Infections after lung transplantation
Introduction
Infection is a common complication after lung transplantation, its recognition may be difficult, and signs and symptoms may sometimes be misleading. Since lifelong immunosuppression is mandatory to prevent acute and chronic rejection, immune system impairment contributes to increased patient vulnerability to infectious agents. At least four clinical scenarios are classically indicated as possible high-risk situations: recipients can host infections from a wide range of microorganisms (especially among patients with cystic fibrosis) or they may become colonized with nosocomial organisms; lung grafts could promote the transmission of infections from donors and, finally, transplanted patients are prone to major infection from agents that are relatively innocuous in an immunocompetent host (1).
Time is a determining factor for the development of infection after lung transplantation; infections are the second cause of mortality within the first 30 days after transplantation (19.2%) but reach the first position (37.3%) between 30 days and 1 year. After one year from surgery, the mortality rate for infections decreases modestly, though always remaining among the main causes of graft failure (2). Time also affects the category of infections the transplant patient can develop: in the first post-operative month, the etiologic cause of the infection is often to be found in germs present in the donor or recipient. Nosocomial infections are frequent in this period, as are infections related to technical problems [catheter infections, surgical site infections (SSI), dehiscence of bronchial anastomoses]. From 1 to 6 months after transplantation, opportunistic agents as well as reactivation of latent infections are common. Six months after transplantation, infections due to community-acquired pathogens are the major concern (1).
The diagnosis of infection is a difficult task in lung transplantation since symptoms such as fever, fatigue, loss of appetite, night sweats, chills, and pain may be unremarkable or absent due to immunosuppressive therapy. Loss of lung function may be observed in lung infection, but is also common in acute and chronic rejection. In addition, white blood cell count is frequently altered from concomitant corticosteroid use. On the contrary, evidence supports the utility of serum procalcitonin to diagnose infections among solid organ transplant recipients, with accuracy rates similar to those of the general population (3).
Considering that pneumonia is the most common form of infection after lung transplantation, chest computed tomography (CT) is a useful instrument for the diagnosis of most of these disorders. Parenchymal consolidations, pleural effusions, micronodules, and interlobular septa thickening are CT common findings in bacterial infections. Invasive aspergillosis presents a CT pattern consisting of pulmonary opacities, with or without excavation, surrounded by a ground glass halo. Ground glass opacities with scattered micronodules (tree-in-bud pattern), bronchiectasis and consolidations are the most common CT findings in viral infections. Pneumocystis infection shows diffuse ground glass opacities that often spare the sub-pleural portion of the lung; focal consolidations, interlobular septa thickening (“crazy paving” pattern) and micronodules are common. Cystic lesions in the upper lobes complicated by pneumothorax or pneumomediastinum are also possible in opportunistic Pneumocystis infection (4).
Bronchoalveolar lavage and transbronchial biopsies have an excellent diagnostic yield for bacteria and opportunistic pathogens. Given that Aspergillus colonization is a demonstrated risk factor for severe airway complications and invasive aspergillosis, surveillance with flexible bronchoscopy is advisable during the first year after transplantation (5).
Pretransplantation infections and vaccinations
Before transplantation, it is essential to identify patient airway colonization and to investigate former infectious disease history. Such information is usually available in cystic fibrosis patients, given constant monitoring. Patients with other indications for transplantation should be carefully investigated for possible infectious diseases, even in latent form. At the time of listing, a wide panel of laboratory tests is recommended for candidates; such panel includes serological tests for cytomegalovirus (CMV), Epstein-Barr virus (EBV), hepatitis B (HBV) and C viruses, herpes simplex virus (HSV), human immunodeficiency virus, Treponema pallidum and varicella-zoster virus (VZV). Furthermore, bronchoalveolar lavage may provide information on bronchial flora to guide antibiotic therapy before and/or after transplantation. Patients who are discovered to be methicillin-resistant Staphylococcus aureus carriers should receive an eradication protocol for the upper and/or lower respiratory tract (6).
If the estimated rate of methicillin-resistant Staphylococcus aureus colonization in patients evaluated for solid organ transplantation is 8.5%, the estimated vancomycin-resistant enterococcus colonization rate reaches 12% of candidates (7). The driver for this high prevalence is exposure to healthcare settings; therefore, meticulous respect for hygiene rules among health professionals must be constantly stressed. Vancomycin-resistant enterococcus is not considered highly pathogenic but careful surveillance is mandatory especially after transplantation. Finally, carbapenem-resistant enterobacteriaceae (including carbapenemase producing Klebsiella pneumoniae—KPC) are highly resistant Gram-negative bacteria that can colonize the recipient bowel exposing the patient to severe infection with high mortality rates. Currently, no decolonization strategies have been developed but fecal microbiota transplantation is a procedure that creates hope for the future (8).
History of possible tuberculosis (TB) must be carefully investigated; recent migration flows must increase the degree of alert, even towards low risk citizens from western countries. Should active TB be identified in a potential lung transplant candidate, proper therapy should be completed prior to listing. Tuberculin skin testing and/or QuantiFERON Gold TB test is recommended in all patients at listing.
Mycobacterium abscessus complex, a group of rapidly growing non-tuberculous mycobacteria (NTM), has emerged as a major problem, particularly in cystic fibrosis candidates for lung transplantation. According to the American Thoracic Society and the Infectious Diseases Society of America criteria, the diagnosis of non-tuberculous mycobacterium disease must include radiological signs (excavated opacities and/or solid nodules, multifocal bronchiectasis, “tree-in-bud”) and positive culture from bronchoalveolar lavage or lung biopsy or at least two separated expectorated sputum samples (9). Many centers consider patients with Mycobacterium abscessus disease not eligible for lung transplantation. Centers that accept patients with this condition require the infection be under control and that treatment be tolerated by the patients. In addition, the surgical procedure needs special precautions: complete hilar and mediastinal lymphadenectomy, pleural cavity washing with amikacin solution and change of surgical gloves after pneumonectomy. Postoperative antimycobacterial antibiotic treatment should last for at least 1 month, if not lifelong (10).
Severe disease and graft rejection could affect young transplant recipients due to infection from vaccine-preventable agents; despite this evidence, immunization is sometimes inadequate in young patients who are listed for lung transplantation mainly for fear of vaccine-related side effects. It is advisable that transplant centers develop a specific vaccination guideline or, at least, follow the national vaccination program prior to lung transplantation. HBV, pneumococcal and meningococcal vaccinations are among the uncommon immunizations that should be implemented, bearing in mind that a time lapse of at least 3 months is advisable between vaccination and transplantation. Monitoring of immunization to vaccine-preventable infections before transplantation is quite common among transplantation centers and offers the opportunity to proceed with opportune vaccinations if needed (11). Finally, influenza vaccination is highly recommended both before and after transplantation for the patient as well as for family contacts.
SSI and antibacterial prophylaxis
Following CDC 1992 indications, infections after surgery are defined as SSI and classified into superficial incisional SSI, deep incisional SSI and organ and/or space SSI (12). To prevent SSI following lung transplantation meticulous attention to asepsis rules is mandatory: accurate skin preparation with products containing iodophors or chlorhexidine gluconate in aqueous or alcohol-based solution is needed; the sterile field should be prepared as close as possible to the time of use; once sterile drapes are placed they should not be rearranged. Renewal of sterile drapes may be considered after a number of hours of surgery. In recipients with a high bacterial burden, appropriate washing of the pleural cavity as well as the recipient trachea and bronchial stumps with iodophors aqueous solution or antibiotic solution prior to implantation of the graft is indicated. Anesthesiologists are requested to substitute suction catheters and the bronchoscope after the graft implantation, particularly in recipients with prior airway colonization. Closed wound suction units with a suitable catheter are advisable in patients with thick adipose tissue or large breasts, to prevent incisional SSI.
There is a sufficient consensus for the use of perioperative antibiotics in general thoracic surgery even though the appropriate duration of the antibiotic administration is not fully shared (13). Unfortunately, neither guidelines nor standard treatments exist regarding the choice of perioperative antibiotic in lung transplantation, but regular prophylaxis is recommended for all recipients. For patients without septic disease who receive a graft free from documented bacterial colonization, ceftazidime is considered the first choice; administration should begin before the incision and be repeated 3 times a day according to renal function. Generally, a period of 48–72 hours is considered appropriate. Piperacillin-tazobactam or cefepime are good alternatives to ceftazidime; levofloxacin could be used in patients with beta-lactam allergy. Vancomycin is generally used, in association with ceftazidime, to cover Gram-positive bacteria. Like ceftazidime, vancomycin should be started before incision; administration should continue twice a day for 48–72 hours. When the postoperative course is uneventful and intubation time is short, the initial antibiotic pattern can be interrupted as scheduled, but it is advisable to perform regular bronchoscopic controls (every seven days for the first month in our department). If the postoperative course presents complications, antibiotic prophylaxis should be continued and eventually adapted to identified pathogens. Patients who receive lungs from donors infected with known pathogens should obviously be treated with appropriate antibiotic scheme for a reasonable period (at least until two consecutive bronchoalveolar lavages are negative).
