
Prognostic value of lactate dehydrogenase in non-small cell lung cancer patients with brain metastases: a retrospective cohort study
Introduction
Lung cancer is the leading cause of malignancy-related death worldwide, accounting for approximately 18% of all cancer-related deaths (1). Non-small cell lung cancer (NSCLC) accounts for 80–85% of all lung cancers. The prognosis of NSCLC is poor, but with continued advances in treatment modalities, the 5-year survival rate for patients with NSCLC has improved to 19.8% (2). The brain is the most common metastatic site, which can lead to disease progression and a poor prognosis (3), seriously affecting the survival and quality of life of patients. There are a variety of known molecular markers for the prognosis of brain metastases from NSCLC. Carcinoembryonic antigen (CEA) is a tumor associated glycoprotein. It was found that CEA levels were significantly higher in patients with NSCLC brain metastases and related to worse prognosis (4,5). As a tumor carbohydrate antigen, the level of CA125 was closely related to the stage and degree of invasion of lung cancer. It had certain value in predicting and evaluating the development and prognosis of brain metastases. NSE is a key enzyme in glycolysis (6). The serum NSE level was higher in lung cancer patients with brain metastases which may be related to the damage of brain tissue caused by tumor brain metastasis. The reduction of NSE expression level also indicated better therapeutic effect. Thus serum NSE could be used as a prognosis marker for lung cancer patients with brain metastases (7). Biomarkers mentioned above have some advantages in the prognosis of brain metastasis of NSCLC, but their sensitivity and specificity are still insufficient, thus a single tumor marker has certain limitations. At present, the generally accepted prognostic scoring system for NSCLC brain metastasis is the diagnosis-specific graded prognostic assessment (DS-GPA), which includes four prognostic indicators: age, Karnofsky Performance Status (KPS) score, extracranial metastatic lesions, and the number of brain metastatic lesions (8,9). Nevertheless, DS-GPA score is not perfect in evaluating survival. Even in the two most favorable groups, occasional patients survive for less than 3 months. Moreover, in the unfavorable group, survival beyond 12 months has been recorded as well. In other words, marked heterogeneity in outcomes for patients with brain metastases exists (10). Therefore, the development of new markers is urgently needed.
Some serum tumor markers are associated with the prognosis of lung cancer patients (11). The detection of serum markers has many advantages, including its non-invasive nature and ability to obtain rapid and easily repeatable measurements. Lactate dehydrogenase (LDH) is a key enzyme in glycolysis metabolism, which acts as a catalyst for the conversion of pyruvate to lactic acid (12). Tumor cells require 30 times more glucose and produce 40 times more lactate through glycolysis than normal cells (13), and this feature is more prominent in patients with brain metastases. In addition, LDH can also promote tumor angiogenesis as well as cell migration and metastasis by promoting the expression of vascular endothelial growth factor (VEGF) (14). This may ultimately lead to a poor prognosis and shorter survival times in patients. Several previous studies have shown that LDH is associated with the prognosis of several cancers, including breast (15), cervical (16), lung (17), and gastric (18) cancers. However, there are currently few studies on NSCLC patients with brain metastases. Therefore, the present study aimed to evaluate whether LDH is a prognostic factor in NSCLC patients with brain metastases. We present the following article in accordance with the STARD reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1502/rc).
Methods
Patients
This study is a retrospective analysis of NSCLC patients diagnosed with brain metastases at Tumor Hospital of Yunnan Province between January 2006 and June 2020. The survival data of patients, serum LDH level and other confounding factors that may affect the prognosis of patients were collected to explore the effect of LDH level on the prognosis of patients. The patients were divided into two groups using a restricted cubic spline (RCS) model according to the level of LDH level. Univariate, multivariate, subgroup and sensitivity analyses were used to analyze the risk factors affecting the prognosis of patients. The following variables were collected for analysis: age, sex, extracranial metastases (lung/chest/liver/bone/adrenal), KPS score, number of brain metastases, smoking status, American Joint Committee on Cancer (AJCC) T stage, AJCC N stage, AJCC M stage, treatment (i.e., whether the primary tumor was treated with surgery/chemotherapy/targeted therapy), and pathological type. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the ethics committee of Tumor Hospital of Yunnan Province (No. KYLX2022130). The requirement for individual consent for this retrospective analysis was waived.
