branches are commonly missed in 3D images. Some workstations that depict pulmonary arteries and veins based on single-phase enhanced CT may frequently mistakenly identify a pulmonary artery as a vein and vice versa. Segmentation is based on the particular program’s algorithms which may include taking the geometric means of two bronchi, but these algorithms may not reflect actual anatomical intersegments. Most importantly, CT images and the consequent 3D model do not reflect the deflated lung, which is the surgical reality. Accordingly, the required resection margin is often underestimated during surgery.
Unlike liver surgery where interactive augmented reality has progressed, introducing this technology into pulmonary resection techniques such as anatomical segmentectomy has been hindered by the challenge of lung collapse and deformity. As such, thoracic surgeons should use 3D-imaging technology to plan and simulate anatomical segmentectomy carefully, recognizing the limitations and pitfalls of current 3D imaging and simulation options.
Intraoperative identification of the target segment (I): inflation-deflation lines
Intersegmental planes can be distinguished by creating inflation-deflation lines. The segment to be resected can be either deflated or inflated. Both methods are relatively easy to perform and are commonly used (Table 2).
Full table
One of the major limitations is that the border can be obscure or misleading because of collateral ventilation, which results in inflation of the area to be left deflated. This problem likely depends on inflation pressure and the nature of the underlying lung, including emphysema. Special attention is required when a strict surgical margin depends on an intersegmental line. Additionally, secretions remaining in the airway could interfere with appropriate inflation of the lung. Reproducibility of an appropriate inflation-deflation line could be the major concern when this method is applied alone.
Another limitation is that even if a clear inflation-deflation line is developed, if the intersegmental planes are divided by electrocautery, air escapes from the cauterized intersegmental surface and inflated lung may deflate. Once this happens, reproducing the same inflation-deflation line might be difficult.
Deflation of the target segment
This is the most conventional method to develop an intersegmental plane (Figure 6A). While the lung on the operation side is deflated, the target bronchus is tied or resected. Ventilation is temporarily resumed, and the lung other than the target segment is inflated while the target segment remains deflated. This method requires no special preparation and is easiest to conduct intraoperatively. A major limitation is interference with the surgical view, especially during thoracoscopic segmentectomy. Another issue is collateral ventilation, especially because the target bronchus is already tied or cut; once the target segment is inflated by collateral ventilation, it is difficult to deflate the segment. It is true that, even in such cases, if ventilation in one lung can be resumed, the lung to be left can be deflated while the target segment remains inflated (see the method described in “Oncological validity”). However, it is typically time-consuming to deflate an emphysematous lung, and reliability of the intersegmental planes is a concern, especially when the surgical margins must be strictly delineated.
Inflation of the target segment
Contrary to the conventional method to develop inflation/deflation lines, the target segment can be inflated, instead (Figure 6B). Okada et al. reported a method to inflate the target segment using a jet ventilator (24). Because a jet ventilator may not be readily available, multiple modified methods have been proposed including intubating the target bronchus after resection or applying a slip knot to the target segment bronchus followed by bilateral ventilation; the knot is then slipped to close the bronchus (23). Importantly, inflating the target bronchus by puncturing the bronchus using a needle to inflate the target segment should be strictly avoided. This method has been reported to cause massive air embolism most likely resulting from direct injection of air into an adjacent pulmonary vein (25,26).
Compared with the method to deflate the target segment described in II-1, inflating the target segment requires more preparation and intraoperative manipulation, although the degree of interference with the thoracoscopic view by the inflated lung is much less; therefore, this technique is more advantageous in the current era of thoracoscopic segmentectomy. Moreover, this technique may also be advantageous regarding resection margins because the target segment including the tumor is inflated, and collateral ventilation may help obtain extra margin. Nevertheless, the issue of reproducibility remains because of changes in collateral ventilation and/or airway secretions.
