
Risk factors for aortoesophageal fistula in cT4b esophageal squamous cell carcinoma after definitive radiation therapy
Highlight box
Key findings
• Post-definitive radiotherapy (DRT) esophageal wall thinning on the aortic side and elevated C-reactive protein (CRP) levels were predictive factors for aortoesophageal fistula (AEF) in patients with cT4b esophageal squamous cell carcinoma (ESCC) with obvious or suspected invasion to the aorta.
What is known and what is new?
• Esophageal fistula (EF) is a severe complication of cT4b ESCC with adjacent organ involvement. Among EFs, AEF can be fatal.
• The findings of this study led to the identification of potential risk factors for AEF in patients with cT4b ESCC who underwent DRT.
What is the implication, and what should change now?
• Close monitoring for post-DRT computed tomography scans and CRP levels could aid in the identification of high-risk patients.
• Clinicians should take these risk factors into consideration and implement appropriate interventions to prevent or manage AEF in high-risk patients.
Introduction
Background
The standard treatment in patients with cT4b esophageal squamous cell carcinoma (ESCC) with adjacent organ invasion, localized disease, and no distant metastases is definitive chemoradiotherapy (DCRT) (1-3). Although the prognosis of advanced esophageal cancer with localized cT4b disease is poor, some cases could respond to DCRT, with a reported survival rate of 20–30% at 2 to 3 years (2-4). However, in addition to tumor progression, fistula formation with invading adjacent organs may define the prognosis. The risk of fistula formation in patients with locally advanced ESCC has been reported to be 14–30% (5-7). In the case of esophagobronchial fistula or esophageal perforation, stenting or bypassing may be effective after disease onset (8,9). On the other hand, aortoesophageal fistula (AEF) is an urgent condition that often has a fatal course (10,11).
Rationale and knowledge gap
The usefulness of thoracic endovascular aortic repair (TEVAR) in the setting of AEF has been reported in several cases (12,13). In addition, the efficacy of prophylactic TEVAR has also been reported (14), as AEF could have a rapid and life-threatening course. However, the risk of developing AEF is controversial, and there is no standardized indication for prophylactic TEVAR.
Objective
This retrospective analysis aimed to identify the risk factors for AEF development in patients with cT4b ESCC who received definitive radiation therapy (DRT). We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-848/rc).
Methods
Patients and diagnosis of AEF
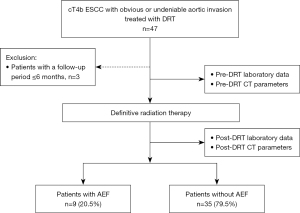
Patients with cT4b ESCC who underwent DRT from December 2004 to December 2021 were included in this study. Of these, patients diagnosed with cT4b due to invasion of organs other than the aorta, without obvious or suspected invasion of the aorta; those with missing data 6 months after DRT initiation were excluded. The patients with cT4b ESCC with aortic invasion who underwent DRT were divided into two groups, those with and without AEF. The risk factors of AEF were determined (Figure 1). AEF was diagnosed based on sudden massive hematemesis, sudden deterioration in hemodynamic status, disruption of the esophageal wall on the aortic side on contrast-enhanced computed tomography (CT), and bleeding into the lumen of the esophagus on aortogram.
All tumors were histologically diagnosed as squamous cell carcinoma based on biopsy samples obtained before treatment. The patients underwent physical examination, standard laboratory tests, chest radiography, esophagography, upper gastrointestinal endoscopy, biopsy, and enhanced CT before and after DRT.
The measured variables were age, sex, Eastern Cooperative Oncology Group Performance Status (ECOG PS), tumor location, tumor-node-metastasis (TNM) classification, histological type, presence of induction chemotherapy (ICT), and irradiation dose. Blood tests included baseline and post-DRT white blood cell counts, serum albumin level, C-reactive protein (CRP), carcinoembryonic antigen (CEA) levels, and squamous cell carcinoma (SCC) antigen levels. Blood tests after DRT were generally performed within 2 weeks after the end of DRT. Additionally, the test data closest to the end of DRT were used for analysis. The median time from the end of DRT to the test date was 0 days (−18 to 18 days from the end of DRT). Three patients had missing data within 2 weeks; thus, data up to 18 days before and after the end of DRT was analyzed. The TNM classification was based on the 8th edition of the Union for International Cancer Control (UICC) (15). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Institutional Review Board of Hiroshima University (Ethical Committee for Epidemiology of Hiroshima University: E-2225). Informed consent was obtained from all participants included in the study.
