The use of transcatheter mitral valve system: can we make mitral regurgitation better again?
Percutaneous treatments of valvular heart disease have evolved over the past two decades. These “minimally invasive” procedures are attractive alternatives to surgery for a growing high-risk population with multiple comorbidities. In 2002, Cribier et al. (1) performed the first transcatheter aortic valve implantation (TAVI) in a patient with aortic stenosis. Currently, TAVI has become a common procedure for high or prohibitive surgical risk patient and is moving rapidly into intermediate risk patients, with over 250,000 devices implanted worldwide. Interest in developing transcatheter mitral valve replacement (TMVR) systems to become a new frontier in structural heart disease interventions has grown, though technology has lagged compared to TAVI. In comparison to the aortic valve, additional challenges exist with the development of new TMVR systems. First, the mitral valve annulus is dynamic, asymmetrical, and leaflet displacement poses a risk of left ventricular outflow tract (LVOT) obstruction given its proximity (2). In addition, the mitral valve is subject to high left ventricular (LV) systolic pressure necessitating a robust valve anchoring system. Further, the relief of severe aortic stenosis clearly improves survival, whereas the relief of severe mitral regurgitation (MR) cannot be clearly linked to improved survival, especially functional MR. Multiple TMVR devices are currently being tested worldwide in small cohorts to determine feasibility and short-term outcomes in high surgical risk patients.
The use of TMVR in MR
MR is the second most common valvular lesion requiring surgery after AVR. Although surgical mitral valve repair for primary MR has excellent outcomes, mitral valve surgery for functional MR is associated with high likelihood of recurrence and the lack of a clear survival advantage (3). In the most recent head to head trial comparing mitral valve replacement to repair in ischemic MR, the former showed significant reduction in MR, lower heart-failure related adverse events but no mortality difference (4). However, one-third to one half of these patients are deemed high risk for undergoing surgery due to advanced age, significant LV dysfunction, and other comorbidities (5). Currently, the only available FDA approved percutaneous treatment option for degenerative MR in the US is edge-to-edge mitral valve repair (MitraClip, Abbott Vascular, Minneapolis, MN, USA). Currently, there are two ongoing studies (RESHAPE, COAPT) evaluating the role of the MitraClip system in patients with symptomatic functional MR. In those patients who have unfavorable anatomy for a clip device and are considered at high surgical risk are being enrolled in ongoing studies using various TMVR systems. To date, six TMVR system devices have been tested in humans (2): Fortis (Edwards Lifesciences, Irvine, CA, USA) (6,7), Tendyne (Abbott, Abbott Park, Illinois, USA) (8-10), NaviGate (NaviGate Cardiac Structures, Inc., Lake Forest, CA, USA), Intrepid (Medtronic, Minneapolis, MN), CardiAQ (Edwards Lifesciences, Irvine, CA, USA) (11), Tiara (Neovasc Inc., Richmond, BC, USA) (12). The common features of these systems include: the need of transapical access, a nitinol self-expanding frame, bovine trileaflet valve, and a sealing cuff (13). These studies have included patients with severe MR who were considered at high or prohibitive surgical risk.