Intraoperative identification of the target segment (II): ICG
ICG is a green dye visible under regular white light and visible as fluorescence by near-infrared light. Using this property, fluorescence thoracoscopy can be used to delineate intersegmental planes depicted by ICG. However, fluorescence thoracoscopy is not yet widely available (Table 2).
Intravenous injection of ICG
Misaki et al. reported injecting ICG intravenously intraoperatively after clamping or resecting pulmonary arteries perfusing the target segment (27). Using fluorescence thoracoscopy, the target segment is visualized as a dark area while the lung perfused with ICG appears as a bright area (Figure 6C). A major advantage is that intravenous ICG injection can easily be performed by an anesthetist. Although injected ICG remains in circulation for only a few minutes, injections can be repeated multiple times in the same surgery. Importantly, if an incorrect pulmonary artery is resected, the result is permanent. Thus, if questions remain regarding hilar anatomy, the candidate artery should be clamped using a tourniquet or Bulldog clump, rather than cut, to see if the indicated segment is a reasonable consideration for resection. Intraoperative ICG angiography may also play a role in avoiding ischemic complication after segmentectomy by visualizing unexpectedly devascularized segments or areas (19). An important advantage of this technique is that it can clearly depict the intersegmental lines independent of airways where collateral ventilation and airway secretions are sometimes problematic when reproducing the intersegmental lines. However, because emphysematous lungs are less-perfused, the indicated intersegmental line may be misleading or confusing following ICG injection (27). Moreover, even without emphysema, collateral circulation or diffusion of ICG may limit correct understanding of the target segment to be resected. Reportedly, ICG diffused into the segment to be removed in 12% of patients, interfering with correct understanding of target segments; however, the phenomenon was not associated with a specific site or segment, or pulmonary emphysema (19). In addition to collateral circulation and method failure, there is sometimes an accessory branch of the pulmonary artery with a supply that does not coincide with bronchial branching, resulting in misleading ICG staining (e.g., an accessary artery to S10a branching from S6 with regular bronchial branching) (28). Careful examination of 3D images is helpful to understand the patient’s particular anatomy. Given these considerations, reproducibility of the intersegmental line remains a potential concern with intraoperative ICG, especially if the resection margin is strictly dependent on the intersegmental plane.
Intrabronchial injection of ICG
This technique was originally reported by Sekine et al. (29), intending to stain the target segment by injecting ICG through a bronchoscope preoperatively or through an intubated target bronchus intraoperatively. The authors reported using fluorescence thoracoscopy to identify the intersegmental planes, while Oh et al. reported using a standard thoracoscope to identify the blue/green color of ICG (29). Compared with intravenous injection of ICG, the dye stays in the alveoli longer with intrabronchial injection; therefore, no repeated injection is required. This technique provides clear intersegmental lines during hilar dissection and resection of the pulmonary parenchyma using electrocautery (30).
Careful and accurate identification of a target bronchus is mandatory with this technique. If dye is injected through an incorrect bronchus, results are confusing, and repeat injection is not possible. Similar to developing inflation-deflation lines, airway secretions may interfere with staining, which may result in heterogeneous staining and confusing intersegmental planes.
ICG can also be used as an isolated dye mark using a bronchoscopic dye injection technique including using an electromagnetic navigation bronchoscope (ENB) (22). Also, if the procedure is repeated to make multiple marks on the lung, ICG can be used for virtual-assisted lung mapping (VAL-MAP), as we discuss in a later section.
Localization techniques in segmentectomy
Localization techniques for small, barely-palpable pulmonary nodules include (but are not limited to) placing a hookwire or microcoil, or injecting dye, barium, or other contrast medium. Some localization tools are placed transbronchially using a bronchoscope, including an ENB (22) or percutaneously, under CT guidance. Similar to cases where lobectomy is a surgical option if malignancy is demonstrated intraoperatively, localization may sometimes be necessary to perform an unplanned examination before resecting the entire segment concerned, especially if the sublobar resection is high-volume (e.g., basal segmentectomy). Conversely, if the segment is small and limited, initial wedge resection should be avoided in most cases because the technique distorts the lung significantly, making segmentectomy technically challenging and even inaccurate.