Patients who receive lung transplantation for high bacterial burden conditions (cystic fibrosis, bronchiectasis, etc.) require special attention. Generally, continuation of patient specific antibiotic patterns is advisable for at least 30 days; in case of highly resistant germs, treatment may be continued following discharge at home for another 30–60 days. Special attention should be paid to Burkholderia cenocepacia carriers due to the high probability of postoperative uncontrollable systemic virulence. Currently, the majority of transplant centers believe that patients harboring this specific species of Burkholderia are not fit to receive lung transplantation (14).
Antifungal prophylaxis
Lung transplant recipients have a higher risk of fungal infections than other solid-organ recipients. Aspergillus is the most frequent pathogen in lung transplantation whereas Candida is the leading pathogen in other solid-organ transplants. Seldom, other fungi can also cause severe infections; among them Cryptococcus, Fusarium, Scedosporium, mucormycetes and endemic agents (Blastomyces, Coccidioides and Histoplasma).
Invasive Aspergillosis is one of the most feared infectious complications after pulmonary transplantation; it occurs generally within 1 year, but it can affect patients up to 3 years after transplantation. Bronchial anastomotic infections commonly occur within the first 3 months after transplantation and may evolve towards Aspergillus ulcerative tracheobronchitis, which is among the worst complications of the surgical procedure.
Antifungal prophylaxis efficacy is well documented in liver transplantation, on the contrary, there is still a lack of evidence on the optimal strategy in lung transplantation. As a consequence, prophylaxis strategies for reducing fungal infection are heterogeneous among lung transplantation centers. A systematic review and meta-analysis, collecting seven studies (mostly retrospective and monocentric) published in 2016, concluded that universal fungal prophylaxis compared with no or targeted prophylaxis reduces the incidence of invasive fungal infection in lung transplant patients (15).
Administration of a systemic azole, inhaled antifungal medication or the combination of the two strategies are possible approaches to universal antifungal prophylaxis. On the other hand, the administration of antifungal agents to high risk patients (cystic fibrosis, complicated postoperative period, advanced donor age, induction therapy, airway ischemia) or to patients with documented airway colonization are defined as target therapy and preemptive therapy, respectively.
Systemic administration of voriconazole or posaconazole is the most rational strategy for universal prophylaxis, but hepatotoxicity and drug interactions should be carefully monitored. The duration of such prophylaxis is also heterogeneous among centers and it ranges between 6 to 12 months. Liposomal Amphotericin B is the drug generally used for inhaled-strategy prophylaxis; daily administration for 1–2 weeks followed by once weekly for 1–3 months is one of the several protocols in use. In our center, we prefer universal prophylaxis with voriconazole for 6 months with the addition of preemptive therapy when needed.
Pneumocystis pneumonia (PCP) prophylaxis
Pneumocystis are unicellular fungi that constitute important pathogens that may cause severe disease in immunocompromised hosts, defined as PCP. It should be noted that the risk for PCP is higher among lung transplant recipients compared with other organ recipients. Fortunately, since the 1980’s, therapy with trimethoprim-sulfamethoxazole, or parenteral pentamidine, has dramatically improved the outcomes of patients who develop PCP.
Lifelong universal prophylaxis with trimethoprim-sulfamethoxazole once-daily or thrice-weekly is highly recommended. For patients who are intolerant to trimethoprim-sulfamethoxazole inhaled pentamidine is an alternative treatment; if the intolerance was discovered before listing, desensitization should be attempted.
CMV
CMV is a beta herpes virus and, after bacterial pneumonia, is the second most common infection in lung transplant recipients (16), being one of the main cause of morbidity and mortality in these patients. It has been associated with tissue injury and infection, and some authors indicate CMV as a risk factor for acute and chronic rejection (17,18); finally, CMV may play an immunomodulatory role promoting several other opportunistic infections (19).
Clinical manifestations
Individuals are primarily infected through physical contact, which involves direct inoculation of infected cells or body fluids, and then harbour CMV for life. CMV generally produces an asymptomatic or a mild acute illness in immunocompetent patients, whilst it can be the cause of severe non-specific syndrome (with fever, malaise, myalgias, arthralgias, leukopenia and thrombocytopenia) and/or organ disease (pneumonia, encephalitis, retinitis, hepatitis or colitis with ulcerations) in an immunosuppressed host.
After lung transplantation, CMV infection may occur in different ways (16):
- By transmission from the graft from a CMV seropositive donor;
- By reactivation of latent infection in a previously seropositive recipient;
- By contact with a CMV infected individual;
- By transfusion of hemocomponents from a CMV seropositive blood donor.
The lung has been identified as a major site of CMV latency and recurrence; lung transplantation is associated with the transfer of a larger CMV load than other solid organs, increasing the risk of CMV infection and disease in these recipients.
Following the American Transplantation Society definitions, CMV infection is defined by the evidence of CMV replication regardless of symptoms, whilst CMV disease is characterised by the presence of a constitutional symptomatic syndrome or tissue-invasive disease (20).
Diagnosis
At present several diagnostic tests are available for CMV: serology, qualitative and quantitative polymerase chain reaction (PCR), pp65 antigenemia, culture, and histopathology.
CMV serology should be performed before transplantation on both the organ donor (D) and the recipient (R), since their serostatus is a key predictor of infection risk and management, with seronegative recipients of seropositive organs (D+/R−) having the highest risk, D+/R+ and D−/R+ intermediate risk, D−/R− the lowest risk. Other relevant risk factors for CMV infection after transplantation are the use of anti-lymphocytes antibodies as means of immunosuppression, high doses of glucocorticoids and several gene polymorphisms.
After lung transplantation, viral load testing is the cornerstone for diagnosis and monitoring for CMV infection and disease, and this can be achieved by antigenemia testing (pp65) or a quantitative PCR-based assay; the latter is currently the most widely used method because of better precision, broader linear range, faster turnaround time, higher throughput, and less risk of contamination (21). The diagnosis of tissue-invasive CMV disease should be confirmed by immunohistochemistry or in situ DNA hybridization. Serology should not be used to diagnose active CMV infection or disease (20).
Monitoring CMV load is also a useful approach for assessing the likelihood of drug resistance, which should be suspected in case of rising or persistently elevated viral load regardless of ongoing antiviral therapy. Resistance testing is currently performed by means of genotypic assays directly from clinical specimens: the most common mutations affect UL97 phosphotransferase and confer resistance to ganciclovir (22); UL54 DNA polymerase mutations may occur as second-step mutations in patients who already have a UL97 mutation, causing different combinations of resistance to ganciclovir, foscarnet, and/or cidofovir (23).
Recent studies focused on CMV-specific cellular immunity, which plays a crucial role in containing viral replication and can be evaluated performing QuantiFERON-CMV assay on plasma by stimulation of CD8+ T-cell responses and enzyme-linked immunosorbent spot (ELISPOT) assay on whole blood by stimulation of CD4+ and CD8+ T-cell responses; other test available include major histocompatibility complex multimer staining assays and intracellular cytokine staining assays (20).
Prevention
With regard to CMV prevention, two strategies have been used:
- Universal prophylaxis of recipients at high/medium risk for infection (i.e., all but D−/R−), usually with oral valganciclovir;
- Pre-emptive treatment of recipients with infection in order to abort the development of disease, meaning that oral valganciclovir is initiated when viral replication has reached a certain threshold.
Currently available guidelines endorse antiviral prophylaxis against pre-emptive approach based on published evidence on the safety and efficacy of the former strategy (20,24).
The American Society of Transplantation (AST) guidelines recommend 12 months of prophylaxis among CMV D+/R− lung transplant recipients and 6 to 12 months for CMV D+/R+ and D−/R+ lung transplant recipients based on the patient’s risk of reactivation, drug toxicity and viral load monitoring. Prophylactic treatment should start with intravenous ganciclovir 5 mg/kg once daily, with dose adjustment for renal insufficiency; once the patient is absorbing oral medications, it can be switched to oral valganciclovir 900 mg once daily, with dose adjustment for renal insufficiency. Individuals receiving anti-lymphocyte antibodies or pulse steroids should receive CMV prophylaxis for at least 1 to 3 months after this antirejection regimen is completed. Finally, CMV immune globulin may be used as an adjunct to conventional antiviral agents in high-risk patients, but should not be used alone (25).
A pre-emptive strategy may be useful after the prophylaxis has ended, relying on constant viral load monitoring. Unfortunately, no effective vaccination is yet available for CMV.
Treatment
The approach to treatment of active disease is based on intravenous ganciclovir and oral valganciclovir; it is important to give appropriate doses, since both these drugs should be adjusted for renal function but inadequate dosing may reduce efficacy and lead to resistance (20); their most common and significant adverse effect is myelotoxicity, in particular leuko-neutropenia and thrombocytopenia.