DRT
One patient received radiotherapy alone due to comorbidities, and 43 patients received DCRT. Radiotherapy consisted of 60–66 Gy (30–33 fractions). Moreover, concurrent chemotherapy comprised 5-fluorouracil, docetaxel, cisplatin, or a combination of these agents, as previously described (16). Furthermore, patients with elevated serum creatinine levels were administered nedaplatin instead of cisplatin. Concurrent chemotherapy regimens were 5-fluorouracil, docetaxel with 5-fluorouracil, cisplatin with 5-fluorouracil, or nedaplatin with 5-fluorouracil for 2 (4.5%), 8 (18.2%), 24 (54.5%), and 9 (20.5%) patients, respectively. Four patients received DRT for curative intent after ICT.
CT
The diagnosis of cT4b with aortic invasion was defined as the contact angle with the aorta of more than 90° and disappearance of the fat layer between the aorta and the esophagus on CT, before starting DRT (17). The patients who did not meet the criteria for cT4b with aortic invasion but whose aortic invasion could not be ruled out were treated as cT4b with suspected aortic invasion. In addition, the criteria were that the aorta and esophagus were in close contact at nearly 90° and the fat surface was partially missing, as previously described (18).
CT parameters were measured in the same axial slices with the widest angle in contact with the aorta. The picus angle, CT values of the esophageal wall on the aortic side, and esophageal wall thickness on the aortic side were measured as CT parameters. The angle comprising the bilateral edges of the area where tumor and the aorta came into contact, without the fat layer, and the center of the aorta was measured as the picus angle. The CT values of the esophagus were measured by drawing a circular region of interest (ROI) with the diameter of the esophageal wall on the aortic side and measuring the average of the CT values within the circle. CT parameters obtained from CTs performed before the start of DRT and within 3 months after its completion were included in the analysis.
Statistical analysis
Continuous variables are presented as medians and ranges or mean ± standard deviation (SD), whereas categorical variables are presented as numbers (percentages). Continuous variables were compared using the Mann-Whitney U test, and categorical variables were compared using the χ2 test. Statistical significance was set at a P value of less than 0.05. In the logistic regression analysis to predict AEF, multivariate analysis was performed using forward selection (likelihood ratio) for factors that showed significant differences (P<0.05) in the univariate analysis. All statistical analyses were performed using SPSS version 28.0 (IBM Corp., Armonk, NY, USA).
Results
Patient selection and outcomes after the onset of AEF
During the study period, DRT was performed in 134 patients, of which, 47 patients diagnosed with cT4b due to invasion of organs other than the aorta, 87 patients without obvious or suspected invasion of the aorta; and 3 with missing data 6 months after the start of DRT were excluded. Therefore data of 44 cases was used for the final analysis. Among 44 patients with ESCC with cT4b who underwent DRT, 9 (20.5%) developed AEF. The time from the end of DRT to the onset of AEF was 151±116 days (mean ± SD).
Of the 9 patients with AEF, 6 patients were not salvageable and 3 underwent TEVAR after hemorrhage. All patients who underwent TEVAR achieved successful hemostasis. Of the patients who underwent TEVER, two underwent emergent TEVAR after developing AEF, one of whom experienced re-bleeding 3 months after TEVAR and expired, and another underwent esophageal by-pass after TEVAR but developed stent infection and expired 6 months after TEVAR. The other patient was scheduled for prophylactic TEVAR due to esophageal wall thinning after CRT. However, the day before surgery, the patient developed massive hematemesis and cardiopulmonary arrest and underwent TEVAR, which could not rescue the patient.