Localization techniques have also been used in anatomical segmentectomy to assist determination of the resection lines (31,32). Localization techniques in anatomical segmentectomy are usually complementary to methods used to identify intersegmental planes. If the intersegmental planes are accurately and clearly identified without uncertainty, and if the nodule and surrounding resection margin is completely included in the target segment, theoretically, there is no need to use a localization technique; anatomical resection of a segment should guarantee successful resection of the nodule, similar to lobectomy. However, in reality, uncertainty remains regarding any methods to identify intersegmental planes, which implies the necessity of using a localization technique in anatomical segmentectomy.
A localization technique such as placing a hook wire is usually used in combination with another technique to identify intersegmental planes such as an inflation-deflation (Figure 6D). To obtain sufficient resection margins, the resection area could be extended beyond the anatomical segment (i.e., extended segmentectomy) based on the information obtained by the localization technique.
Intraoperative identification of the target segment and localization: VAL-MAP
VAL-MAP is a bronchoscopic multi-spot dye-marking technique developed in 2012 (33). In VAL-MAP, virtual bronchoscopy is used to plan multiple lung marking and then dye (indigo carmine in the original method) is injected through an atraumatic catheter. Similar to other localization techniques, this method was originally developed to localize a barely-palpable pulmonary nodule, essentially as an alternative to CT-guided marking where air embolism is a major concern (34,35). However, the multiple marks on the lung in VAL-MAP were found to provide geometric information on the lung, and thus, the application was extended to segmentectomy (Figure 6E) (15,36).
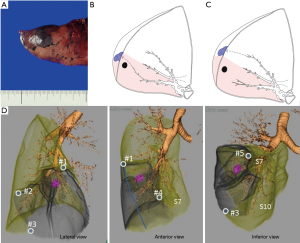
A bronchoscopic dye mark can be made close to the intersegmental plane, either from a bronchial branch inside the targeted segment or from one outside the target segment (Figure 7). Similar to intrabronchial ICG injection, dye remains in a segment and identifies an approximate intersegmental line. Dye marks can be placed at the corner of a targeted segment. Moreover, a dye mark made during VAL-MAP can be placed free of an anatomical segment, especially to indicate appropriate resection margins. In this way, VAL-MAP in segmentectomy is an extension of the combined localization technique (see section “Localization techniques in segmentectomy”). Using VAL-MAP, resection lines can be easily and flexibly designed beyond conventional anatomical segments. Furthermore, this technique emphasizes “reproducibility” of resection lines. Post-mapping CT scans are considered mandatory with this technique (37). By confirming the location of the actual marks and their relationship with existing structures such as the tumor and the targeted segment, VAL-MAP is considered a highly reliable and reproducible technique.
Conversely, a technical limitation of this method is that it requires more preparation than for other techniques used to identify intersegmental planes and/or localization techniques. An ENB can be used to partially overcome the challenges.
Another important limitation is that dye marks made during VAL-MAP are made only on the lung surface, which may not sufficient to acquire resection margins in the deep pulmonary parenchyma even if the resection line on the lung surface is appropriate. Because this limitation is common among different techniques to identify intersegmental planes, we discuss this further in the next section.
Resection margins jeopardized by segmentectomy for deep lesions
Most intersegmental lines, regardless of technique, are generally limited to the lung surface because surgeons see the lung intraoperatively only from the surface. We recently found that this technical limitation has an important negative impact on the surgical margins in anatomical segmentectomy (38). Although segmentectomy appears to provide sufficient resection margins because of hilar dissection compared with wedge resection, segmentectomy and wedge resection showed similar resection failure rates (i.e., insufficient macroscopic resection margins) when resecting deep tumors, in our previous study. In the study, frozen section was not performed routinely, but if positive resection margins were suspected or surgeons felt the margin was insufficient, additional resection of adjacent segments or conversion to lobectomy was performed, as previously described (10). However, all cases requiring changes to the resection plan were considered “resection failure” because the study’s purpose was to evaluate the efficacy of surgical planning and reproducibility using VAL-MAP (38). Importantly, we consider this limitation common to anatomical segmentectomy using other techniques to identify intersegmental planes such as inflation-deflation. The similar failure rates probably represent the danger of developing intersegmental planes (or lines) in a cone-shaped segment, jeopardizing the resection margin for deep lesions (Figure 8A,B).