We selected patients based on the following inclusion criteria: (I) age >18 years; (II) pathological diagnosis of NSCLC; (III) presence of brain metastases diagnosed by surgical pathology or imaging; (IV) currently undergoing chemotherapy, targeted therapy, and primary surgery; and (V) survival time (defined as the time interval between the date of diagnosis and the date of death) of more than 30 days. The exclusion criteria were as follows: (I) presence of other malignant tumors; (II) inaccurate information on primary tumor surgery, chemotherapy, and targeted therapy; and (III) absence of important data. For Cox regression analysis of survival data, outcome events should be at least 5–10 times the number of independent variables. Finally, 224 patients were selected for further analysis considering the included variables according to the above-mentioned inclusion and exclusion criteria.
Patients were followed up for survival status every 3 months after the diagnosis of NSCLC brain metastasis, then every 6 months for 3 years, and once a year for 5 years. Follow-up was carried out by outpatient and inpatient re-examination and telephone inquiry.
Clinical characteristics
The overall survival (OS) time was defined as the time from the first diagnosis of lung cancer brain metastases (rather than the first diagnosis of lung cancer) to the last follow-up or death. Definition of outcome indicators: surviving patients were marked as 1, and non-surviving patients were marked as 0. The survival time and status were also collected. The 8th version of the AJCC TNM staging system was used for staging. Patients who had smoked no more than 100 cigarettes in their lifetime were defined as non-smokers. Smokers were defined as current smokers or individuals who had quit smoking within 1 year before diagnosis. The LDH cutoff values were obtained using a RCS model, and the patients were divided into two groups according to the optimal cut-off value (180 U/L). Based on the baseline LDH levels in selected patients, 107 patients with LDH ≤180 (47.77%) and 117 patients with LDH >180 (52.23%) were identified.
Statistical analysis
A combined RCS model was established using the rms package of R software (R Core Group, Vienna, Austria) to explore the dose-response relationship between continuous changes in LDH and patient prognosis, and the continuous variable LDH was converted into a binary variable. Kaplan-Meier survival curves were drawn using the survival package, and the log-rank test was used to compare survival differences between the two groups.
Univariate and multivariate analyses of the predictors were performed using the Cox proportional hazards (PHs) regression model with the forestplot package. The prognostic value of LDH was explored through Kaplan-Meier curves and Cox regression.
Our study may be affected by many other unknown confounding factors. Even if we have collected enough known confounding factors based on previous research, there may still be unknown confounding factors that affect the reliability of our results. From the perspective of epidemiology, we need to know how stable or reliable our results are, that is, if there are one or several unknown confounding factors, how much “power” these confounding factors need to fully explain the results of our study. The sensitivity analysis of the EValue package developed by Ding and VanderWeele (19-21) was used to further evaluate the robustness of the main analysis results. Sensitivity analysis method E-value takes relative risk (RR) as the main research indicator and constructs a statistical model for RR sensitivity analysis, so as to predict the minimum strength of association between unknown confounding factors and exposure factors i.e., serum LDH level or outcomes i.e., prognosis that can be explained by the RR value.
Results
Correlation between the LDH level and prognosis of NSCLC patients with brain metastasis
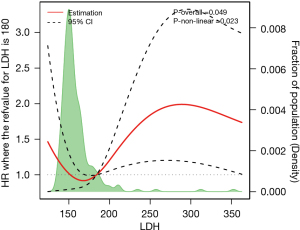
To verify the relationship between LDH levels and the prognosis of NSCLC patients with brain metastases, we constructed an RCS model and found that LDH levels were linearly associated with the prognosis of NSCLC patients with brain metastases (overall association test: P=0.049; non-linear association test: P=0.023). The RCS model showed that for LDH ≤180, the risk of death changed slowly with increasing LDH; for LDH =180, the cutoff point/inflection point was hazard ratio (HR) ≈1; and for LDH >180, the risk of death increased significantly with increasing LDH. After removing two extreme LDH values, it was found that the LDH level of NSCLC patients with brain metastases exhibited a skewed distribution (Figure 1).