Valganciclovir has been showed non-inferior to intravenous ganciclovir in non-life threating CMV disease (26) and therefore should be the regimen of choice (900 mg twice daily) in these cases. However, in severe and/or invasive disease, IV ganciclovir (5 mg/kg every 12 hours) is preferable; the efficacy of antiviral treatment can be augmented by reducing the intensity of immunosuppression (21) and/or, possibly, with administration of CMV immune globulin (limited evidence).
Treatment should be administered until resolution of symptoms or viremia (two consecutive negative serum CMV load 1 week apart) occurs and/or for a minimum of 2 weeks; it should be followed by secondary suppression with oral valganciclovir 900 mg once daily for 1 to 3 months.
In case of documented ganciclovir resistance, alternatives may be IV foscarnet or cidofovir, even if the use of the latter is limited by poor clinical experience and potential severe nephrotoxicity.
Community acquired respiratory viruses (CARV)
CARV infections are of concern in lung transplantation both in terms of associated morbidity and mortality (27), and for the potential subsequent increase risk of acute and chronic lung allograft dysfunction.
The viral agents classifying as CARV generally include the following: influenza, parainfluenza (PIV), human rhinovirus (HRV), adenovirus (ADV), respiratory syncytial virus (RSV), and coronaviruses (COVs), but may be extended to other more recently identified agents such as human metapneumovirus (hMPV) and bocavirus (BCV).
The reported incidence of CARV infections among lung transplant recipients is very diverse and ranges between 7.7% and 64% (28-30). The reasons for this large variation in reported incidence are largely dependent on the applied diagnostic techniques and on seasonality issues (particularly for influenza and RSV). Studies based on serology or viral cultures alone tend to report lower detection rates whereas the more recent application of molecular biology techniques has largely expanded the diagnostic identification of these viruses. Several rapid diagnostic tools [PCR assays for serum, swab, bronchoalveolar lavage (BAL) and other fluids] are currently available to help diagnosis and prompt management. A further development is the availability of multiplex PCR assays, that allow testing for a panel of viruses in a single determination (31).
Treatment
For a large number of viruses there is no currently available effective pharmacological treatment and management is largely supportive. For those viruses for which treatment options are available, it has shown that timely initiation is essential in order to limit complications such as ICU admission and death (32). The development of neuraminidase inhibitors (oseltamivir and zanamivir) has provided a major breakthrough in the treatment and prevention of influenza infection. These agents have largely supplanted previous drugs such as the M2 inhibitor amantadine towards which the vast majority of influenza strains (up to 90%) have developed resistance. Ribavirin is a synthetic nucleoside analogue that has been used in the treatment of different viral agents of the paramyxovirus family. It is generally administrated intravenously or in nebulised form. Table 1 summarises available therapeutic options for CARV infections. The possible therapeutic role of steroids in the context of viral infections still remains to be completely understood.
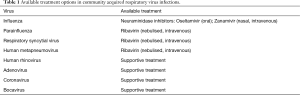
Full table
Different studies have brought evidence both for and against the association between CARV infections and acute or chronic rejection in lung transplant recipients. A systemic review with pooled analysis involving 34 studies on CARV infections in lung transplant recipients failed to detect any association between viral infections and acute rejection (33). Data is as yet insufficient to accept or refute the association between CARV infections and the development of chronic rejection (34).
EBV
EBV is a gamma herpes virus (human herpes virus 4) and is the etiologic agent of infectious mononucleosis, persisting asymptomatically for life in most adults (90–95% in the world). Humans are the only known host for EBV. In the general population EBV is transmitted by exposure to infected body fluids such as during coughing or sneezing, or by sharing drinking or eating utensils. Following solid organ transplantation, EBV transmission from a seropositive donor to a young seronegative recipient is an important source of infection. Recent evidence indicates that approximately 10% of lung transplant recipients present EBV mismatch (D+/R−) (35).
Acute EBV infection causes a polyclonal expansion of B cells hosting the virus and viral antigens expressed by these B cells elicit a T cell response against the majority of the infected B cells. However, a small proportion of infected B cell may escape immune surveillance; even if reactivation is not a prominent issue, in lung and other solid organ transplant recipients, when T cell immunity is waned by immunosuppressive regimen, these latently infected B cells can initiate posttransplant lymphoproliferative disorders (PTLD) (36). Recent studies suggest routine monitoring of EBV viral load by PCR on blood specimens from transplanted patients to detect possible PTLD at an early stage (37). Younger patients, particularly if EBV seronegative prior to lung transplantation are at particular risk for developing post-transplant PTLD. The rate of PTLD in lung transplant recipients may range between 5% and 15% (38).
Some transplant centers apply prophylactic antiviral treatment consisting of acyclovir or ganciclovir in high risk patients for primary EBV infection following surgery (EBV donor+, recipient EBV−), although there is no proof that this strategy has any impact on the development of post-transplant PTLD.
VZV
VZV is an exclusively human virus that is acquired either through direct contact with a skin lesion of an infected person or through airborne spread of respiratory droplets through coughing or sneezing. The vast majority of adults are seropositive having acquired the infection during infancy or adolescence, although a growing number of young adults derived VZV seropositivity from vaccination during childhood. First contact with VZV leads to acute varicella also known as chickenpox. This condition presents with systemic symptoms such as fever and malaise in addition to a diffuse vesicular, pruritic skin rash. Following initial infection, VZV is embedded in the cranial nerves and the dorsal root ganglia, establishing lifelong latency. Reactivation is possible years to decades after the initial infection in the form of herpes zoster (HZ), a flare of vesicular lesions with dermatomeric distribution associated with often intractable neuritic pain (39).
Lung transplant recipients are at increased risk for severe VZV related complications, including cutaneous dissemination and visceral end organ involvement (pneumonia, hepatitis, encephalitis), leading to a life-threatening condition (40). Reactivation of VZV typically occurs later than CMV or HSV and cutaneous lesions may be delayed or atypical with haemorrhage. Given the severity of VZV disease following transplantation, pre-transplant evaluation of recipient VZV immune status is highly advisable. It is suggested that non-immune recipients undergo VZV vaccination prior to transplantation. As for all live-attenuated vaccines, VZV vaccination post-transplantation is discouraged.
Treatment
Treatment of both post-transplant primary VZV infection and HZ reactivation should be managed with currently available antiviral agents. Localised HZ reactivation is generally treated with oral preparations and managed outside the hospital, whereas both disseminated HZ reactivation and primary NZV infection require intravenous treatment and hospitalisation. Table 2 summarises proposed treatment regimens for VZV infection or reactivation following lung transplantation. Antiviral treatment is usually prolonged for 7 days or until crust formation on skin lesions.
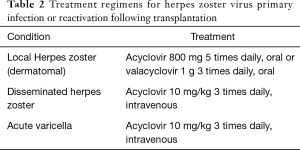
Full table
Prevention
Following lung transplantation, acyclovir prophylaxis to minimise the risk of HZ reactivation is generally unnecessary, as concomitant administration of CMV prophylactic agents (for example valganciclovir) is likely to prevent varicella reactivation. However, with the growing diffusion of Interferon gamma-based assays (for example CMV quantiFERON) as a basis for tailored or preemptive CMV prophylaxis, it is likely that the number of transplant recipients receiving such coverage will diminish. There is as yet no demonstration that administration of oral acyclovir in this setting may be useful to prevent HZ reactivation, although this strategy has been shown useful in other immunosuppressed populations (41). Conversely, in HZV seronegative lung transplant recipients who become exposed to an acute varicella patient, prophylaxis is recommended, given the high risk of developing a potentially life-threatening primary infection. Drugs of choice are acyclovir or valacyclovir for 7 days, beginning roughly a week after varicella exposure.
Fungi
Despite the widespread use of antimold prophylaxis, fungal infections are a frequent complication in lung transplant recipients (15–35% of patients receiving lung allografts) (42), with high morbidity, mortality and a possible role in the development of chronic lung allograft dysfunction (43). The predominant pathogens are Aspergillus spp., Candida species, Cryptococcus spp., the agents of mucormycosis, endemic fungi (Histoplasma, Coccidioides, and Blastomyces spp.), Scedosporium spp. and Fusarium.
Aspergillus spp.
Aspergillus is the most common cause of fungal infection in lung transplant recipients (44); different species have been associated to disease in this set of patients: firstly, A. fumigatus; then, A. flavus, A. niger and A. terreus (45). Aspergilli are ubiquitous in the environment and are acquired by inhalation.
Pulmonary invasive aspergillosis is defined by invasion of lung parenchyma; it generally presents with non-specific symptoms (pleuritic chest pain, cough, fever, dyspnoea, and haemoptysis) and its development may be enhanced by several risk factors, such as allograft dysfunction, immunosuppression levels, single lung transplant, environmental exposure, colonisation (both pre- and post-transplant), hypogammaglobulinemia and CMV infection. Mortality is constantly decreasing thanks to novel antifungal treatment (45). Disseminated disease (with extension to central nervous system, joints, bone, skin, and eye) is becoming increasingly rare.
