Endovascular repair of residual intimal tear or distal new entry after frozen elephant trunk for type A aortic dissection
Introduction
With advances in the surgical techniques and availability of ‘frozen elephant trunk’ (FET) prostheses, more and more centers have adopted the technique of FET and total aortic arch replacement in the management of patients with type A aortic dissection (TAAD). However, following the FET repair, late complications requiring reoperations may still occur (1,2). These are often related to residual intimal tear, new entry or aortic dilation distal to the FET in the descending thoracic aorta. After surgical aortic repair, a residual entry tear may lead to a patent false lumen in the downstream aorta, which will significantly affect long-term prognosis with decreased survival and increased distal aortic events (3-5). Reports on such patients in large series with longer follow-ups are scarce, especially regarding management of distal new entry tear (4,6).
In this study, we seek to evaluate the efficacy of thoracic endovascular aortic repair (TEVAR) for residual or new intimal tears in the descending aorta following FET in 23 patients with TAAD.
Methods
Patients
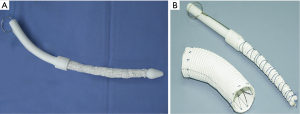
Between May 2003 and April 2013, we performed total arch replacement and FET implantation for 1,003 patients with TAAD using an open stented graft, Cronus® (100 and 120 mm in length; MicroPort Medical, Shanghai, China; Figure 1). The details of the procedure were described previously (7,8). Operative mortality was 7.0% (70/1,003) and 25 hospital survivors were lost to follow-up (2.7%). Among those with follow-up CT scans, a residual intimal or new entry tear distal to FET in the descending thoracic aorta distal to the FET was detected in 41 patients. Of these, 18 patients were asymptomatic and without growth in the aortic size, who were managed medically and closely followed up; 23 patients with symptoms or aortic size increase were managed with TEVAR, which comprises the patient population of the present study.
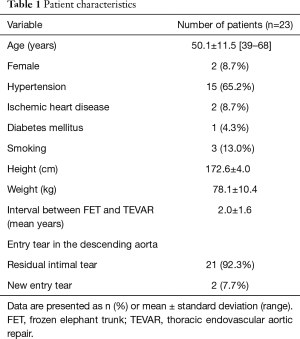
For these 23 patients with residual or new entry tears, reintervention with TEVAR was performed at mean 2.0±1.6 (median, 1.6; range, 0.3–6.7) years after the initial FET procedure; 20 of 23 (87.0%) TEVARs were performed over 1 year following the FET procedure. Mean age was 50.1±11.5 (range, 39–68) years and 21 were male (91.3%). Hypertension was present in 15 (65.2%) patients, smoking in 3 (13.0%) and diabetes in 1 (4.3%). Table 1 lists the patient profile and details of the aortic pathology and initial procedure.
Full table
The indications for reintervention with TEVAR after FET included: a residual intimal tear or new entry tear in descending thoracic aorta distal to the FET, and either one of the following two conditions: (I) the descending thoracic aorta is aneurysmal or an increase in diameter of greater than 5 mm a year; (II) symptoms of recurrent chest/abdominal pain; or malperfusion of visceral organs or lower extremity.
All 23 cases of TEVAR were performed selectively. The indication for reintervention with TEVAR was new entry tears distal to the FET in 2 (8.7%) and residual intimal tear in the descending aorta in 21 (91.3%) patients (which was detected before the initial FET procedure), respectively. The dissection flap extended to the abdominal aorta in all patients before TEVAR. The false lumen was patent or partially thrombosed in 23 patients.
Two patients with Marfan syndrome developed new entry tears in the distal segment of the descending aorta and were treated with TEVAR in local hospitals. Prior to TEVAR, computed tomographic angiography (CTA) was done in all patients to evaluate the anatomic features of the residual false lumen and new entry tear in the descending aorta.