In this editorial, we refer to a global registry study published in the Journal of American College of Cardiology by Muller et al. (9). They reported the results of 30 patients enrolled at eight different sites (November 2014 till March 2016) who underwent TMVR for severe MR using Tendyne valve (Tendyne Mitral Valve System, Abbott Vascular, Roseville, Minnesota). They included adult, symptomatic patients with 3–4+ MR (either structural (10%) or functional (76%) who were deemed as high surgical risk. The valve is a self-expanding prosthesis with porcine pericardial leaflets and 2 frames. The outer frame is D-shaped, nitinol based, whereas the inner frame is circular providing a large orifice area >3 cm2. It is delivered transapically and is held in place by a tether from the valve to the LV apex, designed to reduce paravalvular leak. Patient’s population included an elderly (mean age 75.6 years) predominantly male. LV ejection fraction was moderately impaired in 48%, and normal in 41% of the cohort. The Society of Thoracic Surgeons Predicted Risk of Mortality was 7.3%±5.7%. The device was successfully implanted in 28 patients (93%), and patients were commenced on a single antiplatelet agent along with coumadin with target INR of 2.5–3.5 for ≥3 months post-implantation. Only one patient died due hospital-acquired pneumonia. The primary performance endpoint (successful implant, freedom of cardiac mortality, stroke, or device dysfunction) was achieved in 86.6% of the cohort. No MR was noted in all but ONE patient who had mild MR. There was significant decrease in LV ejection fraction, LV end-systolic and end-diastolic indices. Overall freedom from major adverse events was 83%, and there was significant improvement in New York Heart Association functional class (75% having no or mild symptoms) and quality of life (9).
The authors have conducted an important study in the era of evolving TMVR therapies, and are to be congratulated for their pioneering effort to expand the learning curve of this evolving technology. This represents the third in-human study using this device system (8,10), but the largest cohort of TMVR studies ever published to date of all systems. Table 1 summarizes patient’s characteristics, hemodynamic and clinical outcomes using different TMVR systems used in-human to date for severe MR.
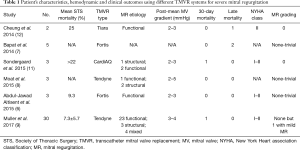
Full table
The current global registry demonstrated technical feasibility of implanting this device with low peri-procedural risks and favorable 30-day outcomes. One of the few advantages of the current TMVR system include: (I) the ability to reposition and retrieve the valve; (II) the double frame system that allows proper adaptability and less risk of paravalvular leak; (III) the presence of apical tether mechanistically aims to reduce the risk of device migration, LVOT obstruction and paravalvular leak; (IV) the device can be implanted without the need of rapid RV pacing.
The study was unique in few aspects. First, over 70% of patients had functional MR, in which current surgical therapies have shown conflicting data with regards to mortality benefit, and unoperated patients have high mortality (14) (20% 1-year mortality rate and 50% 5-year mortality rate). Second, both early and late (30 days) mortality were low with only one death was due to hospital-acquired pneumonia. Early studies using TMVR systems have shown increased periprocedural mortality, likely due to highly comorbid conditions and post-operative complications leading to multi-system organ failure, and suboptimal technical quality (11,12). Third, a very important consideration before performing TMVR is the assessment of LV reserve adequacy. Patient’s with LVEF <30% were excluded from this study, as these patients tend to have higher events and less likely to benefit from mitral intervention. This was not the case in other TMVR studies where severely depressed LV function was studied. Fourth, these device systems consist of circumferentially covered stent struts and can encroach on the LVOT or cause SAM by interacting with the anterior mitral valve leaflet. Hence, a neo-LVOT is created by the device (13,15). Integrating CT data pre-operatively and the retrievability of the device system are critical to avoid this complication at the time of implantation.
The limitations of this study are that it was conducted in highly selected population cohort to improve procedural success. This was a non-randomized study in a small cohort population with short-term follow up, where more data is needed to define durability and long term outcomes. The current technology is yet to evolve with most of these procedures requiring trans-apical approach, which in part more evidence adapted from the TAVR studies showing that it adversely affects LV wall motion and function (16), while other approaches (transatrial, transseptal) are aim of efforts in innovation.
In summary, TMVR is a safe, technically feasible alternative in high surgical risk patient population with MR. Further experience with larger patient cohorts enrolled in randomized studies with these devices is needed to reveal durability and whether these treatments improve longer-term outcomes and quality of life.
Acknowledgements
None.
Footnote
Conflicts of Interest: Dr. Reardon serves on the executive committee for the Medtronic Intrepid TMVR system. The other authors have no conflicts of interest to declare.