Aspergillus tracheobronchitis is seen only in lung transplant recipients and is the most common form of aspergillus infection after this transplant; it usually affects the bronchial anastomosis (with possible stenosis, dehiscence and bleeding) (46), causing productive cough, dyspnoea, fever, stridor/wheeze, haemoptysis, or acute respiratory distress.
Finally, Aspergillus colonisation is defined by lack of probable or proven Aspergillus disease; however, it has been proven a risk factor for acute and chronic rejection and for progression to invasive disease (47).
Surveillance by chest CT scan and bronchoscopy is highly recommended in lung transplant recipients, not just to screen for rejection but also to provide an extensive microbiologic sampling of the graft.
Definitive diagnosis of invasive pulmonary aspergillosis requires a biopsy demonstrating tissue invasion, but evidence of a mold in the airway is suggestive of invasive infection; CT scan findings may vary from consolidation to cavitary lesions or nodular or mass-like lesions. Detection of galactomannan antigen may help to increase sensitivity, but has a very low specificity for invasive disease (48). A novel and promising diagnostic technique may be based on an Aspergillus PCR assay, which is currently under investigation.
Tracheobronchial aspergillosis requires systemic therapy with voriconazole in combination with nebulized amphotericin B for at least 3 months; debridement of the bronchial anastomosis may be indicated in case of abundant necrotic tissue. Voriconazole is the treatment of choice for invasive aspergillosis as well; in case of severe disease, the Infectious Diseases Society of American (IDSA) guidelines recommend combination therapy with an echinocandin (48). Like other azoles, voriconazole has significant interactions with calcineurin inhibitors and m-TOR inhibitors, which should be constantly monitored for dose reduction based on their serum levels.
Mucormycosis
The agents of Mucormycosis, which account approximately for 2% of all invasive fungal infections in transplant recipients, include the following: Rhizopus, Mucor, Rhizomucor, Lichtheimia, Cunninghamella, Apophysomyces, and Saksenaea (49). Risk factors are diabetes, renal impairment and recent rejection.
Mucormycosis is characterized by infarction and necrosis of host tissues that result from invasion of the vasculature by fungal hyphae. Pulmonary disease (consolidation/mass lesion, nodules and cavities found on the chest CT scan) is the most frequent presentation in lung transplant recipients, even if sino-orbital, cutaneous, and disseminated disease have also been reported (50). Diagnosis requires both histopathology and culture.
The overall mortality is very high, ranging from 49% to 90%. Management of mucormycosis can be very challenging: intravenous administration of lipid formulation of amphotericin B is the cornerstone of therapy in these cases, together with the reduction of immunosuppression and consideration for surgical debridement. Once the patient has stabilized, therapy may be switched to oral posaconazole (51).
Scedosporium spp.
Scedosporium spp. is a hyaline mold found in the environment, with two species capable of causing disease in humans: S. apiospermum and S. prolificans. The most common manifestations are invasive pulmonary infection and disseminated disease. Due to the intrinsic resistance to amphotericin B, voriconazole is the drug of choice in these cases, even if surgical resection may often be necessary (52).
Other
Fusarium is ubiquitous and is a notorious plant pathogen. F. solani and F. oxysporum are the most common human pathogenic species, but fusariosis is quite uncommon in lung transplant recipients, causing primarily lung involvement with or without disseminated disease (53). Fusarium is often resistant to azoles and therefore difficult to treat; amphotericin B may be an effective choice together with reducing immunosuppression.
Mycobacteria
Mycobacterial infections must be considered amongst the differential diagnoses of a lung transplant recipient presenting with infection. Both infection with Mycobacterium tuberculosis (MTB) and non-tuberculous mycobacteria (NTM) may play a role in the setting of lung transplantation. Common to all mycobacterial infections are the problems and delays in diagnosis, due to their fastidious culture requirements, and the complexity of treatment regimens, particularly in the current context of increasing antimicrobial resistance.
MTB
TB is still a major health threat to mankind being associated with over eight million TB-associated deaths per year, and over 2 billion people harboring latent tubercular infection worldwide (54).
Solid organ transplants recipients overall are at an increased risk of post-transplant TB compared to the general population (55). This risk is highest in the subgroup of patients undergoing lung transplantation, with reported incidence rates ranging from 6.4% to 10% (55-57). Differences in prevalence rates are partly related to the underlying degree of TB diffusion in the reporting country. Over 90% of TB cases develop within the first year following transplantation, and roughly three quarters involve the lungs (56). Crude mortality rates for post-transplant TB are in the order of 20–30%, with an attributable mortality of 10% (56,58).
Lung transplant recipients are at risk for development of TB through a number of ways. These include (in decreasing order of frequency): immunosuppression-induced reactivation of latent TB in the recipient, acquired infection by transmission of MTB from a contagious person, or acquired from the donor during transplant. Clinical presentation of active TB generally involves systemic signs and symptoms in conjunction with respiratory symptoms, as the lung is by far the most commonly involved site, as previously mentioned. Diagnosis is hampered by heavy reliance on traditional time-consuming microbiological procedures such as culture, although the introduction of nucleic amplification tests can help both in obtaining rapid results and in differentiating MTB from NTM species.
Treatment
Treatment regimens for lung transplant recipients that develop active TB are based on the same drugs employed for MTB infection in the immunocompetent host. The two cornerstones of treatment are: a multidrug regimen in order to avoid the development of resistance, and prolonged treatment in order to achieve bacterial eradication. Treatment involves two phases: an intensive phase consisting of four drugs to be continued for 2 months or until antibiotic sensitivity testing is obtained, and a continuation phase so as to complete 6 months of treatment (59). The most effective regimen requires daily dosing throughout the entire treatment course; although alternative regimens including combinations with twice weekly or 3 times weekly drug intake are acceptable, though carry a lesser degree of efficacy. Depending on the degree of immunosuppression, prolongation of the aggressive phase to 4 months, and/or extending the continuation phase to a total of 9–12 months may be considered on an individual basis, particularly in the presence of additional risk factors such as cavitation on presenting radiograph and persisting culture positivity after 2 months of treatment (59,60). Furthermore, reduction of immunosuppressive treatment is often suggested, particularly during the early phases of treatment. Table 3 summarizes the currently proposed treatment regimens for drug susceptible TB.
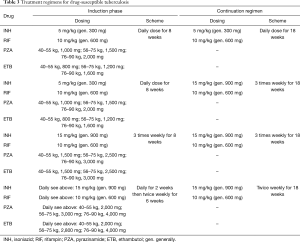
Full table
Given the number of drugs used, and the length of treatment for active TB, discontinuations due to drug induced toxicity are common even in the immunocompetent host. All the more so, following transplantation there is a considerable risk of significant drug interactions between antitubercular agents and immunosuppressants and of drug toxicity. Regarding the former, rifampin in particular induces hepatic enzymatic activity that promotes more rapid metabolism of immunosuppressive drugs and is potentially associated with increased risk of acute rejection (61,62). Considerable increases in the doses of calcineurin inhibitors may be required (up to 3–5 fold) and in certain occasions 3 times daily administration may be preferable to twice daily calcineurin inhibitor administration. Particularly during the 4-drug aggressive phase, drug toxicity (primarily drug-induced liver injury) may be an important issue, particularly considering potential concomitant use of other hepatotoxic drugs (azathioprine, azoles, etc.). Indications for treatment discontinuation are >3-fold increase in alanine aminotransferase (ALT) levels in the presence of symptoms (nausea, vomiting, and abdominal pain), or >5-fold increase in ALT in the absence of symptoms. Drug reintroduction procedures once liver enzymes are back to baseline levels are as yet insufficiently standardized.
With the antitubercular drugs currently available, successful treatment of active TB caused by drug susceptible strains may be observed in the vast majority of patients, even after organ transplantation. Nonetheless, there is growing concern regarding the emergence of drug resistance among TB strains across the world. Multidrug-resistant (MDR) TB is defined as resistance to both isoniazid and rifampin, whereas extremely drug-resistant (XDR) TB is defined as resistance to isoniazid, rifampin, fluoroquinolones and at least one injectable drug (i.e., amikacin, kanamycin and capreomycin) (63). There is considerable country-to-country variation in the rates of both MDR and XDR, with eastern European countries showing alarmingly high rates of both forms of resistance. There is limited consensus on the optimal management of drug resistance TB in immunocompetent hosts, and only a limited number of such MDR or XDR cases have been reported following lung transplantation. General indications include using four to six drugs for the aggressive phase, involving injectable antimicrobials such as streptomycin, amikacin, kanamycin or capreomycin, and linezolid or other second-line drugs (64). It is suggested that treatment should be prolonged for up to two years following culture conversion, and is best managed with the assistance of specialists with expertise in the treatment of drug resistant TB.