Baseline characteristics of the participants
Figure 2 displays the patient selection flow diagram. After applying the inclusion and exclusion criteria, we finally selected 224 participants for analysis. Of the included patients, 147 survived and 77 died. We constructed two baseline data tables based on the outcome indicators and LDH levels. Among the included variables, the proportion of patients who underwent primary tumor surgery was significantly different between the surviving and non-surviving patients (P<0.05) (Table S1). Based on the baseline LDH levels in selected patients, 107 patients with LDH ≤180 (47.77%) and 117 patients with LDH >180 (52.23%) were identified. There were significant differences between the low and high LDH groups in terms of the clinical stage (including N and M stages, P=0.034 and 0.006), primary tumor operation (P<0.001), and extracranial metastasis (lung, liver, bone, and adrenal gland, P=0.011, 0.044, 0.002 and 0.018). There were no statistically significant differences in age, sex, body mass index (BMI), KPS score, total number of metastases, smoking status, AJCC T stage, chemotherapy, targeted therapy, pathological type, or DS-GPA between the two groups (Table 1).
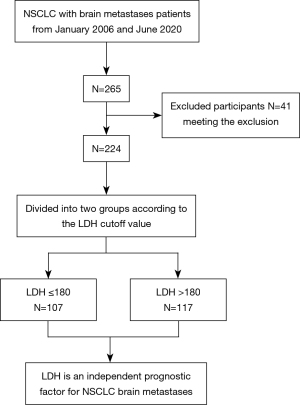
Table 1
| Variables | Total (n=224) | LDH ≤180 (n=107) | LDH >180 (n=117) | P |
|---|---|---|---|---|
| Age_cat, n (%) | 0.057 | |||
| ~50 | 78 (34.82) | 45 (42.06) | 33 (28.21) | |
| 51–60 | 73 (32.59) | 34 (31.78) | 39 (33.33) | |
| 61~ | 73 (32.59) | 28 (26.17) | 45 (38.46) | |
| Sex, n (%) | 0.138 | |||
| Female | 84 (37.5) | 46 (42.99) | 38 (32.48) | |
| Male | 140 (62.5) | 61 (57.01) | 79 (67.52) | |
| BMI_cat, n (%) | 0.478 | |||
| Normal | 163 (72.77) | 75 (70.09) | 88 (75.21) | |
| Overweight | 61 (27.23) | 32 (29.91) | 29 (24.79) | |
| KPS, n (%) | 0.319 | |||
| ~70 | 13 (5.8) | 4 (3.74) | 9 (7.69) | |
| 70–80 | 60 (26.79) | 32 (29.91) | 28 (23.93) | |
| 80~ | 151 (67.41) | 71 (66.36) | 80 (68.38) | |
| Metnum, n (%) | 0.189 | |||
| 1 | 108 (48.21) | 54 (50.47) | 54 (46.15) | |
| 2–3 | 60 (26.79) | 32 (29.91) | 28 (23.93) | |
| 4~ | 56 (25.0) | 21 (19.63) | 35 (29.91) | |
| Smoking, n (%) | 0.143 | |||
| No | 130 (58.04) | 68 (63.55) | 62 (52.99) | |
| Yes | 94 (41.96) | 39 (36.45) | 55 (47.01) | |
| AJCC.T, n (%) | 0.103 | |||
| T1 | 29 (12.95) | 14 (13.08) | 15 (12.82) | |
| T2 | 85 (37.95) | 48 (44.86) | 37 (31.62) | |
| T3 | 32 (14.29) | 9 (8.41) | 23 (19.66) | |
| T4 | 64 (28.57) | 29 (27.1) | 35 (29.91) | |
| Tx | 14 (6.25) | 7 (6.54) | 7 (5.98) | |
| AJCC.N, n (%) | 0.034 | |||
| N0 | 33 (14.73) | 21 (19.63) | 12 (10.26) | |
| N1 | 25 (11.16) | 16 (14.95) | 9 (7.69) | |
| N2 | 86 (38.39) | 41 (38.32) | 45 (38.46) | |
| N3 | 67 (29.91) | 25 (23.36) | 42 (35.9) | |
| Nx | 13 (5.8) | 4 (3.74) | 9 (7.69) | |
| AJCC.M, n (%) | 0.006 | |||
| M1 | 17 (7.59) | 6 (5.61) | 11 (9.4) | |
| M1a–M1b | 73 (32.59) | 46 (42.99) | 27 (23.08) | |