Thoracic endovascular aortic repair
Our anatomic selection criteria for TEVAR include the two following conditions:
- The diameter of the descending thoracic aorta is less than 40 mm;
- The residual intimal or new entry tear is located at least 2 cm away from the celiac trunk.
The procedure was done under local anesthesia through a groin incision with open femoral arteriotomy. When wire access to the true lumen was confirmed in the ascending aorta, arch angiography was performed to confirm the location of intimal tear and measure the diameter of the landing zone for the endograft. The average of the two measurements (from CTA and aortic angiogram) was then taken as the diameter of the landing zone.
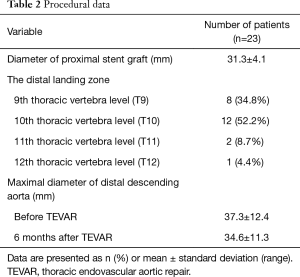
The endografts for TEVAR were selected according to: (I) anatomic selection criteria; (II) measurements from CTA and aortic angiogram; and (III) the diameter and length of the previous FET. We routinely oversize the diameter of endograft by 10–15% of the previous FET. The proximal landing zone of the endograft was implanted 3–6 cm inside the distal end of the FET. To avoid injury to the intima distal to the endograft, the diameter of distal end was tapered by 0–4 mm of the proximal end of the endograft according to the diameter of the segmental true lumen. Details of the TEVAR procedures were listed in Table 2. Because TEVAR was performed under local anesthesia, we did not take any specific measures to prevent the occurrence of spinal cord injury. Patients are conscious throughout the procedure and management will be initiated immediately any symptom or sign of paresthesia occurs. After TEVAR, the sensory and motor functions of the lower extremities were closely monitored and cerebrospinal fluid drainage was initiated in time if there were any changes in the sensory and motor functions.
Full table
Patient follow-up
Patients were clinically followed up by clinic visits, letters or emails, phone calls or by the local referring physician to monitor survival, reintervention and adverse events, such as stroke and spinal cord ischemia. Radiographic follow-up with CTA was performed before discharge, at 1 month, 6 months, 1 year and annually afterwards to detect stent graft patency, thrombosis and obliteration of the false aneurysm, sizes of the true lumen and aorta, endoleak and other complications.
Results
Five types of stent grafts were used, including Valiant (Medtronic Vascular, CA, USA) in 9 cases, Hercules-T (Microport, Shanghai, China) in 13, E-vita (Jotec GmBH, Hechingen, German) in 5, Ankura (Lifetech, Shenzhen, China) in 2 and Relay (Bolton Medical, Spain) in 2. The distal landing zone was at the level of the 9th thoracic vertebra (T9) in 8 patients, T10 in 12, T11 in 2 and T12 in 1 patient (Table 2).
The endografts were successfully deployed in all patients (100%). Completion aortography showed restoration of blood flow in the true lumen and enhanced flow to the abdominal arteries. No early death and no paraplegia or stroke or renal failure occurred after TEVAR. No endoleak of any subtype was detected in the early postprocedural period. The average length of hospital stay was 1.4±1.2 days.
Clinical and radiological follow-up was complete in 23 patients (100%) with a mean duration of 2.8±1.7 years (range, 0.3–6.4). One non-Marfan patient (4.35%) died at 4 months after TEVAR due to aortic rupture distal to the endograft. No cases of stroke or paraplegia occurred by the latest follow-up. Specifically, no late events or death occurred in the two patients with Marfan syndrome. Overall survival was 95.7% (95% CI, 72.9–99.4%) at 3 and 5 years, respectively.