Nontuberculous mycobacteria (NTM)
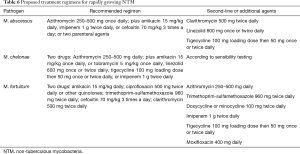
NTM are an ever-growing family of currently over 140 organisms that play an increasing role in pre- and post-transplant troublesome infections. NTM are free-living saprophytic organisms ubiquitous in the environment, being commonly found in soil, water supplies, dust, and plant material. Based on growth rate in culture they are usually classified as rapid growers, which generally form visible colonies in culture within a week, and slow growers that behave more like MTB in culture. Table 4 lists currently known MTB associated with disease in lung transplant recipients. Contrary to MTB, there is no human-to-human transmission of NTM, at least in the immunocompetent host, whereas this may only be partially true in the presence of structural lung damage or immunosuppression (65).

Full table
Several disease conditions that may eventually require lung transplantation develop pulmonary structural derangements associated with acquisition of NTM infection prior to transplantation. These include, COPD, pneumoconiosis and cystic fibrosis. Currently, roughly 12% of cystic fibrosis patients present a positive NTM culture prior to transplantation (61). Reported overall incidence of NTM infection among lung transplant recipients varies between 3.8% to 22.4%, but not all cases necessarily require treatment (66,67). Infection generally occurs at a considerable time distance from transplantation (averaging 2 years) indicating that most infections are acquired following transplantation, although persistence of NTM harbored prior to transplantation is an issue in cystic fibrosis patients, and in COPD patients who undergo single lung transplantation. It is thought that transplant recipients may be at higher risk of acquiring nosocomial NTM infections from contaminated water supplies (68).
NTM infections in solid organ transplant recipients as a class may develop as disseminated disease, pulmonary infection, skin and soft tissues infection, musculoskeletal infections, or catheter-related infection. Conversely, following lung transplantation pulmonary involvement is more common, but not exclusive. Additionally, lung transplant recipients may develop both surgical wound and bronchial and vascular anastomotic NTM infections in the early perioperative period, particularly with M. abscessus. Pulmonary involvement may present as nodules, pulmonary infiltrates, abscesses and cavitating nodules. Systemic symptoms such as fever are not universally present, whereas respiratory symptoms may include chronic cough, sputum production, dyspnea and, occasionally, hemoptysis (69). Diagnosis is based on typical imaging findings for pulmonary NTM, in addition to positive cultures, or biopsy of involved tissues (e.g., skin).
Pre-transplant culturing of M. abscessus, an increasingly common event in cystic fibrosis patients, has become a growing concern in deciding eligibility for transplantation, based both on the bacterium’s intrinsic resistance to most antibiotics, and the risk of disseminated uncontrolled infection post-surgery. Whether M. abscessus infection negatively impacts on lung transplant survival is still a matter of debate. The latest international consensus on the indications for lung transplantation suggests that presence of NTM infection prior to surgery, including M. abscessus, may be considered a contraindication for transplantation if there is progressive disease despite optimal therapy, or when such treatment is not tolerated due to side effects (70). More properly we should now refer to the M. abscessus complex (MABSC), which includes three closely related species of NTM: M. abscessus sensu stricto (referred to as M. abscessus), M. massiliense, and M. bolletii (71). The latter is relatively uncommon accounting for roughly 10% of isolates, whereas M. abscessus and M. massilense make up the remaining 90% of isolates and are fairly evenly distributed between them. When MABSC is cultured in a potential lung transplant candidate, genotyping is important in order to distinguish between species. In fact, M. massiliense generally presents a lesser degree of antimicrobial resistance compared to M. abscessus due to the absence of the erythromycin ribosomal methylase gene, erm (41), which encodes for inducible clarithromycin resistance (72). In addition, pre-transplant infection with M. massiliense is more likely to clear following transplantation compared to M. abscessus (73). Microbiological differentiation between species is therefore important in considering eligibility for lung transplantation.
Treatment
Treatment of NTM infection is based on the same basic principles that guide management of MTB infection: a multiple drug regimen in order to reduce the risk of developing resistance, and prolonged treatment in order to obtain bacterial eradication. It has been suggested that treatment of MABSC should involve an intensive phase followed by a continuation phase, similarly to MTB infection, although duration of the intensive phase is as yet undefined and based on the severity of infection (74). Tables 5,6 summarize indications for treatment regimens for slow growing and fast growing NTM, respectively (9,74). Optimal duration of treatment, particularly in the setting of lung transplantation is as yet undetermined. In some skin and soft tissue infections 6 months of treatment may be sufficient, whereas in pulmonary and disseminated disease treatment should be prescribed for 12 months following culture negativity. Drug toxicity may be a significant problem due to the multi drug treatment regimens and length of treatment. Periodic evaluation for potential drug toxicities (hearing loss, visual loss, renal impairment and liver function tests) should be performed throughout treatment. In adjunction to medical therapy, surgical excision and reduction of immunosuppressive levels may be considered.
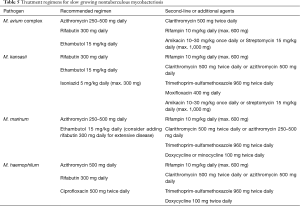
Full table
Full table
Nocardia
Nocardia is saprophytic gram-positive aerobic actinomycetes, ubiquitous in the environment (75). Over 80 Nocardia species have currently been identified, of which 30 have been associated with disease in humans.
Nocardia infections may cause both localized and systemic suppurative disease (76); it can disseminate to any organ (in particular the central nervous system) and tends to relapse or progress despite therapy. It can be acquired by inhalation, direct inoculation and/or ingestion. Nocardiosis is typically considered an opportunistic infection and its risk is highest in the first year following organ transplantation, especially for lung recipients. Recent data indicate a 3.5% rate of Nocardia infections amongst lung transplant recipients (77). N. nova and N. farcinica generally account for more than 75% of the infections and the majority of patients are affected by pulmonary disease. Other commonly encountered nocardia species in the setting of solid organ transplantation include N. asteroides sensu stricto, N. brasiliensis, N. otitidiscaviarum, and N. transvalensis. Hospital outbreaks have occurred among transplant recipients, diffusion being likely mediated by healthcare worker hands, air contamination, or vicinity with construction sites. Nocardia infection should be suspected particularly in febrile events with pulmonary or extrapulmonary involvement that initially respond to antibiotic treatment but relapse on termination of standard duration schemes. Whenever such an infection is suspected, special staining (modified acid-fast bacillus) and cultures should be promptly requested.
Treatment
Treatment of nocardial infections following lung transplantation is often based on a combination of more than one antimicrobial agent, given the risk of antimicrobial resistance, and the high associated morbidity and mortality in this setting (78). Table 7 provides guidance for antimicrobial choices in treating nocardial infections. Individualised treatment is however common based on antimicrobial sensibility testing and the development of drug toxicity may dictate antimicrobial substitution during therapy. Prolonged treatment is usually required, although optimal duration is as yet undefined. Standard regimens in immunocompromised hosts suggest 6–12 months of treatment, although shorter courses have been successful, provided there be an initial phase of parenteral antibiotic management. In presence of nocardial soft tissue abscesses, surgical drainage and reduction in immunosuppression may be useful adjuncts to antimicrobial therapy.

Full table
Prevention
Most lung transplant recipients receive prolonged Pneumocystis jirovecii prophylaxis with co-trimoxazole. Given the activity of this agent towards Nocardia species, it may be reasonable to assume that this would provide sufficient coverage to prevent the development of nocardia infections. Published case series however indicate that most lung transplant patients who develop nocardia infections do so while receiving co-trimoxazole prophylaxis (79). Reasons for insufficient prophylactic coverage probably involve increases in antimicrobial resistance over time and the failure to obtain adequate serum levels for nocardial prevention with the 3 times weekly regimen employed for pneumocystis prevention. Increasing co-trimoxazole dosing regimen so as to provide primary prevention towards nocardia species is an as yet untested possibility that must be counterbalanced with the increased risk of drug toxicity. Similarly, long term co-trimoxazole has been used as secondary prophylaxis following nocardia infection (79), although there is no consensus on optimal dosing regimens.
Clostridium difficile (C. difficile)
Clostridium difficile (C. difficile) is a spore-forming, anaerobic, Gram positive bacillus. In the general population it is reported to cause 6–25% of cases of antibiotic associated diarrhea, over 70% of antibiotic-associated colitis, and over 90% of cases of antibiotic-associated pseudomembranous colitis (80). Intestinal tissue damage is mediated through toxin A and toxin B, which trigger a cytotoxic response, neutrophilic infiltrate and cytokine release.
C. difficile infection is a common problem in lung transplant recipients, with an estimated incidence of 7–31% (81). Several risk factors have been identified: prolonged hospitalization and ICU stay, intense immunosuppression, exposure to antimicrobial agents with gram positive activity. Presentation is often atypical, with little diarrhoea; abdomen CT scan may be useful to rule out severe disease (pseudomembranous colitis), which is associated with a high risk of bowel perforation.