Clinical characteristics of patients with and without AEF
Table 1 presents the clinical characteristics of the patients with and without AEF. There were no significant differences in age, ECOG PS, histological type, TNM stage, and radiation dose between the two groups. However, the patients with AEF were significantly more likely to be female (55.6% with AEF versus 17.1% without AEF; P=0.030) and significantly more likely to have received ICT (33.3% with AEF versus 2.9% without AEF; P=0.023).
Table 1
| Variables | cT4b with AEF, n=9 | cT4b without AEF, n=35 | P |
|---|---|---|---|
| Age (years) | 63 [49–77] | 65 [44–82] | 0.731 |
| Sex | 0.030* | ||
| Male | 4 (44.4) | 29 (82.9) | |
| Female | 5 (55.6) | 6 (17.1) | |
| ECOG PS | 0.494 | ||
| PS 0−1 | 9 (100.0) | 32 (91.4) | |
| PS 2 | 0 | 3 (8.6) | |
| Histology (biopsy) | 0.443 | ||
| POR | 1 (11.1) | 7 (20.0) | |
| Others | 8 (88.9) | 28 (80.0) | |
| Tumor diameter (mm) | 70 [50–110] | 75 [30–120] | 0.954 |
| Location | 0.418 | ||
| Ut | 3 (33.3) | 6 (17.1) | |
| Mt | 6 (66.7) | 26 (74.3) | |
| Lt | 0 | 3 (8.6) | |
| cT | 0.514 | ||
| cT4b (invasion to aorta) | 6 (66.7) | 21 (60.0) | |
| cT4b (invasion to other organs, suspected aortic invasion) | 3 (33.3) | 14 (40.0) | |
| cN | 0.578 | ||
| cN0−1 | 6 (66.7) | 22 (62.9) | |
| cN2−4 | 3 (33.3) | 13 (37.1) | |
| cM | 0.241 | ||
| cM0 | 5 (55.6) | 26 (73.3) | |
| cM1 (LYM) | 4 (44.4) | 9 (25.7) | |
| Induction chemotherapy | 0.023* | ||
| Without | 6 (66.7) | 34 (97.1) | |
| With | 3 (33.3) | 1 (2.9) | |
| Radiation dose | 0.278 | ||
| ≤60 Gy | 6 (66.7) | 17 (48.6) | |
| >60 Gy | 3 (33.3) | 18 (51.4) |
Qualitative variables are expressed as median [range] or number (%). *, indicates statistical significance (P<0.05). AEF, aortoesophageal fistula; ECOG PS, Eastern Cooperative Oncology Group Performance Status; POR, poorly differentiated; Ut, upper thoracic esophagus; Mt, middle thoracic esophagus; Lt, lower thoracic esophagus; cM1 (LYM), clinical metastasis to the supraclavicular lymph node.
The relationship between AEF and laboratory data
Table 2 summarizes a comparison of pre- and post-DRT laboratory tests in patients with cT4b with and without AEF. In the nine patients who developed AEF, pre-treatment white blood cell counts were significantly higher than those of patients without AEF (P=0.032). Serum albumin, CRP, CEA, and SCC levels were not significantly different between the two groups. Regarding laboratory tests after DRT, there were no significant differences in white blood cell counts and serum albumin, CEA, and SCC levels between the patients with or without AEF. However, CRP levels in the patients with AEF were significantly higher compared with those in those without AEF (median CRP, 5.3 mg/dL with AEF versus 0.9 mg/dL without AEF; P=0.001).