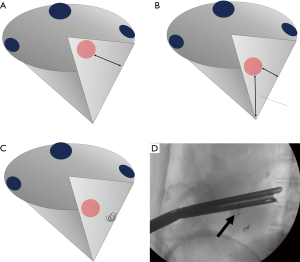
To overcome the challenges of segmentectomy (and the challenges of VAL-MAP), we recently developed a technique to combine dye marks on the lung with bronchoscopic placement of platinum microcoils deep in the lung (Figure 8C) (39). Bronchoscopic microcoil placement is a proposed localization technique by the Tokushima University group (40,41). Similar to combining a localizing technique with a technique to develop intersegmental planes, our new technique, “VAL-MAP 2.0”, applies the principles of VAL-MAP to microcoil placement. Specifically, a microcoil(s) is placed some distance from the tumor to indicate appropriate resection lines rather than near the tumor itself (Figure 8C,D). Our preliminary study showed excellent outcomes (39), and we are conducting a multicenter prospective study in Japan. Hopefully, this new technique overcomes the common challenges in anatomical segmentectomy.
In conclusion, there are many technical tips and issues to consider when planning appropriate anatomical segmentectomy. Although there are several different techniques to identify intersegmental planes, these address only some of the challenges. There is room for further investigation and development to improve anatomical segmentectomy.
Acknowledgements
We thank Jane Charbonneau, DVM from Edanz Editing (ww.edanzediting.com/ac), for editing a draft of this manuscript.
Funding: This work was supported by the Japan Agency for Medical Research and Development (18ck0106244h0003).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615-22; discussion 622-3. [Crossref] [PubMed]
- Blasberg JD, Pass HI, Donington JS. Sublobar resection: a movement from the Lung Cancer Study Group. J Thorac Oncol 2010;5:1583-93. [Crossref] [PubMed]
- Nakamura K, Saji H, Nakajima R, et al. A phase III randomized trial of lobectomy versus limited resection for small-sized peripheral non-small cell lung cancer (JCOG0802/WJOG4607L). Jpn J Clin Oncol 2010;40:271-4. [Crossref] [PubMed]
- Altorki NK, Yip R, Hanaoka T, et al. Sublobar resection is equivalent to lobectomy for clinical stage 1A lung cancer in solid nodules. J Thorac Cardiovasc Surg 2014;147:754-62; discussion 762-4. [Crossref] [PubMed]
- Wolf AS, Richards WG, Jaklitsch MT, et al. Lobectomy versus sublobar resection for small (2 cm or less) non-small cell lung cancers. Ann Thorac Surg 2011;92:1819-23; discussion 1824-5.
- Okada M, Koike T, Higashiyama M, et al. Radical sublobar resection for small-sized non-small cell lung cancer: a multicenter study. J Thorac Cardiovasc Surg 2006;132:769-75. [Crossref] [PubMed]
- Howington JA, Blum MG, Chang AC, et al. Treatment of stage I and II non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e278S-e313S.