CDI is diagnosed by confirming the presence of toxigenic C. difficile in the stool of a symptomatic patient. Indications for treatment options based on severity of disease have recently been issued for organ transplant recipients (82). In the absence of complications such as ileus, toxic megacolon or multiorgan failure, oral metronidazole 500 mg 3 times daily for 10–14 days is the initial treatment of choice. Conversely, in presence of the above complications, combination treatment with intravenous metronidazole and oral vancomycin 500 mg 4 times daily should be initiated. In uncomplicated cases started on oral metronidazole, failure to respond within 5 days should prompt switch to oral vancomycin 125 mg 4 times daily. Once treatment has been discontinued, should symptoms relapse, retreatment may be initiated with either metronidazole or vancomycin, based on the severity of symptoms (82).
Pneumocystis jirovecii pneumonia
Pneumocystis jirovecii (previously Pneumocystis carinii), is a component of the Taphrinomycotina branch of the fungal kingdom. Pneumocystis spp. are ubiquitous in nature, and apparently infect children in early life with probable airborne person to person transmission (83). Immunocompetent persons usually clear the infection in the absence of any symptoms; but in transplanted patients PCP progresses to severe inflammatory pneumonia with respiratory failure and death. In these immunocompromised patients, PCP has a mortality rate ranging between 20% and 40%, which is the double that reported for HIV patients (84). In the past PCP rates among solid organ transplant recipients were in the order of 5–15%, and up to 20–40% in lung transplant recipients. However, the widespread use of sulfamethoxazole-trimethoprim prophylaxis has now dramatically reduced infection rates to 0.3–2.6% (85). Prior to prophylaxis, risk of PCP was highest during the first 6 months following transplantation, thus leading to the recommendation to sustain prophylaxis for 6–12 months post-surgery (86).
Clinical presentation of PCP includes fever, dry cough, dyspnea and chest pain, night sweats and weight loss. Pneumothorax is a possible complication. Patients generally present marked hypoxemia, out of proportion compared to physical findings. Symptoms may rapidly evolve in 1–2 days, or sometimes be more slowly protracted over 7–10 days. Initial chest X-ray may be unremarkable in about 30% of patients. if there are signs, these typically include bilateral diffuse symmetric finely granular infiltrates with characteristic central location and fast progression to the peripheral parts of the lungs. Lung nodules (which may cavitate) and hilar lymphadenopathy are possible findings; furthermore, upper lobes may present cysts. Chest CT may be highly suggestive presenting bilateral, asymmetric patchy mosaic appearance with ground-glass pattern, thickening of lobular septa, nodules with possible excavation, pneumatoceles, hilar lymphadenopathy, pleural effusion and pneumothorax.
High values of serum lactate dehydrogenase are a suggestive indicator of PCP in HIV-infected patients, but this does not apply in transplanted patients. Elevated serum levels of beta-D-glucan (a fungal cell-wall element) could support the diagnosis of PCP; the negative predictive value of beta-D-glucan is consistently high, even though a high rate of false positive results has been described. Immunofluorescent microscopic visualization of Pneumocystis in bronchoalveolar lavage is the standard method for the laboratory diagnosis of PCP (87). Unfortunately, transplant patients often develop PCP with a lower fungal burden than patients with other forms of immunodeficiency, consequently a consistent number of patients without Pneumocystis identification on the microscopic smear are effectively affected by PCP. PCR techniques have been developed to identify Pneumocystis DNA in bronchoalveolar lavage (or other biological fluid); in particular, single-copy real-time PCR may distinguish patients with infection from those with colonization (88).
Treatment
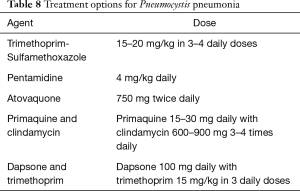
If PCP is suspected on clinical and radiological grounds and beta-D-glucan values are high, adequate therapy initiation should not be delayed while awaiting Pneumocystis identification on bronchoalveolar lavage. Intravenous trimethoprim-sulfamethoxazole is the treatment of choice; it must be adjusted on renal function and generally continued for 3 weeks. In patients who are allergic to the drug or who develop toxicity during treatment, alternative antimicrobial regimens are available (89) (see Table 8). Corticosteroid treatment is added in hypoxemic patients (PaO2 <70 mmHg on room air) as this has been shown to exert beneficial survival effects in HIV patients, although the additional benefit has never been tested in solid organ recipients. Reduction of immunosuppressive levels is generally recommended.
Full table
In the current era of 1-year PCP prophylaxis, it has been found that a considerable number of delayed onset PCP cases may develop during the second year post lung transplantation, supporting the notion that prolonged PCP prophylaxis may be necessary in lung transplant recipients (90).
Toxoplasmosis
In developed countries, Toxoplasma gondii is one of the most common parasites; France is particularly involved, since more than 50% of citizens are infected (91). Toxoplasma gondii is an obligate apicomplexan intracellular protozoan that can infect a large number of warm-blooded animals; cats are the final host, where the organisms undergo sexual reproduction determining the environmental dissemination of highly resistant oocysts with the feces. In the human body, an intermediate host, the oocysts’ wall is degraded by intestinal proteolytic enzymes; then the protozoa enter the intestinal epithelium where they differentiate into tachyzoites, which represent the cellular stage of Toxoplasma gondii. Tachyzoites can quickly multiply and move through tissues; in no more than 10 days from ingestion, the brain and muscles are contaminated with Toxoplasma gondii cysts.
Immunocompetent persons present a subclinical course when infected from Toxoplasma; on the contrary, in immunocompromised individuals this parasite could develop life-threatening disease. Among transplantation procedures, heart transplantation has the greatest risk for toxoplasmosis; myocarditis generally occurs within 6 months from surgery, but brain abscess, pneumonia and disseminated infections are also possible. Serologically mismatched patients (seropositive donor/seronegative recipients) have the highest risk of developing the disease (92). Toxoplasmosis is less common in lung transplantation since anti-PCP prophylaxis is extensively applied in all centers. When the lungs are involved, radiological signs include bilateral ground-glass infiltrates, septal and peribronchial thickening, miliary multiple nodules, lymph node enlargement and pleural effusion. The diagnosis of toxoplasmosis is typically made by serologic testing but identification of the parasite in bronchoalveolar lavage, or even histologic examination showing necrotizing inflammation and characteristic organisms are needed for definitive diagnosis.
The therapeutic regimen is bases on pyrimethamine with the addition of leucovorin calcium and sulfadiazine or clindamycin; treatment generally lasts 6 weeks.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Fishman JA. Infection in solid-organ transplant recipients. N Engl J Med 2007;357:2601. [Crossref] [PubMed]
- Yusen RD, Edwards LB, Dipchand AI, et al. The Registry of the International Society for Heart and Lung Transplantation: Thirty-third Adult Lung and Heart-Lung Transplant Report-2016; Focus Theme: Primary Diagnostic Indications for Transplant. J Heart Lung Transplant 2016;35:1170-84. [Crossref] [PubMed]
- Yu XY, Wang Y, Zhong H, et al. Diagnostic value of serum procalcitonin in solid organ transplant recipients: a systematic review and meta-analysis. Transplant Proc 2014;46:26-32. [Crossref] [PubMed]
- Hemmert C, Ohana M, Jeung MY, et al. Imaging of lung transplant complications. Diagn Interv Imaging 2014;95:399-409. [Crossref] [PubMed]
- Lehto JT, Koskinen PK, Anttila VJ, et al. Bronchoscopy in the diagnosis and surveillance of respiratory infections in lung and heart-lung transplant recipients. Transpl Int 2005;18:562-71. [Crossref] [PubMed]
- Solís A, Brown D, Hughes J, et al. Methicillin-resistant Staphylococcus aureus in children with cystic fibrosis: An eradication protocol. Pediatr Pulmonol 2003;36:189-95. [Crossref] [PubMed]
- Ziakas PD, Pliakos EE, Zervou FN, et al. MRSA and VRE colonization in solid organ transplantation: a meta-analysis of published studies. Am J Transplant 2014;14:1887-94. [Crossref] [PubMed]
- Davido B, Batista R, Michelon H, et al. Is faecal microbiota transplantation an option to eradicate highly drug-resistant enteric bacteria carriage? J Hosp Infect 2017;95:433-7. [Crossref] [PubMed]
- Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 2007;175:367-416. Erratum in: Am J Respir Crit Care Med 2007;175:744-5. [Crossref] [PubMed]
- Smibert O, Snell GI, Bills H, et al. Mycobacterium abscessus Complex - a Particular Challenge in the Setting of Lung Transplantation. Expert Rev Anti Infect Ther 2016;14:325-33. [Crossref] [PubMed]
- Benden C, Danziger-Isakov LA, Astor T, et al. Variability in immunization guidelines in children before and after lung transplantation. Pediatr Transplant 2007;11:882-7. [Crossref] [PubMed]
- Horan TC, Gaynes TP, Martone WJ, et al. CDC Definitions of Nosocomial Surgical Site Infections, 1992: A Modification of CDC Definitions of Surgical Wound Infections. Infect Control Hosp Epidemiol 1992;13:606-8. [Crossref] [PubMed]
- Edwards FH, Engelman RM, Houck P, et al. The Society of Thoracic Surgeons Practice Guideline Series: Antibiotic Prophylaxis in Cardiac Surgery, Part I: Duration. Ann Thorac Surg 2006;81:397-404. [Crossref] [PubMed]
- Nash EF, Coonar A, Kremer R, et al. Survival of Burkholderia cepacia sepsis following lung transplantation in recipients with cystic fibrosis. Transpl Infect Dis 2010;12:551-4. [Crossref] [PubMed]
- Pilarczyk K, Haake N, Heckmann J, et al. Is universal antifungal prophylaxis mandatory in adults after lung transplantation? A review and meta-analysis of observational studies. Clin Transplant 2016;30:1522-31. [Crossref] [PubMed]
- Zamora MR. Cytomegalovirus and lung transplantation. Am J Transplant 2004;4:1219. [Crossref] [PubMed]
- Estenne M, Maurer JR, Boehler A, et al. Bronchiolitis obliterans syndrome 2001: an update of the diagnostic criteria. J Heart Lung Transplant 2002;21:297. [Crossref] [PubMed]
- Sharples LD, McNeil K, Stewart S, et al. Risk factors for bronchiolitis obliterans: a systematic review of recent publications. J Heart Lung Transplant 2002;21:271. [Crossref] [PubMed]
- Razonable RR, Limaye RR. Cytomegalovirus infection after solid organ transplantation. In: Bowden RA, Ljungman P, Snydman DR. editors. Transplant Infections, 3rd edition. Philadelphia: Lippincott Williams and Wilkins, 2010:328.