Table 2
| Variables | cT4b with AEF, n=9 | cT4b without AEF, n=35 | P |
|---|---|---|---|
| Pre-DRT laboratory data | |||
| WBC (/μL) | 10,450 (6,910–18,800) | 8,500 (4,210–18,740) | 0.032* |
| Alb (g/dL) | 3.6 (2.2–4.4) | 3.8 (2.4–4.7) | 0.456 |
| CRP (mg/L) | 2.0 (0.4–11.0) | 1.0 (0.1–5.6) | 0.075 |
| CEA (ng/mL) | 4.1 (1.3–7.9) | 3.2(0.8–91.8) | 0.528 |
| SCC (ng/mL) | 2.4 (0.6–46.9) | 2.0 (0.6–14) | 0.829 |
| Post-DRT laboratory data | |||
| WBC (/μL) | 4,030 (1,170–6,070) | 4,270 (1,610–15,520) | 0.606 |
| Alb (g/dL) | 2.8 (2.5–3.6) | 3.4 (1.7–4.1) | 0.067 |
| CRP (mg/L)† | 5.3 (0.4–20.3) | 0.9 (0.09–14.0) | 0.001* |
| CEA (ng/mL) | 3.2 (1.2–5.2) | 3.1(0.7–59.1) | 0.562 |
| SCC (ng/mL) | 1.2 (0.6–2.9) | 1.4 (0.4–3.9) | 0.591 |
Continuous variables are expressed as median (range). *, indicates statistical significance (P<0.05); †, post-DRT CRP was missing in one case in the group with AEF. AEF, aortoesophageal fistula; DRT, definitive radiation therapy; WBC, white blood cell count; Alb, serum albumin level; CRP, C-reactive protein; CEA, carcinoembryonic antigen; SCC, squamous cell carcinoma antigen.
CT parameters in the patients with and without AEF
Representative CT images in the patients with and without AEF before and after DRT are shown in Figure 2. Table 3 presents a comparison between the CT parameters in the patients with and without AEF. There was no significant difference in any of the pre-treatment CT parameters between the two groups. However, regarding post-treatment CT parameters, the contact angle with the aorta was significantly larger in the patients with AEF than in those without AEF (median, 116.2° with AEF versus 95.1° without AEF; P=0.047). In addition, CT values of the esophageal wall on the aortic side were significantly lower in the patients with AEF than those without it [median, 56.2 Hounsfield unit (HU) with AEF versus 69.9 HU without AEF; P=0.017], and the esophageal wall thickness on the aortic side was significantly thinner (median, 6 mm with AEF versus 7 mm without AEF; P=0.012) after DRT in the patients with AEF than in those without it.

Table 3
| Variables | cT4b with AEF, n=9 | cT4b without AEF, n=35 | P |
|---|---|---|---|
| Pre-DRT CT parameters | |||
| Picus angle (°) | 108 [71.5–175] | 101.3 [65.8–159.4] | 0.373 |
| Esophageal wall thickness on aortic side (mm) | 10 [7–14] | 11 [3–32] | 0.405 |
| CT value of esophageal wall on aortic side (HU) | 81.1 [75.9–90.1] | 78.3 [51.8–106.7] | 0.350 |
| Post-DRT CT parameters | |||
| Picus angle (°) | 116.2 [78.7–205.6] | 95.1 [60.3–214] | 0.047* |
| Esophageal wall thickness on aortic side (mm) | 6 [3–9] | 7 [4–16] | 0.012* |
| CT value of esophageal wall on aortic side (HU) | 56.2 [27.5–87.9] | 69.9 [50.5–98.8] | 0.017* |
Continuous variables are expressed as median [range]. *, indicates statistical significance (P<0.05). CT, computed tomography; AEF, aortoesophageal fistula; DRT, definitive radiation therapy; HU, Hounsfield unit.
Risk factors for developing AEF
Table 4 presents the optimal cutoff values established by receiver operating characteristic (ROC) curve analysis using continuous variables that show significant differences between the patients with and without AEF. In addition to sex and presence of ICT, which showed significant differences between the two groups using the univariate analysis, multivariate logistic regression analysis was performed using CT parameters calculated based on ROC curve analysis and cutoff values of blood tests (Table 5). The multivariate analysis identified elevated CRP levels after DRT [odds ratio (OR): 30.7; 95% confidence interval (CI): 2.92–323.2; P=0.004] and thinning of the esophageal wall on the aortic side after DRT (OR: 13.2; 95% CI: 1.24–140.1; P=0.033) as significant risk factors for the development of AEF.