- Cao C, Tian DH, Fu B, et al. The problem with sublobar resections. J Thorac Dis 2018;10:S3224-6. [Crossref] [PubMed]
- Cao C, Chandrakumar D, Gupta S, et al. Could less be more?-A systematic review and meta-analysis of sublobar resections versus lobectomy for non-small cell lung cancer according to patient selection. Lung Cancer 2015;89:121-32. [Crossref] [PubMed]
- Gossot D, Lutz JA, Grigoroiu M, et al. Unplanned Procedures During Thoracoscopic Segmentectomies. Ann Thorac Surg 2017;104:1710-7. [Crossref] [PubMed]
- Gu Z, Wang H, Mao T, et al. Pulmonary function changes after different extent of pulmonary resection under video-assisted thoracic surgery. J Thorac Dis 2018;10:2331-7. [Crossref] [PubMed]
- Duan L, Jiang G, Yang Y. One hundred and fifty-six cases of anatomical pulmonary segmentectomy by uniportal video-assisted thoracic surgery: a 2-year learning experience. Eur J Cardiothorac Surg 2018;54:677-82. [Crossref] [PubMed]
- Hernandez-Arenas LA, Purmessur RD, Gonzalez-Rivas D. Uniportal video-assisted thoracoscopic segmentectomy. J Thorac Dis 2018;10:S1205-14. [Crossref] [PubMed]
- Tsubota N, Ayabe K, Doi O, et al. Ongoing prospective study of segmentectomy for small lung tumors. Study Group of Extended Segmentectomy for Small Lung Tumor. Ann Thorac Surg 1998;66:1787-90. [Crossref] [PubMed]
- Sato M. Virtual assisted lung mapping: navigational thoracoscopic lung resection. Cancer Res Front 2016;2:85-104. [Crossref]
- Sato M. Techniques to Identify Inter-segmental Planes. Kyobu Geka 2018;71:862-7. [PubMed]
- Sato M, Murayama T, Nakajima J. Thoracoscopic stapler-based "bidirectional" segmentectomy for posterior basal segment (S10) and its variants. J Thorac Dis 2018;10:S1179-86. [Crossref] [PubMed]
- Traibi A, Grigoroiu M, Boulitrop C, et al. Predictive factors for complications of anatomical pulmonary segmentectomies. Interact Cardiovasc Thorac Surg 2013;17:838-44. [Crossref] [PubMed]
- Bédat B, Triponez F, Sadowski SM, et al. Impact of near-infrared angiography on the quality of anatomical resection during video-assisted thoracic surgery segmentectomy. J Thorac Dis 2018;10:S1229-34. [Crossref] [PubMed]
- Oizumi H, Kanauchi N, Kato H, et al. Anatomic thoracoscopic pulmonary segmentectomy under 3-dimensional multidetector computed tomography simulation: a report of 52 consecutive cases. J Thorac Cardiovasc Surg 2011;141:678-82. [Crossref] [PubMed]
- Saji H, Inoue T, Kato Y, et al. Virtual segmentectomy based on high-quality three-dimensional lung modelling from computed tomography images. Interact Cardiovasc Thorac Surg 2013;17:227-32. [Crossref] [PubMed]
- Seguin-Givelet A, Grigoroiu M, Brian E, et al. Planning and marking for thoracoscopic anatomical segmentectomies. J Thorac Dis 2018;10:S1187-94. [Crossref] [PubMed]
- Endoh M, Oizumi H, Kato H, et al. Determination of the intersegmental plane using the slip-knot method. J Thorac Dis 2018;10:S1222-8. [Crossref] [PubMed]
- Okada M, Mimura T, Ikegaki J, et al. A novel video-assisted anatomic segmentectomy technique: selective segmental inflation via broncho fiberoptic jet followed by cautery cutting. J Thorac Cardiovasc Surg 2007;133:753-8. [Crossref] [PubMed]
- Otsuka T, Nakamura Y, Harada A, et al. Extremely rare but potential complication of diffuse brain edema due to air embolism during lung segmentectomy with selected segmental inflation technique by syringe needle during video-assisted thoracoscopic surgery. J Thorac Cardiovasc Surg 2011;142:e151-2. [Crossref] [PubMed]