- Kotton CN, Kumar D, Caliendo AM, et al. Updated international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation 2013;96:333. [Crossref] [PubMed]
- Piiparinen H, Hockerstedt K, Gronhagen-Riska C, et al. Comparison of two quantitative CMV PCR tests, Cobas Amplicor CMV Monitor and TaqMan assay, and pp65-antigenemia assay in the determination of viral loads from peripheral blood of organ transplant patients. J Clin Virol 2004;30:258. [Crossref] [PubMed]
- Chou S, Marousek GI, Van Wechel LC, et al. Growth and drug resistance phenotypes resulting from cytomegalovirus DNA polymerase region III mutations observed in clinical specimens. Antimicrob Agents Chemother 2007;51:4160-2. [Crossref] [PubMed]
- Hakki M, Chou S. The biology of cytomegalovirus drug resistance. Curr Opin Infect Dis 2011;24:605-11. [Crossref] [PubMed]
- Razonable RR, Humar A, Infectious Diseases AST. Community of Practice. Cytomegalovirus in solid organ transplantation. Am J Transplant 2013;13 Suppl 4:93-106. [Crossref] [PubMed]
- Valantine HA, Luikart H, Doyle R, et al. Impact of cytomegalovirus hyperimmune globulin on outcome after cardiothoracic transplantation: a comparative study of combined prophylaxis with CMV hyperimmune globulin plus ganciclovir versus ganciclovir alone. Transplantation 2001;72:1647-52. [Crossref] [PubMed]
- Asberg A, Humar A, Rollag H, et al. Oral valganciclovir is noninferior to intravenous ganciclovir for the treatment of cytomegalovirus disease in solid organ transplant recipients. Am J Transplant 2007;7:2106-13. [Crossref] [PubMed]
- Shalhoub S, Husain S. Community-acquired respiratory viral infections in lung transplant recipients. Curr Opin Infect Dis 2013;26:302-8. [Crossref] [PubMed]
- Gottlieb J, Schulz TF, Welte T, et al. Community-acquired respiratory viral infections in lung transplant recipients: a single season cohort study. Transplantation 2009;87:1530-7. [Crossref] [PubMed]
- Garbino J, Soccal PM, Aubert JD, et al. Respiratory viruses in bronchoalveolar lavage: a hospital-based cohort study in adults. Thorax 2009;64:399-404. [Crossref] [PubMed]
- Speich R, van der Bij W. Epidemiology and management of infections after lung transplantation. Clin Infect Dis 2001;33:S58-65. [Crossref] [PubMed]
- Mahony J, Chong S, Merante F, et al. Development of a respiratory virus panel test for detection of twenty human respiratory viruses by use of multiplex PCR and a fluid microbead-based assay. J Clin Microbiol 2007;45:2965-70. [Crossref] [PubMed]
- Muthuri SG, Myles PR, Venkatesan S, et al. Impact of neuraminidase inhibitor treatment on outcomes of public health importance during the 2009–2010 influenza A(H1N1) pandemic: a systematic review and meta-analysis in hospitalized patients. J Infect Dis 2013;207:553-63. [Crossref] [PubMed]
- Vu DL, Bridevaux PO, Aubert JD, et al. Respiratory viruses in lung transplant recipients: a critical review and pooled analysis of clinical studies. Am J Transplant 2011;11:1071-8. [Crossref] [PubMed]
- Allyn PR, Duffy EL, Humphries RM, et al. Graft Loss and CLAD-onset is hastened by viral pneumonia after lung transplantation. Transplantation 2016;100:2424-31. [Crossref] [PubMed]
- Kotton CN, Huprikar S, Kumar D. Transplant Infectious Diseases: A review of the scientific registry of transplant recipients published data. Am J Transplant 2017;17:1439-46. [Crossref] [PubMed]
- Kieff E, Rickinson AB. Epstein Barr virus and its replication. In: Knipe DM, Howley PM. editors. Fields Virology. 5th edition. Philadelphia: Lippincott Williams and Wilkins, 2007. pp2603.
- Tsai DE, Douglas L, Andreadis C, et al. EBV PCR in the diagnosis and monitoring of post transplant lymphoproliferative disorder: results of a two-arm prospective trial. Am J Transplant 2008;8:1016-24. [Crossref] [PubMed]
- Neuringer IP. Posttransplant lymphoproliferative disease after lung transplantation. Clin Dev Immunol 2013;2013. [Crossref] [PubMed]
- Pergam SA, Limaye AP, Infectious Diseases AST. Community of Practice. Varicella zoster virus in solid organ transplantation. Am J Transplant 2013;13 Suppl 4:138-46. [Crossref] [PubMed]
- Miller GG, Dummer JS. Herpes simplex and varicella zoster viruses: forgotten but not gone. Am J Transplant 2007;7:741-7. [Crossref] [PubMed]
- Erard V, Guthrie KA, Varley C, et al. One-year acyclovir prophylaxis for preventing varicella-zoster virus disease after hematopoietic cell transplantation: No evidence of rebound varicella-zoster virus disease after drug discontinuation. Blood 2007;110:3071-7. [Crossref] [PubMed]
- Christie JD, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: 29th adult lung and heart-lung transplant report-2012. J Heart Lung Transplant 2012;31:1073-86. [Crossref] [PubMed]
- Husain S, Singh N. Bronchiolitis obliterans and lung transplantation: evidence for an infectious etiology. Semin Respir Infect 2002;17:310-4. [Crossref] [PubMed]
- Pappas PG, Alexander BD, Andes DR, et al. Invasive fungal infections among organ transplant recipients: results of the Transplant-Associated Infection Surveillance Network (TRANSNET). Clin Infect Dis 2010;50:1101-11. [Crossref] [PubMed]
- Steinbach WJ, Marr KA, Anaissie EJ, et al. Clinical epidemiology of 960 patients with invasive aspergillosis from the PATH Alliance registry. J Infect 2012;65:453-64. [Crossref] [PubMed]
- Nunley DR, Gal AA, Vega JD, et al. Saprophytic fungal infections and complications involving the bronchial anastomosis following human lung transplantation. Chest 2002;122:1185-91. [Crossref] [PubMed]
- Weigt SS, Elashoff RM, Huang C, et al. Aspergillus colonization of the lung allograft is a risk factor for bronchiolitis obliterans syndrome. Am J Transplant 2009;9:1903-11. [Crossref] [PubMed]
- Patterson TF, Thompson GR 3rd, Denning DW, et al. Practice Guidelines for the Diagnosis and Management of Aspergillosis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis 2016;63:e1-60. [Crossref] [PubMed]
- Kwon-Chung KJ. Taxonomy of fungi causing mucormycosis and entomophthoramycosis (zygomycosis) and nomenclature of the disease: molecular mycologic perspectives. Clin Infect Dis 2012;54 Suppl 1:S8-15. [Crossref] [PubMed]
- Sun HY, Aguado JM, Bonatti H, et al. Zygomycosis Transplant Study Group. Pulmonary zygomycosis in solid organ transplant recipients in the current era. Am J Transplant 2009;9:2166-71. [Crossref] [PubMed]
- Bhaskaran A, Hosseini-Moghaddam SM, Rotstein C, et al. Mold Infections in Lung Transplant Recipients. Semin Respir Crit Care Med 2013;34:371-9. [Crossref] [PubMed]
- Cortez KJ, Roilides E, Quiroz-Telles F, et al. Infections caused by Scedosporium spp. Clin Microbiol Rev 2008;21:157-97. [Crossref] [PubMed]
- Carneiro HA, Coleman JJ, Restrepo A, et al. Fusarium infection in lung transplant patients: report of 6 cases and review of the literature. Medicine (Baltimore) 2011;90:69-80. [Crossref] [PubMed]
- Zumla A, George A, Sharma V, et al. The WHO 2014 global tuberculosis report--further to go. Lancet Glob Health 2015;3:e10-2. [Crossref] [PubMed]
- Muñoz P, Rodriguez C, Bouza E. Mycobacterium tuberculosis infection in recipients of solid organ transplants. Clin Infect Dis 2005;40:581-7. [Crossref] [PubMed]