Table 4
| Variables | Cut-off value | AUC | 95% CI | P |
|---|---|---|---|---|
| Pre-DRT parameters | ||||
| WBC | 9,800 | 0.732 | 0.56–0.91 | 0.034 |
| Post-DRT parameters | ||||
| CRP (mg/dL) | 3.3 | 0.852 | 0.69–1.00 | 0.002 |
| Contact angle to aorta (°) | 150 | 0.717 | 0.52–0.91 | 0.046 |
| Esophageal wall thickness on aortic side (mm) | 6 | 0.770 | 0.60–0.94 | 0.013 |
| CT value of the esophageal wall on aortic side (HU) | 64 | 0.760 | 0.56–0.96 | 0.018 |
ROC, receiver operating characteristic; AEF, aortoesophageal fistula; AUC, area under curve; 95% CI, 95% confidence interval; DRT, definitive radiation therapy; WBC, white blood cell count; CRP, C-reactive protein; CT, computed tomography; HU, Hounsfield unit.
Table 5
| Variables | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | ||
| Female | 6.04 | 1.24–29.4 | 0.026 | – | – | – | |
| With induction chemotherapy | 17.00 | 1.51–191.9 | 0.022 | – | – | – | |
| Pre-DRT WBC ≥9,800 | 5.78 | 1.19–28.0 | 0.030 | – | – | – | |
| Post-DRT CRP ≥3.3 | 21.3 | 3.45–132.0 | <0.001 | 30.7 | 2.92–323.2 | 0.004* | |
| Post-DRT contact angle to aorta ≥150° | 27.2 | 2.41–295.0 | 0.007 | – | – | – | |
| Post-DRT esophageal wall thickness on aortic side ≤6 mm | 8.75 | 1.55–49.6 | 0.014 | 13.2 | 1.24–140.1 | 0.033* | |
| Post-DRT CT value of esophageal wall on aortic side ≤64 HU | 8.75 | 1.55–49.6 | 0.014 | – | – | – | |
*, indicates statistical significance (P<0.05). AEF, aortoesophageal fistula; OR, odds ratio; 95% CI, 95% confidence interval; DRT, definitive radiation therapy; WBC, white blood cell count; CRP, C-reactive protein; CT, computed tomography; HU, Hounsfield unit.
Among the factors identified as risk factors for AEF, post-DRT CRP levels of 3.3 or greater were identified in nine patients, six of whom developed AEF (66.7%). The thickness of the esophageal wall on the aortic side after DRT was less than 6 mm in 17 patients, 7 of whom developed AEF (41.2%). Five patients had both post-DRT CRP levels of 3.3 or greater and a post-DRT thickness of esophageal wall on the aortic side of 6 mm or less, and four of them developed AEF (80%).
Discussion
Key findings
Esophageal fistula (EF) is a condition causing a variety of complications that directly affect prognosis due to worse outcomes and delays of anti-tumor treatment. The patients who developed EF are reported to have a poor prognosis (19-21), and several risk factors for EF formation in esophageal squamous cell carcinoma have been reported. The risk factors for EF formation are as follows: T4 lesions (19,22), formation of ulcers (19,23), total circumferential lesions (20,21), esophageal stricture (6,22), low body mass index (24), tumor length and diameter (21), high platelet-to-lymphocyte ratio (25), and hypocholesterolemia (5).
Among EFs, AEFs that form fistulas with the aorta could cause rapid and massive hemorrhage and have a fatal course. In acute bleeding due to AEF, salvage TEVAR has been reported to provide effective temporary hemostasis (26). However, AEF could cause circulatory deterioration due to rapid bleeding, which may result in failure in time for surgery. Furthermore, when AEF develops, the direct contact between the esophageal lumen and the artificial stent may cause intractable infections and other problems after TEVAR (27,28). Recently, elective TEVAR has been reported to contribute to reduced complications and improved prognosis compared with salvage TEVAR cases after the onset of AEF (14). Therefore, predicting AEF is an urgent issue, and we assessed the risk factors for the development of AEF in patients with cT4b ESCC who received DRT.
Strengths and limitations
In this study, we identified the risk factors for AEF in cases where judging the indication for prophylactic TEVAR could be challenging in real-world clinical practice. Notably, only the patients with cT4b ESCC whose primary lesion was widely adjacent to the aorta were selected for evaluation, excluding cases in which cT4b was diagnosed due to the invasion of other organs without suspected aortic invasion. To the best of our knowledge, there have been no reports of AEF-specific risk factors in only obvious or suspected invasion of the aorta after DRT.
