Atrial septal defect with pulmonary hypertension: when/how can we consider closure?
Introduction
Atrial septal defect (ASD) is one of the common congenital heart diseases with a prevalence of 1.6 per 1,000 live births and accounting for 8–10% of all congenital heart defects (CHD) (1). Pulmonary arterial hypertension (PAH), defined as pulmonary arterial systolic pressures (PASP) ≥40 mmHg, has been noted in 6% to 35% of patients with secundum ASD (2-5). Moderate to severe pulmonary hypertension (PH) in ASD is seen in 9–22% cases (2,3,6). PH in un-operated patients with ASD has been associated with functional limitations, atrial tachyarrhythmias, heart failure and increased mortality (7). Preoperative PH remains predictive of mortality, heart failure, and arrhythmias even after closure of the ASD (8,9).
PH in the setting of an ASD can be secondary to various etiologies. Post capillary PH can be due to elevated left ventricular (LV) end diastolic pressure as seen in elderly patients with hypertension, ischemic heart disease, diabetes mellitus and chronic kidney disease or due to mitral valve disease as in Lutembacher’s syndrome. Pre-capillary PH (PAH) can be secondary to a large shunt or in some cases may be disproportionate to the magnitude of shunt. At one end of the spectrum are patients with reversible PAH who will clearly benefit from closure of the shunt. At the other end are those with irreversible PAH with shunt reversal (Eisenmengerisation) in whom closure of ASD will be counterproductive and therefore will need to be managed medically. The challenge is to precisely identify the subset of ASD patients with reversible PAH who will benefit with shunt closure.
In this review we attempt to identify the various subset of patients with PH who can benefit from closure of their ASD.
ASD with post capillary PH
Two common scenarios under this subset include ASD with mitral stenosis (MS) (Lutembacher’s syndrome) and ASD with elevated LV end diastolic pressure. Both the scenarios lead to elevated left atrial (LA) pressure eventually resulting in increased left to right shunt and increased Qp:Qs (10,11).
The original case of Lutembacher’s syndrome was a 61-year-old woman who had been pregnant 7 times (12). Females have a predilection for Lutembacher’s syndrome since female predominance is seen for each lesion individually. The incidence of MS in patients with ASD is 4%, and conversely, ASD is seen in 0.6–0.7% of patients with MS (13).
The combined transcatheter therapy as a rescue procedure for Lutembacher’s syndrome was first described in 1992 by Ruiz et al., who utilized Lock’s clamshell occluder along with mitral and aortic balloon valvotomies (14). Later, Joseph et al. described successful combined transcatheter therapy in 1999 using Amplatzer septal occluder and Joseph mitral balloon catheter (15). Since then, several successful cases have been reported (16,17). Indications for transcatheter therapy include ASD with Qp/Qs ratio >1.5 with adequate rims, symptomatic moderate to severe MS with valve morphology favorable for balloon mitral valvotomy, and any degree of PH as long as the shunt across the ASD remains left to right (18).
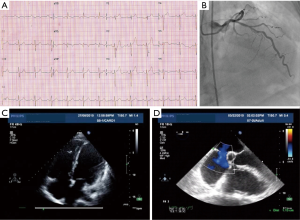
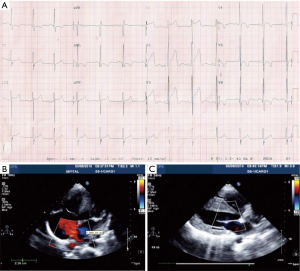
With aging, the shunt volume through an ASD can increase due to a rise in left-sided filling pressures secondary to the development of hypertensive or ischemic heart disease (Figure 1) with or without other risk factors such as diabetes mellitus, atrial fibrillation and chronic kidney disease. Hence in the elderly, an anatomically small defect can lead to significant left to right flow resulting in right ventricular volume overload with or without PH. In the elderly, an ASD with 1 cm or more of effective diameter on echocardiography can be hemodynamically important (11). Jategaonkar et al. reported 96 adults, aged 60–84 years, undergoing ASD closure mainly for correction of right heart overload where the mean defect diameter on transesophageal echocardiography was 15±5 mm, and the corresponding Qp:Qs was 2:0 (19). Much less commonly, LV restrictive physiology is seen in association with hypertrophic cardiomyopathy (Figure 2).