- Torre-Cisneros J, Doblas A, Aguado JM, et al. Tuberculosis after solid-organ transplant: incidence, risk factors and clinical characteristics in the RESITRA (Spanish Network of Infection in Transplantation) cohort. Clin Infect Dis 2009;48:1657-65. [Crossref] [PubMed]
- Meije Y, Piersimoni C, Torre-Cisneros J, et al. Mycobacterial infections in solid organ transplant recipients. Clin Microbiol Infect 2014;20:89-101. [Crossref] [PubMed]
- Holty JE, Sista RR. Mycobacterium tuberculosis infection in transplant recipients: Early diagnosis and treatment of resistant tuberculosis. Curr Opin Organ Transplant 2009;14:613-8. [Crossref] [PubMed]
- Nahid P, Dorman SE, Alipanah N, et al. Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America Clinical Practice Guidelines: Treatment of Drug-Susceptible Tuberculosis. Clin Infect Dis 2016;63:e147-95. [Crossref] [PubMed]
- Aguado JM, Torre-Cisneros J, Fortun J, et al. Tuberculosis in solid-organ transplant recipients: consensus statement of the group for the study of infection in transplant recipients (GESITRA) of the Spanish Society of Infectious Diseases and Clinical Microbiology. Clin Infect Dis 2009;48:1276-84. [Crossref] [PubMed]
- Pena T, Klesney-Tait J. Mycobacterial infections in solid organ and hematopoietic stem cell transplantation. Clin Chest Med 2017;38:761-70. [Crossref] [PubMed]
- Modry DL, Stinson EB, Oyer PE, et al. Acute rejection and massive cyclosporine requirements in heart transplant recipients treated with rifampin. Transplantation 1985;39:313-4. [Crossref] [PubMed]
- Kliiman K, Altraja A. Predictors of extensively drug-resistant pulmonary tuberculosis. Ann Intern Med 2009;150:766-75. [Crossref] [PubMed]
- Falzon D, Jaramillo E, Schunemann HJ, et al. WHO guidelines for the programmatic management of drug-resistant tuberculosis: 2011 update. Eur Respir J 2011;38:516-28. [Crossref] [PubMed]
- Aitken ML, Limaye A, Pottinger P, et al. Respiratory outbreak of Mycobacterium abscessus subspecies massiliense in a lung transplant and cystic fibrosis center. Am J Respir Crit Care Med 2012;185:231-2. [Crossref] [PubMed]
- Kesten S, Chaparro C. Mycobacterial infections in lung transplant recipients. Chest 1999;115:741-5. [Crossref] [PubMed]
- Knoll BM, Kappagoda S, Gill RR, et al. Non-tuberculous mycobacterial infection among lung transplant recipients: a 15-year cohort study. Transpl Infect Dis 2012;14:452-60. [Crossref] [PubMed]
- Baker AW, Lewis SS, Alexander BD, et al. Two-phase hospital-associated outbreak of Mycobacterium abscessus: investigation and mitigation. Clin Infect Dis 2017;64:902-11. [PubMed]
- Keating MR, Daly JS. Nontuberculous mycobacterial infections in solid organ transplantation. Am J Transplant 2013;13:77-82. [Crossref] [PubMed]
- Weill D, Benden C, Corris PA, et al. A consensus document for the selection of lung transplant candidates: 2014--an update from the Pulmonary Transplantation Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 2015;34:1-15. [Crossref] [PubMed]
- Leao SC, Tortoli E, Euzéby JP, et al. Proposal that Mycobacterium massiliense and Mycobacterium bolletii be united and reclassified as Mycobacterium abscessus subsp. bolletii comb. nov., designation of Mycobacterium abscessus subsp. abscessus subsp. nov. and emended description of Mycobacterium abscessus. Int J Syst Evol Microbiol 2011;61:2311-3. [Crossref] [PubMed]
- Koh WJ, Jeon K, Lee NY, et al. Clinical Significance of Differentiation of Mycobacterium massiliense from Mycobacterium abscessus. Am J Respir Crit Care Med 2011;183:405-10. [Crossref] [PubMed]
- Roux AL, Catherinot E, Soismier N, et al. Comparing Mycobacterium massiliense and Mycobacterium abscessus lung infections in cystic fibrosis patients. J Cyst Fibros 2015;14:63-9. [Crossref] [PubMed]
- Piersimoni C. Nontuberculous mycobacteria infection in solid organ transplant recipients. Eur J Clin Microbiol Infect Dis 2012;31:397-403. [Crossref] [PubMed]
- Lerner PI. Nocardiosis. Clin Infect Dis 1996;22:891-903. [Crossref] [PubMed]
- Lederman ER, Crum NF. A case series and focused review of nocardiosis: clinical and microbiologic aspects. Medicine (Baltimore) 2004;83:300. [Crossref] [PubMed]
- Peleg AY, Husain S, Qureshi ZA, et al. Risk factors, clinical characteristics, and outcome of Nocardia infection in organ transplant recipients: A matched case–control study. Clin Infect Dis 2007;44:1307-14. [Crossref] [PubMed]
- Clark NM, Reid GE, Infectious Diseases AST. Community of Practice. Nocardia infections in solid organ transplantation. Am J Transplant 2013;13 Suppl 4:83-92. [Crossref] [PubMed]
- Poonyagariyagorn HK, Gershman A, Avery R, et al. Challenges in the diagnosis and management of Nocardia infections in lung transplant recipients. Transpl Infect Dis 2008;10:403-8. [Crossref] [PubMed]
- Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol 2010;31:431-55. [Crossref] [PubMed]
- Paudel S, Zacharioudakis IM, Zervou FN, et al. Prevalence of Clostridium difficile infection among solid organ transplant recipients: a meta-analysis of published studies. PLoS One 2015;10. [Crossref] [PubMed]
- Dubberke ER, Burdette SD, Infectious Diseases AST. Community of Practice. Clostridium difficile infections in solid organ transplantation. Am J Transplant 2013;13 Suppl 4:42-9. [Crossref] [PubMed]
- Morris A, Wei K, Afshar K, et al. Epidemiology and clinical significance of pneumocystis colonization. J Infect Dis 2008;197:10-7. [Crossref] [PubMed]
- Mansharamani NG, Garland R, Delaney D, et al. Management and outcome patterns for adult Pneumocystis carinii pneumonia, 1985 to 1995: comparison of HIV-associated cases to other immunocompromised states. Chest 2000;118:704-11. [Crossref] [PubMed]
- Iriart X, Challan Belval T, Fillaux J, et al. Risk factors of Pneumocystis pneumonia in solid organ recipients in the era of the common use of posttransplantation prophylaxis. Am J Transplant 2015;15:190-9. [Crossref] [PubMed]
- Martin SI, Fishman JA. Pneumocystis pneumonia in solid organ transplant recipients. Am J Transplant 2009;9:S227-33. [Crossref] [PubMed]
- Procop GW, Haddad S, Quinn J, et al. Detection of Pneumocystis jiroveci in respiratory specimens by four staining methods. J Clin Microbiol 2004;42:3333-5. [Crossref] [PubMed]
- Wilson JW, Limper AH, Grys TE, et al. Pneumocystis jirovecii testing by real-time polymerase chain reaction and direct examination among immunocompetent and immunosuppressed patient groups and correlation to disease specificity. Diagn Microbiol Infect Dis 2011;69:145-52. [Crossref] [PubMed]
- Smego RA Jr, Nagar S, Maloba B, et al. A meta-analysis of salvage therapy for Pneumocystis carinii pneumonia. Arch Intern Med 2001;161:1529-33. [Crossref] [PubMed]
- Wang EHZ, Partovi N, Levy RD, et al. Pneumocystis pneumonia in solid organ transplant recipients: not yet an infection of the past. Transpl Infect Dis 2012;14:519-25. [Crossref] [PubMed]
- Flegr J, Prandota J, Sovičková M, et al. Toxoplasmosis--a global threat. Correlation of latent toxoplasmosis with specific disease burden in a set of 88 countries. PLoS One 2014;24;9:e90203.
- Derouin F, Pelloux H. ESCMID Study Group on Clinical Parasitology. Prevention of toxoplasmosis in transplant patients. Clin Microbiol Infect 2008;14:1089-101. [Crossref] [PubMed]