Our study has several limitations. First, this was a single-center, retrospective study with a relatively small sample size. Second, this study included blood tests and CT scans, but there were minor variations in the dates of the tests due to the retrospective nature of the study. Third, concurrent chemotherapy regimens and treatment strategies varied depending on the date and the concomitant disease. This study included four patients who received ICT, three of whom (75%) developed AEF. The patients with DRT after ICT had higher CRP levels (median 6.6 versus 0.9 mg/dL, P=0.018) and thinner esophageal walls (median 5 versus 7 mm, P=0.027) after DRT than did those with DRT alone. It is possible that DRT after ICT, which is considered stronger treatment intensity, might have caused greater tumor shrinkage and ulceration. In addition, reduced systemic immunity due to stronger treatment induced infection, resulting in elevated CRP. However, due to the small number of ICT cases in this study, ICT was not a significant risk factor for AEF using the multivariate analysis, and further study is needed to evaluate this hypothesis.
Comparison with similar researches
CT scans, which are relatively easy to perform, are frequently used in clinical practice, and there have been several reports of their usefulness in predicting EFs. Gui et al. reported on the prediction of EF based on nomograms including gross tumor volume (GTV) obtained from CT scans before radiotherapy and found an increase in the maximum diameter of the GTV was a risk factor for EF (29). Shi et al. reported an increased tumor thickness and formation of deep ulcerations on baseline CT imaging in patients with EF (30). They reported that the ulcer depth was a risk factor for EF. In the present study, no significant predictors of AEF could be identified on pretreatment CT parameters, but thinning of the aortic side of the esophageal wall of less than 6 mm after DRT was identified as a predictor of AEF in cT4b with clear or undeniable involvement of the aorta. Although pre-DRT esophageal ulcerations could not be identified on pre-DRT CT, post-DRT thinning of the aortic side of the esophageal wall may reflect that esophageal ulcerations appeared deeper and more apparent after DRT.
Additionally, we identified post-DRT CRP levels of 3.3 mg/dL or greater as a risk factor for AEF. Kawakami et al. reported baseline elevated CRP levels as risk factors for EF in patients who received DCRT (20). They suggested that elevated CRP levels may reflect tissue damage. Similarly, in our analysis, baseline CRP levels were higher in patients who developed AEF, but the difference was not significant.
Explanations of the findings
In our study, post-DRT CRP levels and thinning of the aortic side of the esophageal wall after DRT were significantly higher and were identified as a significant risk factor of AEF in the multivariate analysis. The possibility that infection of ulcerative lesions increases the risk of EF has been reported (30), and the elevated CRP levels and thinning of the esophageal wall at the end of DRT that we identified may reflect the manifestation of esophageal ulcer and concomitant infection.
Implications and actions needed
This study identified risk factors that are indications for potential prophylactic TEVAR in cT4b esophageal squamous cell carcinoma with or suspected aortic invasion. The presence of elevated CRP and esophageal wall thickness at the end of DRT may indicate the possibility of developing AEF within a few months. Therefore, close follow-up should be switched and prophylactic TEVAR should be considered in case of worsening of these findings. Since the timing of follow-up after DRT varied, we compared CT and hematological findings at the start and end of DRT, and found that the findings at the end of DRT were risk factors for subsequent development of AEF. Since there is a relatively long period of time between the end of DRT and AEF onset, it is undeniable that there may be other findings and optimal timing of examinations that could more accurately reflect AEF onset, other than the findings at the end of DRT that were identified as risk factors for AEF in this study. Therefore, a prospective study with a standardized post-DRT examination protocol is needed to validate the usefulness of the risk factors identified in this study.
Conclusions
In conclusion, we found that thinning of the esophageal wall on the aortic side on CT scan and elevated CRP levels on blood tests at the end of DRT were predictive factors for AEF in cT4b ESCC with obvious or suspected invasion of the aorta. Particularly, patients meeting both of these risk factors developed AEF at a high frequency of 80%. We suggest that if these risk factors are present at the end of treatment, prophylactic TEVAR may be considered.