References
- Cribier AG. The Odyssey of TAVR from concept to clinical reality. Tex Heart Inst J 2014;41:125-30. [Crossref] [PubMed]
- Krishnaswamy A, Mick S, Navia J, et al. Transcatheter mitral valve replacement: A frontier in cardiac intervention. Cleve Clin J Med 2016;83:S10-S17. [Crossref] [PubMed]
- Mihaljevic T, Lam BK, Rajeswaran J, et al. Impact of mitral valve annuloplasty combined with revascularization in patients with functional ischemic mitral regurgitation. J Am Coll Cardiol 2007;49:2191-201. [Crossref] [PubMed]
- Goldstein D, Moskowitz AJ, Gelijns AC, et al. Two-Year Outcomes of Surgical Treatment of Severe Ischemic Mitral Regurgitation. N Engl J Med 2016;374:344-53. [Crossref] [PubMed]
- Mirabel M, Iung B, Baron G, et al. What are the characteristics of patients with severe, symptomatic, mitral regurgitation who are denied surgery? Eur Heart J 2007;28:1358-65. [Crossref] [PubMed]
- Abdul-Jawad Altisent O, Dumont E, Dagenais F, et al. Initial Experience of Transcatheter Mitral Valve Replacement With a Novel Transcatheter Mitral Valve: Procedural and 6-Month Follow-Up Results. J Am Coll Cardiol 2015;66:1011-9. [Crossref] [PubMed]
- Bapat V, Buellesfeld L, Peterson MD, et al. Transcatheter mitral valve implantation (TMVI) using the Edwards FORTIS device. EuroIntervention 2014;10 Suppl U:U120-8.
- Moat N, Duncan A, Lindsay A, et al. Transcatheter mitral valve replacement for the treatment of mitral regurgitation: in-hospital outcomes of an apically tethered device. J Am Coll Cardiol 2015;65:2352-3. [Crossref] [PubMed]
- Muller DW, Farivar RS, Jansz P, et al. Transcatheter Mitral Valve Replacement for Patients With Symptomatic Mitral Regurgitation: A Global Feasibility Trial. J Am Coll Cardiol 2017;69:381-91. [Crossref] [PubMed]
- Lutter G, Lozonschi L, Ebner A, et al. First-in-human off-pump transcatheter mitral valve replacement. JACC Cardiovasc Interv 2014;7:1077-8. [Crossref] [PubMed]
- Sondergaard L, Brooks M, Ihlemann N, et al. Transcatheter mitral valve implantation via transapical approach: an early experience. Eur J Cardiothorac Surg 2015;48:873-7; discussion 877-8. [Crossref] [PubMed]
- Cheung A, Webb J, Verheye S, et al. Short-term results of transapical transcatheter mitral valve implantation for mitral regurgitation. J Am Coll Cardiol 2014;64:1814-9. [Crossref] [PubMed]
- Blanke P, Dvir D, Cheung A, et al. Mitral Annular Evaluation With CT in the Context of Transcatheter Mitral Valve Replacement. JACC Cardiovasc Imaging 2015;8:612-5. [Crossref] [PubMed]
- Goel SS, Bajaj N, Aggarwal B, et al. Prevalence and outcomes of unoperated patients with severe symptomatic mitral regurgitation and heart failure: comprehensive analysis to determine the potential role of MitraClip for this unmet need. J Am Coll Cardiol 2014;63:185-6. [Crossref] [PubMed]
- Blanke P, Naoum C, Webb J, et al. Multimodality Imaging in the Context of Transcatheter Mitral Valve Replacement: Establishing Consensus Among Modalities and Disciplines. JACC Cardiovasc Imaging 2015;8:1191-208. [Crossref] [PubMed]
- Tang GH, George I, Hahn RT, et al. Transcatheter mitral valve replacement: design implications, potential pitfalls and outcomes assessment. Cardiol Rev 2015;23:290-6. [PubMed]