A modest increment in LA pressure is often seen after closure of an ASD, but large changes in pressure are uncommon and seen primarily in older patients with hypertension and/or LV dysfunction (Figure 3) (20-22). Before proceeding with ASD closure in such patients, consideration should be given to test occlusion of the ASD to evaluate the potential for a deleterious increase in LA pressure (Figures 4 and 5). Holzer et al. suggest that an LA mean pressure increase exceeding 3 mmHg during test occlusion should prompt a consideration for the use of a fenestrated occluder (Figure 6) that reduces, but does not abolish, the left to right interatrial shunt (20). This approach by decreasing the shunt flow and reducing the volume load on the right heart lowers the LA pressure than would be expected with a complete closure (22). Humenberger et al. used an LA mean pressure increment of 10 mmHg (during test occlusion) to identify high-risk patients (21). The exact rise in LV end diastolic pressure after temporary occlusion of the ASD that would identify patients who might benefit from a fenestrated occluder rather than a complete closure of the defect remains unknown.
ASD with PAH
ASD associated with PAH can present with different clinical scenarios. One includes adults without or only with mild pulmonary vascular disease and a large shunt. These are patients who can safely undergo shunt closure. Another includes adults with large defect, severe, irreversible pulmonary vascular disease, shunt reversal and chronic cyanosis, that is, Eisenmenger syndrome. These are patients who need to be managed medically. Still another includes small defect with severe disproportionate PAH where the ASD is more likely to be an innocent bystander and not the true etiology for elevated pulmonary artery pressure (PAP). In this subset as well, the closure of ASD is likely to be deleterious and will need to be treated with pulmonary vasodilators
However, the most difficult subset is the one where the large left to right shunt at the atrial level is associated with severe PAH.
There are no set guidelines as to the exact cut off level of PAP or pulmonary vascular resistance index (PVRI), which will preclude closure of an ASD in the presence of severe PAH. As per the American College of Cardiology (ACC) and American Heart Association (AHA) 2008 guidelines for management of adults with CHD (23), closure of an ASD, either percutaneously or surgically, may be considered in the presence of net left to right shunting, PAP less than two thirds systemic levels, PVR less than two thirds systemic vascular resistance, or when responsive to either pulmonary vasodilator therapy or test occlusion of the defect (Figure 7). Unfortunately, these guidelines fail to define the precise criteria for responsiveness.
Despite the lack of solid data, acute pulmonary vasodilator testing is commonly recommended in cases with a baseline PVR index of 6–9 Wu.m2 to test the residual dilatatory capacity of the pulmonary vascular bed. A decrease of 20% in PVR, a decrease of 20% in PVR:SVR ratio, a final PVR index <6 Wu.m2 and a final PVR:SVR ratio of <0.33 are considered to indicate a favorable outcome after shunt closure (24). The major limitation of these recommendations is that they are based on data derived from case series or from expert opinion and are solely dependent on numbers obtained at cardiac catheterization without taking into consideration clinical, radiological, electrocardiographic and echocardiographic parameters.
In our practice, we use a multidimensional approach on a case-by-case basis to decide on the operability. Assessment of pre-tricuspid shunts with severe PHT from the point of operability appears to be much more difficult as compared to post-tricuspid shunts. This is due to the fact that the post tricuspid shunts are either systolic or continuous and therefore tend to become bidirectional at rest or during exercise when PA pressure becomes systemic with irreversible increase in PVR (25). In the case of an ASD, since the shunt is predominantly diastolic, it may continue to remain left to right despite significantly elevated PAP and PVR. The shunt becomes bidirectional only when the RV diastolic pressure rises. Thus, absence of desaturation either at rest or on exercise does not rule out irreversible PAH. Similarly, in some patients with more than mild tricuspid regurgitation, there might be desaturation at rest or on exertion without there being irreversible PAH due to the TR jet being directed towards the left atrium. These issues tend to complicate the selection process clinically.