Acknowledgments
We would like to thank all the individuals of the Department of Surgical Oncology and Radiation Oncology, Hiroshima University, for advice on preparing this manuscript.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-848/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-848/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-848/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-848/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Institutional Review Board of Hiroshima University (Ethical Committee for Epidemiology of Hiroshima University: E-2225). Informed consent was obtained from all participants included in the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ohtsu A, Boku N, Muro K, et al. Definitive chemoradiotherapy for T4 and/or M1 lymph node squamous cell carcinoma of the esophagus. J Clin Oncol 1999;17:2915-21. [Crossref] [PubMed]
- Ishida K, Ando N, Yamamoto S, et al. Phase II study of cisplatin and 5-fluorouracil with concurrent radiotherapy in advanced squamous cell carcinoma of the esophagus: a Japan Esophageal Oncology Group (JEOG)/Japan Clinical Oncology Group trial (JCOG9516). Jpn J Clin Oncol 2004;34:615-9. [Crossref] [PubMed]
- Shinoda M, Ando N, Kato K, et al. Randomized study of low-dose versus standard-dose chemoradiotherapy for unresectable esophageal squamous cell carcinoma (JCOG0303). Cancer Sci 2015;106:407-12. [Crossref] [PubMed]
- Slabber CF, Nel JS, Schoeman L, et al. A randomized study of radiotherapy alone versus radiotherapy plus 5-fluorouracil and platinum in patients with inoperable, locally advanced squamous cancer of the esophagus. Am J Clin Oncol 1998;21:462-5. [Crossref] [PubMed]
- Taniguchi H, Yamazaki K, Boku N, et al. Risk factors and clinical courses of chemoradiation-related arterio-esophageal fistula in esophageal cancer patients with clinical invasion of the aorta. Int J Clin Oncol 2011;16:359-65. [Crossref] [PubMed]
- Tsushima T, Mizusawa J, Sudo K, et al. Risk Factors for Esophageal Fistula Associated With Chemoradiotherapy for Locally Advanced Unresectable Esophageal Cancer: A Supplementary Analysis of JCOG0303. Medicine (Baltimore) 2016;95:e3699. [Crossref] [PubMed]
- Taniyama TK, Tsuda T, Miyakawa K, et al. Analysis of fistula formation of T4 esophageal cancer patients treated by chemoradiotherapy. Esophagus 2020;17:67-73. [Crossref] [PubMed]
- Hihara J, Hamai Y, Emi M, et al. Esophageal bypass operation prior to definitive chemoradiotherapy in advanced esophageal cancer with tracheobronchial invasion. Ann Thorac Surg 2014;97:290-5. [Crossref] [PubMed]
- Ohtsuka T, Kato D, Tsukamoto Y, et al. Esophagobronchial fistula successfully managed with a self-expandable metallic stent followed by fixation using a silicon Y stent. Thorac Cancer 2022;13:2908-10. [Crossref] [PubMed]
- Kubota S, Shiiya N, Shingu Y, et al. Surgical strategy for aortoesophageal fistula in the endovascular era. Gen Thorac Cardiovasc Surg 2013;61:560-4. [Crossref] [PubMed]
- Okita Y, Yamanaka K, Okada K, et al. Strategies for the treatment of aorto-oesophageal fistula. Eur J Cardiothorac Surg 2014;46:894-900. [Crossref] [PubMed]
- Matsumoto A, Kanaoka Y, Baba T, et al. Result of Thoracic Endovascular Aortic Repair for Patients with Esophageal Cancer. World J Surg 2018;42:1551-8. [Crossref] [PubMed]
- Matono S, Fujita H, Tanaka T, et al. Thoracic endovascular aortic repair for aortic complications after esophagectomy for cancer: report of three cases. Dis Esophagus 2011;24:E36-40. [Crossref] [PubMed]
- Lin SH, Lee JM, Wu IH. Comparison of Clinical Outcomes between Salvage and Elective Thoracic Endovascular Aortic Repair in Patients with Advanced Esophageal Cancer with Aortic Invasion: A Retrospective Cohort Study. Biomedicines 2021;9:1889. [Crossref] [PubMed]
- Rice TW, Patil DT, Blackstone EH. 8th edition AJCC/UICC staging of cancers of the esophagus and esophagogastric junction: application to clinical practice. Ann Cardiothorac Surg 2017;6:119-30.