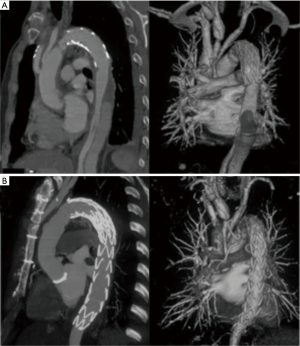
Follow-up CTA at 6 months after TEVAR showed a decrease in the size of the thrombosed false lumen in all patients (Table 2). Thrombosis of the false lumen around the endograft stented aortic segments without compressing the true lumen was seen in 21 patients (91.3%) (Figure 2). Complete false lumen thrombosis in the thoracoabdominal aorta was observed in 2 patients (8.6%).
Discussion
TAAD is a lethal catastrophe with significantly high mortality rate and needs emergency operation to prevent death caused by aortic rupture or tamponade (9-13). With the advances in the surgical techniques and the availability of the FET prostheses, more and more centers, including ours, have adopted the FET and total arch replacement technique for patients with type A dissection. Evidence has been accumulating that the FET procedure can close the false lumen in the proximal descending aorta and reduce the need for reintervention on the downstream aorta (14-20). However, the FET technique may fail to achieve false lumen obliteration at the descending thoracic aorta level in 8–15% of patients (21,22). A distal intimal tear after initial surgery for TAAD was a proven risk factor for patent false lumen and dilation of the descending aorta (4). Kimura and colleagues reported that a patent false lumen significantly decreased survival and increased the incidence of distal aortic events (2). Obviating the residual or new tears in the descending aorta allows for false lumen thrombosis and obliteration, which would promote distal aortic remodeling and improve long-term outcomes.
Open surgical reintervention for distal aortic pathologies following FET are technically challenging, with high mortality rates and morbidity rates. Pichlmaier et al. (23) reported four patients had extensive open replacements of the aorta following frozen elephant operation. Two patients developed renal failure and one of them also sustained partial paraplegia postoperatively. Coselli and colleagues (24) reported that patients undergoing replacement of the entire thoracoabdominal aorta (extent II) had the highest rates of death (6.0%), spinal cord deficit (6.3%), and renal failure (8.3%). As TEVAR has become an alternative treatment for thoracic aortic aneurysms and dissections owing to its lower mortality and morbidity rates compared with open re-intervention, it has been used as an approach to residual intimal tear following endovascular stent grafting and acute aortic dissection repair (25). In a recent study, Rylski and colleagues showed that, endovascular repair descending aortic pathologies after acute aortic dissection repair in 141 consecutive patients was associated with lower in-hospital mortality and better survival, and did not increase the incidence of later reintervention at mid-term follow-up (26). Though the use of TEVAR for treatment of intimal tear in descending aorta (type B and functional type B after type A repair) was previously described in literature, we speculate that a somewhat different point of our technique is the special FET prosthesis (Cronus) we use. It has an extra centimeter of routine Dacron graft at its proximal and distal ends, the latter offering a perfect landing zone for an endovascular stent graft (Figure 1). The landing zone in the distal FET is attached linearly to the aortic wall, thereby eliminating risk of endoleak.
The persistence of a non-resected intimal tear and a patent false lumen have been identified as risk factors for delayed aneurysmal expansion, reoperation, and worse long-term survival (4,27). Esposito et al. (6) reported one death among 65 patients undergoing TEVAR after surgery for type A acute aortic dissections. In the report of Kimura et al. (2), 7 patients (2.5%, 7/280) underwent distal TEVAR to manage a patent false lumen after surgery for acute type A dissection; no 30-day or in-hospital death, stroke and spinal cord ischemia occurred. In our own series of 1,003 patients undergoing FET for type A dissection, residual intimal or new entry tear distal to the FET in the descending aorta was detected in 41 patients on follow-up CT scans. Reintervention with TEVAR was required in 23 patients with rapid aortic growth or symptoms at mean 2.0±1.6 years following the FET repair. Neither death nor any paraplegia or stroke occurred early after reintervention with TEVAR. The present study shows that residual intimal tear and new entry tear can be managed with an additional endograft distal to the FET, which offers an ideal landing zone for the endograft. To completely obliterate the distal intimal tears located far from the FET, two endografts may be used. The distal end of endografts was tapered for 0–8 mm compared with the proximal end according to the diameter of descending aorta. In our center we have successful experience with using two endografts to obliterate the intimal tears in the distal descending aorta in patients with type B dissection (28).