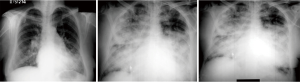
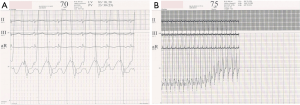
Younger age, symptoms of pulmonary over circulation, absence of cyanosis at rest or on exercise and presence of flow murmur across the tricuspid and pulmonary valve is suggestive of reversible PAH. On the other hand, presence of cyanosis or clubbing, precordial heave, absence of flow murmurs and narrowly split second heart sound (A2-P2), are pointers towards high PVR that is likely to be irreversible. Intensity of the second heart sound in ASD is not a good indicator of the severity of PH leave alone its reversibility (13). Peripheral pruning in the lateral 1/3rd of lung fields on a plain X-ray chest is usually suggestive of irreversible PAH (26). Evidence of qR or monophasic R waves in the lead V1 with T wave inversion beyond V3 is an indicator of systemic or even suprasystemic PA pressure (27). 2D echocardiography with color Doppler can assess the size of the defect, its suitability for transcatheter closure and also give a clear picture about the direction of the shunt. Estimating PO2 post exercise is an important parameter of operability but may not be as reliable as in post tricuspid shunts (28).
Cardiac catheterization has been considered as a gold standard to determine the operability in this subset of patients. It is performed routinely in this subset of patients both on room air, while breathing 100% oxygen and with inhaled or intravenously injected pulmonary vasodilators. But it is essential to know the various limitations of the catheterization data (29). In most centers, including ours, O2 consumption is assumed and not actually measured. While estimating the AV oxygen difference, the dissolved oxygen is assumed to be zero, which is not true especially while the patient is breathing O2. Similarly, for obtaining mixed venous blood oxygen content, various proportions of superior and inferior vena cava oxygen content are assumed. Calculation of PVR may be flawed due to error in the pressure estimate secondary to suboptimum zeroing, balancing and frequency response of pressure measuring transducers (30). The Poiseuille-Hagen equation used for estimating PVR is in the strict sense applicable to steady state Newtonian fluid in a rigid system. Cardiovascular system is non-rigid and blood is a non-Newtonian fluid (31). All these factors are known to introduce errors in various estimates used to determine the operability based on cardiac catheterization data alone. Hence the need to take into consideration other clinical, radiological and echocardiographic parameters as was done in this study.
A detailed assessment and optimal management can make a significant impact on the prognosis of such patients. In a recent study by Manes et al., of the 192 adults, 90 (47%) had Eisenmenger syndrome, 48 (25%) had shunt-induced PAH, 10 (5%) had PAH in the setting of a small defect and 44 (23%) had PAH after corrective surgery. Outcome was worst for patients with PAH after corrective surgery (32). Ten-year Kaplan-Meier survival estimates were 89% (95% CI: 79–94%) for Eisenmenger patients, 93% (95% CI: 76–99%) for shunt-induced PH, 88% (95% CI: 39–98%) for PAH with small defects (50% had small ASD, half had a small ventricular septal defect) and 65% (95% CI: 43–80%) for the patients with PAH after corrective surgery. In comparison, 10-year survival was 46% (95% CI: 38–54%) in a group of adults with idiopathic PAH treated during the same period in the center. These data clearly suggest that closure of ASD in an adult with advanced shunt-induced PH resulting in persistent or recurrent PAH after shunt closure can be hazardous for the patient. In all other scenarios, there is a communication between the pulmonary and the systemic circulation, which allows maintaining cardiac output by acting as relief valve, at the cost of central cyanosis.
Our experience
Between January 2009 to December 2014, 25 cases of ASD with severe PH were referred to us for further management. Using our multi-modality approach, we decided to close the ASD in 6 patients all of whom had a baseline mean PAP >75% of mean systemic PAP.
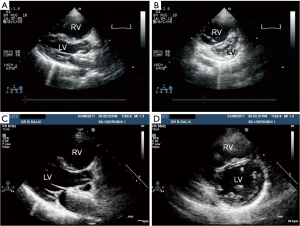
All the patients reported significant symptomatic improvement; 5/6 being in NYHA class I, and 1 in class II. Doppler examination revealed significant drop (>40%) in their PAP at the time of their last follow up although none had complete normalization of their PAP. This was confirmed on cardiac catheterization in 4/6 patients. Impressive reversed remodeling of the right atrium and the right ventricle was seen in all the patients (Figure 8).
All our patients who showed a great deal of symptomatic relief and significant hemodynamic improvement had following variables in common: age <30 years, absence of cyanosis at rest, flow murmurs across the tricuspid and pulmonary valves, mild cardiomegaly without peripheral pruning of vasculature on chest X-ray, SpO2 >95% on room air, anatomically large defect (>25 mm) on TEE with exclusive left to right shunt on color flow mapping and a large baseline shunt with a Qp:Qs of >2.5:1.