- Ochi M, Murakami Y, Nishibuchi I, et al. Long-term results of definitive chemoradiotherapy for unresectable locally advanced esophageal squamous cell carcinoma. J Radiat Res 2021;62:142-8. [Crossref] [PubMed]
- Hamai Y, Hihara J, Emi M, et al. Treatment outcomes and prognostic factors for thoracic esophageal cancer with clinical evidence of adjacent organ invasion. Anticancer Res 2013;33:3495-502.
- Hirohata R, Hamai Y, Hihara J, et al. Evaluation of Neoadjuvant Chemoradiotherapy Followed by Surgery for Borderline Resectable Esophageal Squamous Cell Carcinoma. World J Surg 2022;46:1934-43. [Crossref] [PubMed]
- Hu B, Jia F, Zhou H, et al. Risk Factors Associated with Esophageal Fistula after Radiotherapy for Esophageal Squamous Cell Carcinoma. J Cancer 2020;11:3693-700. [Crossref] [PubMed]
- Kawakami T, Tsushima T, Omae K, et al. Risk factors for esophageal fistula in thoracic esophageal squamous cell carcinoma invading adjacent organs treated with definitive chemoradiotherapy: a monocentric case-control study. BMC Cancer 2018;18:573. [Crossref] [PubMed]
- Wang S, Zhang C, Wang Y, et al. Risk factors and prognosis for esophageal fistula in patients with esophageal squamous cell carcinoma during radiotherapy. Esophagus 2022;19:660-9. [Crossref] [PubMed]
- Pao TH, Chen YY, Chang WL, et al. Esophageal fistula after definitive concurrent chemotherapy and intensity modulated radiotherapy for esophageal squamous cell carcinoma. PLoS One 2021;16:e0251811. [Crossref] [PubMed]
- Chen B, Deng M, Yang C, et al. High incidence of esophageal fistula on patients with clinical T4b esophageal squamous cell carcinoma who received chemoradiotherapy: A retrospective analysis. Radiother Oncol 2021;158:191-9. [Crossref] [PubMed]
- Watanabe S, Ogino I, Kunisaki C, et al. Relationship between nutritional status and esophageal fistula formation after radiotherapy for esophageal cancer. Cancer Radiother 2019;23:222-7. [Crossref] [PubMed]
- Han D, Zhang J, Zhao J, et al. Platelet-to-lymphocyte ratio is an independent predictor of chemoradiotherapy-related esophageal fistula in esophageal cancer patients. Ann Transl Med 2020;8:1163. [Crossref] [PubMed]
- Chakfé N, Diener H, Lejay A, et al. Editor's Choice - European Society for Vascular Surgery (ESVS) 2020 Clinical Practice Guidelines on the Management of Vascular Graft and Endograft Infections. Eur J Vasc Endovasc Surg 2020;59:339-84. [Crossref] [PubMed]
- Ishikawa N, Maruta K, Oi M, et al. Thoracic endovascular repair for aorto-esophageal fistula in patients with esophageal carcinoma: report of 3 cases. Vasc Endovascular Surg 2013;47:65-9. [Crossref] [PubMed]
- Takeno S, Ishii H, Nanashima A, et al. Aortoesophageal fistula: review of trends in the last decade. Surg Today 2020;50:1551-9. [Crossref] [PubMed]
- Gui Z, Liu H, Shi W, et al. A Nomogram for Predicting the Risk of Radiotherapy-Related Esophageal Fistula in Esophageal Cancer Patients. Front Oncol 2021;11:785850. [Crossref] [PubMed]
- Shi YJ, Liu C, Wei YY, et al. Quantitative CT analysis to predict esophageal fistula in patients with advanced esophageal cancer treated by chemotherapy or chemoradiotherapy. Cancer Imaging 2022;22:62. [Crossref] [PubMed]