| M1c | 134 (59.82) | 55 (51.4) | 79 (67.52) | |
| Surgery, n (%) | <0.001 | |||
| No | 145 (64.73) | 56 (52.34) | 89 (76.07) | |
| Yes | 79 (35.27) | 51 (47.66) | 28 (23.93) | |
| Chemotherapy, n (%) | 0.12 | |||
| No | 71 (31.7) | 28 (26.17) | 43 (36.75) | |
| Yes | 153 (68.3) | 79 (73.83) | 74 (63.25) | |
| Targeted, n (%) | 0.944 | |||
| No | 169 (75.45) | 80 (74.77) | 89 (76.07) | |
| Yes | 55 (24.55) | 27 (25.23) | 28 (23.93) | |
| Histologic, n (%) | 0.947 | |||
| LAC | 194 (86.61) | 92 (85.98) | 102 (87.18) | |
| Others | 30 (13.39) | 15 (14.02) | 15 (12.82) | |
| MetLung, n (%) | 0.011 | |||
| No | 111 (49.55) | 63 (58.88) | 48 (41.03) | |
| Yes | 113 (50.45) | 44 (41.12) | 69 (58.97) | |
| MetChest, n (%) | 0.227 | |||
| No | 84 (37.5) | 45 (42.06) | 39 (33.33) | |
| Yes | 140 (62.5) | 62 (57.94) | 78 (66.67) | |
| MetLiver, n (%) | 0.044 | |||
| No | 206 (91.96) | 103 (96.26) | 103 (88.03) | |
| Yes | 18 (8.04) | 4 (3.74) | 14 (11.97) | |
| MetBone, n (%) | 0.002 | |||
| No | 141 (62.95) | 79 (73.83) | 62 (52.99) | |
| Yes | 83 (37.05) | 28 (26.17) | 55 (47.01) | |
| MetAdrenal, n (%) | 0.018 | |||
| No | 191 (85.27) | 98 (91.59) | 93 (79.49) | |
| Yes | 33 (14.73) | 9 (8.41) | 24 (20.51) | |
| DS_GPA, n (%) | 0.056 | |||
| 0 | 5 (2.23) | 2 (1.87) | 3 (2.56) | |
| 0.5 | 8 (3.57) | 3 (2.8) | 5 (4.27) | |
| 1 | 29 (12.95) | 12 (11.21) | 17 (14.53) | |
| 1.5 | 42 (18.75) | 21 (19.63) | 21 (17.95) | |
| 2 | 51 (22.77) | 19 (17.76) | 32 (27.35) | |
| 2.5 | 36 (16.07) | 18 (16.82) | 18 (15.38) | |
| 3 | 27 (12.05) | 16 (14.95) | 11 (9.4) | |
| 3.5 | 14 (6.25) | 5 (4.67) | 9 (7.69) | |
| 4 | 12 (5.36) | 11 (10.28) | 1 (0.85) |
LDH, lactate dehydrogenase; BMI, body mass index; KPS, Karnofsky Performance Status; Metnum, number of metastasis; AJCC, American Joint Committee on Cancer; LAC, lung adenocarcinomas; MetLung, lung metastasis; MetChest, chest metastasis; MetLiver, liver metastasis; MetBone, bone metastasis; MetAdrenal, adrenal metastasis; DS_GPA, diagnosis-specific graded prognostic assessment.
Kaplan-Meier survival curve analysis
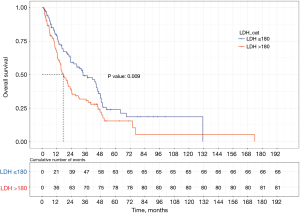
We used the survival package of R language to draw the total survival time Kaplan-Meier curves of the different groups (Figure 3). Compared with the OS of patients in the low LDH expression group, that of patients in the high LDH expression group (HR =1.549, P=0.009) was worse. Moreover, the median survival time of patients in the high and low LDH groups was approximately 16 and 33 months, respectively. The log-rank test indicated that this difference was statistically significant (P=0.009).
Univariate and multivariate Cox regression analyses
In the Schoenfeld residual diagram, the change in the visible curve with time was not obvious, so LDH satisfied the PH hypothesis (P=0.95) (Figure S1), which confirms that the Cox regression model (PHs regression model, proportional risk regression model) is meaningful.