Distal new entry may occur following implantation of either endovascular or open stent grafts. Experience in patients with type B dissection has shown that most endograft-related complications occurred in stent grafts without tapered a distal ends (27). Two patients in this cohort developed a distal new entry after FET, which might be a mechanical complication of FET implantation. In these two patients, the true lumen was very narrow due to compression by the enlarged false lumen and the intima was extremely fragile. The new entry tear might result from the injury to the intima of the descending aorta by the distal end of FET, which may be oversized or kinked. Care should be taken to avoid inducing a distal new entry during FET selection and implantation. A tapered distal end of the FET that can fit within the descending aorta well may help prevent this complication.
Spinal cord injury is a devastating complication following endovascular interventions on the thoracoabdominal aorta (29). In this series, no patient sustained spinal cord ischemia early or late after TEVAR, which may be ascribed to two reasons. First, 87% of TEVARs were performed more than 12 months after the FET procedure. Two-stage interventions on the thoracoabdominal aorta have been suggested to reduce the risk of spinal cord injury, probably by promoting collateral network perfusion, even though the optimal time interval between stages has not yet been determined. The interval between the initial FET and reinterventions with TEVAR in the present cohort was 2.0±1.2 years. During the interval, the thrombus in false lumen is relatively stable and chronic ischemia after the acute dissection phase may precondition the spinal cord to the ischemia induced by TEVAR in patients with intercostal arteries arising from the false lumen. Some collateral flow to the ischemic spinal cord may occur to withstand another episode of ischemia (30). Second, a lower distal landing zone has been associated with an increased risk of paraplegia. Leontyev and colleagues reported that a distal landing zone lower than T10 was a risk factor for spinal cord injury (31). In this cohort, the distal landing zone of the endograft was above T10 in 87.0% (20/23) of patients, which may be helpful in decreasing the risks of early and late spinal cord ischemic injuries.
The present study was limited by its retrospective nature, the small sample size, and the lack of longer follow-up after TEVAR. The lack of a control group makes it difficult to directly interpret the benefits of TEVAR for residual or new entry tear following FET in patients with type A dissection. Although there was a subset of 18 patients with similar aortic pathology, we have been very cautious and selective in recommending any type of reintervention (TEVAR or surgery) for such patients, unless the indications and section criteria are met. The small number of events (41 residual and new entry tears in total, only 23 managed with TEVAR) precludes testing all possible relevant factors for their association with the event (reintervention with TEVAR) for such aortic pathologies. Although favorable results were achieved in this cohort and that of others (32), the use of TEVAR in patients with Marfan syndrome is still controversial. Despite these limitations, the present study has shown the efficacy of TEVAR for distal new entry tear or residual dissections after FET and further studies in large series are warranted.
Conclusions
The results of this study have shown that TEVAR may be an alternative approach to residual intimal tear or distal new entry tear following FET and total arch replacement in patients with TAAD, with acceptable risks and satisfactory early and midterm outcomes. These results argue for the use of TEVAR patients with type A dissection sustaining a residual intimal tear or distal new entry in the descending aorta after the frozen elephant trunk procedure.
Acknowledgements
Funding: This study was supported by grants from the National Key Technologies Research and Development Program (grant 2015BA112B03) and Special Research Fund for Public Health and Welfare (grant 201402009).
Footnote
Conflicts of Interest: Presented at the American Association for Thoracic Surgery Aortic Symposium 2016, New York, NY, USA, May 12–13, 2016.
Ethical Statement: The study was approved by the Ethics Committee of Beijing Anzhen Hospital, Capital Medical University (No. 2013013X).