- Kiribayashi M, Nakasone M, Moriyama N, et al. Multiple cerebral infarction by air embolism associated with remarkable low BIS value during lung segmentectomy with video assisted thoracic surgery (VATS) technique: a case report. Masui 2010;59:480-3. [PubMed]
- Misaki N, Chang SS, Igai H, et al. New clinically applicable method for visualizing adjacent lung segments using an infrared thoracoscopy system. J Thorac Cardiovasc Surg 2010;140:752-6. [Crossref] [PubMed]
- Sequin-Givelet A, Kovac sE, Potenza R, et al. Use of Indocyanine Green as a Mark for Intersegmental Plane During Thoracoscopic Segmentectomies: An Apparent Failure. Available online: https://www.ctsnet.org/article/use-indocyanine-green-mark-intersegmental-plane-during-thoracoscopic-segmentectomies
- Sekine Y, Ko E, Oishi H, et al. A simple and effective technique for identification of intersegmental planes by infrared thoracoscopy after transbronchial injection of indocyanine green. J Thorac Cardiovasc Surg 2012;143:1330-5. [Crossref] [PubMed]
- Oh S, Suzuki K, Miyasaka Y, et al. New technique for lung segmentectomy using indocyanine green injection. Ann Thorac Surg 2013;95:2188-90. [Crossref] [PubMed]
- Abdelsattar ZM, Blackmon SH. Using novel technology to augment complex video-assisted thoracoscopic single basilar segmentectomy. J Thorac Dis 2018;10:S1168-78. [Crossref] [PubMed]
- Yang SM, Lin CK, Chen LW, et al. Combined virtual-assisted lung mapping (VAL-MAP) with CT-guided localization in thoracoscopic pulmonary segmentectomy. Asian J Surg 2019;42:488-94. [Crossref] [PubMed]
- Sato M, Omasa M, Chen F, et al. Use of virtual assisted lung mapping (VAL-MAP), a bronchoscopic multispot dye-marking technique using virtual images, for precise navigation of thoracoscopic sublobar lung resection. J Thorac Cardiovasc Surg 2014;147:1813-9. [Crossref] [PubMed]
- Sakiyama S, Kondo K, Matsuoka H, et al. Fatal air embolism during computed tomography-guided pulmonary marking with a hook-type marker. J Thorac Cardiovasc Surg 2003;126:1207-9. [Crossref] [PubMed]
- Horan TA, Pinheiro PM, Araujo LM, et al. Massive gas embolism during pulmonary nodule hook wire localization. Ann Thorac Surg 2002;73:1647-9. [Crossref] [PubMed]
- Sato M, Murayama T, Nakajima J. Techniques of Stapler-based Navigational Thoracoscopic Segmentectomy using Virtual Assisted Lung Mapping (VAL-MAP). J Thorac Dis 2016;8:S716-30. [Crossref] [PubMed]
- Sato M, Nagayama K, Kuwano H, et al. Role of post-mapping computed tomography in virtual-assisted lung mapping. Asian Cardiovasc Thorac Ann 2017;25:123-30. [Crossref] [PubMed]
- Sato M, Kobayashi M, Kojima F, et al. Effect of virtual-assisted lung mapping (VAL-MAP) in acquisition of surgical margins in sublobar lung resection. J Thorac Cardiovasc Surg 2018;156:1691-701.e5. [Crossref] [PubMed]
- Sato M, Nagayama K, Kobayashi M, et al. Virtual-assisted lung mapping 2.0: preoperative bronchoscopic three-dimensional lung mapping. Ann Thorac Surg 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Toba H, Kondo K, Miyoshi T, et al. Fluoroscopy-assisted thoracoscopic resection after computed tomography-guided bronchoscopic metallic coil marking for small peripheral pulmonary lesions. Eur J Cardiothorac Surg 2013;44:e126-32. [Crossref] [PubMed]
- Miyoshi T, Kondo K, Takizawa H, et al. Fluoroscopy-assisted thoracoscopic resection of pulmonary nodules after computed tomography--guided bronchoscopic metallic coil marking. J Thorac Cardiovasc Surg 2006;131:704-10. [Crossref] [PubMed]