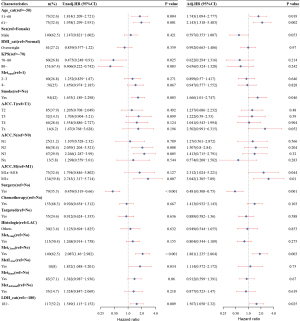
The results showed that in univariate survival analysis, LDH >180 U/L [HR =1.549, 95% confidence interval (95% CI): 1.115–2.152, P=0.009], age (51–60 and >61 years, P=0.004 and 0.001), KPS score (70–80, >80, P=0.025 and 0.003), smoking (P=0.003), AJCC N stage (N2, N3, P=0.008 and 0.005), AJCC M1c stage (P=0.007), primary surgery (P<0.001), thoracic metastasis (P<0.001), and liver metastasis (P=0.034) were risk factors for OS. However, patients with KPS scores ≥70 and primary surgery exhibited a lower risk of death. In the multivariate analysis, high LDH (HR =1.567, 95% CI: 1.058–2.32, P=0.025), age (51–60 and >60 years, P=0.019 and 0.002), smoking (P=0.046), AJCC M1c stage (P=0.01), and thoracic metastasis (P=0.003) were all identified as independent risk factors for OS (Figure 4).
Subgroup analysis
The effects of different covariables including age, sex, extracranial metastasis (lung, breast, liver, bone, and adrenal gland), KPS, number of brain metastases, smoking, AJCC T stage, AJCC N stage, AJCC M stage, BMI, and treatment (primary focus surgery/chemotherapy/targeted therapy) on the prognosis of patients grouped by serum LDH levels (high LDH group vs. low LDH group) were assessed. The likelihood ratio test showed that there was no significant difference between the level of serum LDH and other covariables; that is, there was no significant interaction between LDH and the above covariables (Figure 5).

Sensitivity analysis
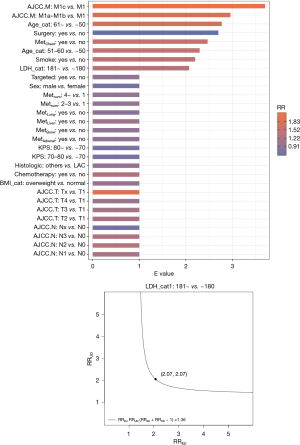
In this study, there may have been unmeasured or unknown confounding factors that could lead to bias in the research results. We used sensitivity analysis to explore the robustness of the research results (Figure 6). The E value of LDH was 2.07 (95% CI: 1.055–2.65) and the RR was 1.36, indicating that the findings were still reliable, despite the possible presence of unmeasured confounding factors.
Discussion
This study evaluated the prognostic value of serum LDH in NSCLC patients with brain metastases. The survival time of patients with brain metastases from NSCLC is limited, and strategies for the detection of representative markers are worthy of further research, as the application of such strategies could prolong the survival time of patients. Our findings suggest that LDH is an independent prognostic factor for OS in NSCLC patients with brain metastases. The findings of this study remained reliable despite the possible presence of unmeasured confounders.
Brain metastasis is a serious complication of NSCLC. Tumor cells use 30 times more glucose and produce 40 times more lactate through glycolysis than normal cells (13), and this feature is more prominent in patients with brain metastases. One of the key enzymes in the glycolytic pathway is LDH, and five isoenzymes are widely present in human tissues. On the one hand, LDH participates in glycolysis during the process of cancer cell proliferation, provides energy for cancer cells, and promotes their growth (22). On the other hand, LDH promotes the immune escape of cancer cells by inhibiting the function of cluster of differentiation 8+ (CD8+) cells and natural kill (NK) cells (23,24). In addition, it can also promote tumor angiogenesis as well as cell migration and metastasis by promoting the expression of VEGF (14). This ultimately leads to a poor prognosis and shorter survival times in patients.
High serum LDH levels are associated with resistance to various chemotherapy regimens, such as bevacizumab 20, platinum-based agents, and programmed cell death protein 1 (PD-1), in advanced NSCLC (25). Resistance is the result of the conversion of lactate to pyruvate by stromal cells, which promotes cancer cell progression and increases their resistance to chemotherapeutic agents (26), thereby reducing patient survival. Patients with advanced NSCLC are usually treated with targeted therapy and chemotherapy. In patients with advanced NSCLC treated with immune checkpoint inhibitors (ICIs), elevated pretreatment LDH is an independent marker of poor prognosis. Moreover, the continuous increase in LDH during treatment is associated with poor OS (27). In advanced NSCLC patients receiving platinum-based chemotherapy, increased LDH (≥20%) and high LDH before treatment are associated with lower OS (28).