References
- Kim JB, Lee CH, Lee TY, et al. Descending aortic aneurysmal changes following surgery for acute DeBakey type I aortic dissection. Eur J Cardiothorac Surg 2012;42:851-6; discussion 856-7. [Crossref] [PubMed]
- Kimura N, Itoh S, Yuri K, et al. Reoperation for enlargement of the distal aorta after initial surgery for acute type A aortic dissection. J Thorac Cardiovasc Surg 2015;149:S91-8.e1. [Crossref] [PubMed]
- Ergin MA, Phillips RA, Galla JD, et al. Significance of distal false lumen after type A dissection repair. Ann Thorac Surg 1994;57:820-4; discussion 825. [Crossref] [PubMed]
- Kim YS, Kim JH, Kim JB, et al. Influence of radiologically evident residual intimal tear on expansion of descending aorta following surgery for acute type I aortic dissection. Korean J Thorac Cardiovasc Surg 2014;47:6-12. [Crossref] [PubMed]
- Unosawa S, Hata M, Niino T, et al. Prognosis of patients undergoing emergency surgery for type A acute aortic dissection without exclusion of the intimal tear. J Thorac Cardiovasc Surg 2013;146:67-71. [Crossref] [PubMed]
- Esposito G, Cappabianca G, Bichi S, et al. Hybrid repair of type A acute aortic dissections with the Lupiae technique: ten-year results. J Thorac Cardiovasc Surg 2015;149:S99-104. [Crossref] [PubMed]
- Li B, Sun L, Chang Q, et al. Total arch replacement with stented elephant trunk technique: a proposed treatment for complicated Stanford type B aortic dissection. J Card Surg 2009;24:704-9. [Crossref] [PubMed]
- Ma WG, Zheng J, Zhu JM, et al. Dr. Sun's procedure for type A Aortic dissection: Total arch replacement using tetrafurcate graft with stented elephant trunk implantation. Aorta (Stamford) 2013;1:59-64. [Crossref] [PubMed]
- Hagan PG, Nienaber CA, Isselbacher EM, et al. The International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease. JAMA 2000;283:897-903. [Crossref] [PubMed]
- Haverich A, Miller DC, Scott WC, et al. Acute and chronic aortic dissections--determinants of long-term outcome for operative survivors. Circulation 1985;72:II22-34. [PubMed]
- Pansini S, Gagliardotto PV, Pompei E, et al. Early and late risk factors in surgical treatment of acute type A aortic dissection. Ann Thorac Surg 1998;66:779-84. [Crossref] [PubMed]
- Chiappini B, Schepens M, Tan E, et al. Early and late outcomes of acute type A aortic dissection: analysis of risk factors in 487 consecutive patients. Eur Heart J 2005;26:180-6. [Crossref] [PubMed]
- Lai DT, Robbins RC, Mitchell RS, et al. Does profound hypothermic circulatory arrest improve survival in patients with acute type a aortic dissection? Circulation 2002;106:I218-28. [PubMed]
- Suto Y, Yasuda K, Shiiya N, et al. Stented elephant trunk procedure for an extensive aneurysm involving distal aortic arch and descending aorta. J Thorac Cardiovasc Surg 1996;112:1389-90. [Crossref] [PubMed]
- Kato M, Ohnishi K, Kaneko M, et al. New graft-implanting method for thoracic aortic aneurysm or dissection with a stented graft. Circulation 1996;94:II188-93. [PubMed]
- Karck M, Chavan A, Hagl C, et al. The frozen elephant trunk technique: a new treatment for thoracic aortic aneurysms. J Thorac Cardiovasc Surg 2003;125:1550-3. [Crossref] [PubMed]
- Karck M, Chavan A, Khaladj N, et al. The frozen elephant trunk technique for the treatment of extensive thoracic aortic aneurysms: operative results and follow-up. Eur J Cardiothorac Surg 2005;28:286-90; discussion 290. [Crossref] [PubMed]