All of them were continued on Phosphodiesterase inhibitors (sildenafil) and/or Endothelin receptor antagonist (bosentan) following closure of ASD. These were well tolerated and continued till the last follow-up. Pulmonary vasodilators are known to reduce the PAP in patients with primary PH (PPH) (33). Going by that logic they would also reduce the PAP in these patients after closure of ASD.
Conclusions
Closing ASD in patients with moderate or severe PAH as well as in those with significant LV restriction is a clinical dilemma. Using data from clinical parameters as well as from multiple modalities of investigations can help in making a reasonably accurate decision. In case of any doubt, it is better to use a fenestrated device or leave the ASD open. Continuing long-term pulmonary vasodilators even after ASD closure may be advisable since PAH can recur after a significant drop of PAP immediately following the device placement.
Acknowledgements
The authors would like to thank Prafulla Kerkar and Biswajit Bandopadhyay for providing images and related materials for the manuscript.
Footnote
Conflicts of Interest: Bharat Dalvi is a Consultant for St. Jude Medical. Shreepal Jain has no conflicts of interest to declare.
References
- van der Linde D, Konings EE, Slager MA, et al. Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis. J Am Coll Cardiol 2011;58:2241-7. [Crossref] [PubMed]
- Engelfriet PM, Duffels MG, Moller T, et al. Pulmonary arterial hypertension in adults born with a heart septal defect: the Euro Heart Survey on adult congenital heart disease. Heart 2007;93:682-7. [Crossref] [PubMed]
- Vogel M, Berger F, Kramer A, et al. Incidence of secondary pulmonary hypertension in adults with atrial septal or sinus venosus defects. Heart 1999;82:30-3. [Crossref] [PubMed]
- de Lezo JS, Medina A, Romero M, et al. Effectiveness of percutaneous device occlusion for atrial septal defect in adult patients with pulmonary hypertension. Am Heart J 2002;144:877-80. [Crossref] [PubMed]
- Yong G, Khairy P, De Guise P, et al. Pulmonary arterial hypertension in patients with transcatheter closure of secundum atrial septal defects: a longitudinal study. Circ Cardiovasc Interv 2009;2:455-62. [Crossref] [PubMed]
- Steele PM, Fuster V, Cohen M, et al. Isolated atrial septal defect with pulmonary vascular obstructive disease--long-term follow-up and prediction of outcome after surgical correction. Circulation 1987;76:1037-42. [Crossref] [PubMed]
- Craig RJ, Selzer A. Natural History and Prognosis of Atrial Septal Defect. Circulation 1968;37:805-15. [Crossref] [PubMed]
- Konstantinides S, Geibel A, Olschewski M, et al. A comparison of surgical and medical therapy for atrial septal defect in adults. N Engl J Med 1995;333:469-73. [Crossref] [PubMed]
- Hörer J, Muller S, Schreiber C, et al. Surgical closure of atrial septal defect in patients older than 30 years: risk factors for late death from arrhythmia or heart failure. Thorac Cardiovasc Surg 2007;55:79-83. [Crossref] [PubMed]
- Vasan RS, Shrivastava S, Kumar MV. Value and limitations of Doppler echocardiographic determination of mitral valve area in Lutembacher syndrome. J Am Coll Cardiol 1992;20:1362-70. [Crossref] [PubMed]
- Webb G, Gatzoulis MA. Atrial septal defects in the adult: recent progress and overview. Circulation 2006;114:1645-53. [Crossref] [PubMed]
- Lutembacher R. De la sténose mitrale avec communication interauriculaire. Arch Mal Coeur 1916;9:237-60.
- Perloff JK. The Clinical Recognition of Congenital Heart Disease, 4th edition. Philadelphia: Saunders, 1994:323-8.