- Uchida N, Shibamura H, Katayama A, et al. Total arch replacement with an open stent graft for acute type A aortic dissection: fate of the false lumen. Eur J Cardiothorac Surg 2009;35:83-8. [Crossref] [PubMed]
- Ma WG, Zhang W, Wang LF, et al. Type A aortic dissection with arch entry tear: Surgical experience in 104 patients over a 12-year period. J Thorac Cardiovasc Surg 2016;151:1581-92. [Crossref] [PubMed]
- Shrestha M, Fleissner F, Ius F, et al. Total aortic arch replacement with frozen elephant trunk in acute type A aortic dissections: are we pushing the limits too far? Eur J Cardiothorac Surg 2015;47:361-6; discussion 366. [Crossref] [PubMed]
- Liu ZG, Sun LZ, Chang Q, et al. Should the "elephant trunk" be skeletonized? Total arch replacement combined with stented elephant trunk implantation for Stanford type A aortic dissection. J Thorac Cardiovasc Surg 2006;131:107-13. [Crossref] [PubMed]
- Di Bartolomeo R, Pantaleo A, Berretta P, et al. Frozen elephant trunk surgery in acute aortic dissection. J Thorac Cardiovasc Surg 2015;149:S105-9. [Crossref] [PubMed]
- Pichlmaier MA, Teebken OE, Khaladj N, et al. Distal aortic surgery following arch replacement with a frozen elephant trunk. Eur J Cardiothorac Surg 2008;34:600-4. [Crossref] [PubMed]
- Coselli JS, Bozinovski J, LeMaire SA. Open surgical repair of 2286 thoracoabdominal aortic aneurysms. Ann Thorac Surg 2007;83:S862-4; discussion S890-2.
- He Y, Wu Z, Zhang H, et al. Repeat endovascular repair for multiple intimal tears after endovascular stent grafting of Stanford type B aortic dissection. Vasc Endovascular Surg 2013;47:245-9. [Crossref] [PubMed]
- Rylski B, Beyersdorf F, Desai ND, et al. Distal aortic reintervention after surgery for acute DeBakey type I or II aortic dissection: open versus endovascular repair. Eur J Cardiothorac Surg 2015;48:258-63. [Crossref] [PubMed]
- Tsai MT, Wu HY, Roan JN, et al. Effect of false lumen partial thrombosis on repaired acute type A aortic dissection. J Thorac Cardiovasc Surg 2014;148:2140-6.e3. [Crossref] [PubMed]
- Huang X, Huang L, Sun L, et al. Endovascular repair of Stanford B aortic dissection using two stent grafts with different sizes. J Vasc Surg 2015;62:43-8. [Crossref] [PubMed]
- Etz CD, Weigang E, Hartert M, et al. Contemporary spinal cord protection during thoracic and thoracoabdominal aortic surgery and endovascular aortic repair: a position paper of the vascular domain of the European Association for Cardio-Thoracic Surgery. Eur J Cardiothorac Surg 2015;47:943-57. [Crossref] [PubMed]
- Nakai M, Shimizu S, Ochi Y, et al. Thoracodorsal artery as a collateral source to the artery of Adamkiewicz after endovascular aneurysm repair for descending thoracic aortic aneurysm. Eur J Vasc Endovasc Surg 2009;37:566-8. [Crossref] [PubMed]
- Leontyev S, Tsagakis K, Pacini D, et al. Impact of clinical factors and surgical techniques on early outcome of patients treated with frozen elephant trunk technique by using EVITA open stent-graft: results of a multicentre study. Eur J Cardiothorac Surg 2016;49:660-6. [Crossref] [PubMed]
- Amako M, Spear R, Clough RE, et al. Total endovascular aortic repair in a patient with Marfan syndrome. Ann Vasc Surg 2017;39:289.e9-289.e12. [Crossref] [PubMed]