- Ruiz CE, Gamra H, Mahrer P, et al. Percutaneous closure of a secundum atrial septal defect and double balloon valvotomies of a severe mitral and aortic valve stenosis in a patient with Lutembacher’s syndrome and severe pulmonary hypertension. Cathet Cardiovasc Diagn 1992;25:309-12. [Crossref] [PubMed]
- Joseph G, Rajpal KA, Kumar KS. Definitive percutaneous treatment of Lutembacher’s syndrome. Catheter Cardiovasc Interv 1999;48:199-204. [Crossref] [PubMed]
- Chau EM, Lee CH, Chow WH. Transcatheter treatment of a case of Lutembacher syndrome. Catheter Cardiovasc Interv 2000;50:68-70. [Crossref] [PubMed]
- Ledesma M, Martinez P, Cázares MA, et al. Transcatheter treatment of Lutembacher syndrome: Combined balloon mitral valvuloplasty and percutaneous atrial septal defect closure. J Invasive Cardiol 2004;16:678-9. [PubMed]
- Aminde LN, Dzudie A, Takah NF, et al. Current diagnostic and treatment strategies for Lutembacher syndrome: the pivotal role of echocardiography. Cardiovasc Diagn Ther 2015;5:122-32. [PubMed]
- Jategaonkar S, Scholtz W, Schmidt H, et al. Percutaneous closure of atrial septal defects: echocardiographic and functional results in patients older than 60 years. Circ Cardiovasc Interv 2009;2:85-9. [Crossref] [PubMed]
- Holzer R, Qi-Ling C, Hijazi ZM. Closure of a moderately large atrial septal defect with a self-fabricated fenestrated Amplatzer septal occluder in an 85-year-old patient with reduced diastolic elasticity of the left ventricle. Catheter Cardiovasc Interv 2005;64:513-518. [Crossref] [PubMed]
- Humenberger M, Rosenhek R, Gabriel H, et al. Benefit of atrial septal defect closure in adults: impact of age. Eur Heart J 2011;32:553-60. [Crossref] [PubMed]
- Bruch L, Winkelmann A, Sonntag S, Scherf F, Rux S, Grad MO, Kleber FX. Fenestrated occluders for treatment of ASD in elderly patients with pulmonary hypertension and/or right heart failure. J Interv Cardiol 2008;21:44-9. [Crossref] [PubMed]
- Warnes CA, Williams RG, Bashore TM, et al. ACC/AHA 2008 guidelines for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Develop Guidelines on the Management of Adults With Congenital Heart Disease). Developed in Collaboration With the American Society of Echocardiography, Heart Rhythm Society, International Society for Adult Congenital Heart Disease, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol 2008;52:e143-263. [Crossref] [PubMed]
- Beghetti M, Galie N, Bonnet D. Can "inoperable" congenital heart defects become operable in patients with pulmonary arterial hypertension? Dream or reality? Congenit Heart Dis 2012;7:3-11. [Crossref] [PubMed]
- Viswanathan S, Kumar RK. Assessment of Operability of Congenital Cardiac Shunts with Increased Pulmonary Vascular Resistance. Catheter Cardiovasc Interv 2008;71:665-70. [Crossref] [PubMed]
- Virmani R, Roberts WC. Pulmonary arteries in congenital heart disease: a structure-function analysis. In: Roberts WC. editor. Adult congenital heart disease. 1st ed. Philadelphia, PA: Davis, 1987:77-130.
- Toscano Barboza E, Brandenburg RO, Swan HJ. Atrial septal defect: the electrocardiogram and its hemodynamic correlation in 100 proved cases. Am J Cardiol 1958;2:698-713. [Crossref] [PubMed]
- Laksmivenkateshiah S, Singhi AK, Vaidyanathan B, et al. Decline in arterial partial pressure of oxygen after exercise: a surrogate marker of pulmonary vascular obstructive disease in patients with atrial septal defect and severe pulmonary hypertension. Cardiol Young 2011;21:292-8. [Crossref] [PubMed]
- Wilkinson JL. Congenital heart disease: hemodynamic calculations in the catheter laboratory. Heart 2001;85:113-20. [Crossref] [PubMed]
- de Vecchi A, Clough RE, Gaddum NR, et al. Catheter-Induced Errors in Pressure Measurements in Vessels: An In-Vitro and Numerical Study. IEEE Trans Biomed Eng 2014;61:1844-50. [Crossref] [PubMed]
- Roos A. Poiseuille’s law and its limitations in vascular systems. Med Thorac 1962;19:224-38. [PubMed]
- Manes A, Palazzini M, Leci E, et al. Current era survival of patients with pulmonary arterial hypertension associated with congenital heart disease: a comparison between clinical subgroups. Eur Heart J 2014;35:716-24. [Crossref] [PubMed]
- Galiè N, Ghofrani HA, Torbicki A, et al. Sildenafil use in Pulmonary arterial hypertension (SUPER) study group. Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med 2005;353:2148. [Crossref] [PubMed]