In this study, we enrolled patients undergoing chemotherapy, targeted therapy, and primary surgery. Their LDH values before imaging diagnosis or surgical pathology were determined, and NSCLC patients with a baseline LDH >180 U/L were selected. NSCLC patients with brain metastases demonstrated a significantly increased risk of death, which is consistent with previous findings. This study also found through univariate analysis that age (51–60 and >61 years), KPS score (70–80, >80), smoking, AJCC N stage (N2, N3), AJCC M1c stage, primary surgery, thoracic metastases, liver metastases, and LDH >180 U/L were significantly associated with a higher risk of death, while a KPS score of ≥70 points and primary tumor surgery were related to increased survival time in patients. After multivariate adjustment, high LDH was still an independent risk factor for OS (HR =1.43, 95% CI: 1.004–2.038, P=0.048); age (51–60 and >60 years), smoking, AJCC M1c stage, and thoracic metastasis were also independent risk factors.
Based on the above findings, patients with high LDH, AJCC M1c stage, thoracic metastases, and age ≥51 years tend to experience worse survival outcomes, and these patients may require better follow-up care. Therefore, the routine detection of LDH levels in patients with brain metastases diagnosed by imaging or by surgical pathology may provide valuable prognostic information. Moreover, for patients who require chemotherapy, the treatment of elevated LDH levels prior to cancer treatment may reduce tumor pressure and improve the efficacy of chemotherapeutic drugs, thereby prolonging the survival time of patients.
Currently, various effective LDH inhibitors have been used in clinical treatment, and the inhibition of LDH has a minimal effect on normal tissues (29). More importantly, reducing LDH activity has been shown to inhibit several other measures of cancer proliferation in vivo (30). Galloflavin, oxalate, and other inhibitors can be used to treat breast cancer (31), hepatocellular carcinoma (32), endometrial cancer (33), pancreatic cancer (34), nasopharyngeal cancer (35), Burkitt lymphoma (36), and other malignant tumors. In the future, more types of LDH inhibitors may be used in patients with advanced NSCLC, thereby improving the survival time of patients.
There are some limitations to this study that should be noted. Firstly, this study suffers from the limitations inherent to retrospective analyses of observational data from a single center. It also lacks LDH gene expression analysis in NSCLC patients with brain metastases, analysis of NSCLC brain metastases before and after chemotherapy, and a comparative study of LDH in patients with metastatic disease. In addition, LDH levels may be affected by other factors. Although this study has certain limitations, it still has a certain guiding significance for the treatment of patients with advanced NSCLC with brain metastases.
Conclusions
The mortality risk increases sharply in NSCLC patients with brain metastases with LDH >180 U/L. High LDH is an independent risk factor for NSCLC patients with brain metastases.
Acknowledgments
Funding: This work was supported by grants from the National Natural Science Foundation, Regional Science Foundation Project (Nos. 81960322, 82160343); the Medical Reserve Personnel Training Program of Yunnan Provincial Health Commission (No. H-2018097); the Joint Program of Applied Basic Research of Yunnan Provincial Department of Science and Technology - Kunming Medical University [Nos. 202101AY070001-158, 2019FE001(-236)]; the Graduate Innovation Fund of Kunming Medical University (No. 2022B15); the “Famous Doctor” Special Project of Ten Thousand People Plan of Yunnan Province (Nos. YNWR-MY-2020-095, CZ0096); and the Medical Leading Talents Training Program of Yunnan Provincial Health Commission (No. L-2019028).
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1502/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1502/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1502/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the ethics committee of Tumor Hospital of Yunnan Province (No. KYLX2022130). The requirement for individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Lu T, Yang X, Huang Y, et al. Trends in the incidence, treatment, and survival of patients with lung cancer in the last four decades. Cancer Manag Res 2019;11:943-53. [Crossref] [PubMed]
- Riihimäki M, Hemminki A, Fallah M, et al. Metastatic sites and survival in lung cancer. Lung Cancer 2014;86:78-84. [Crossref] [PubMed]
- Li WY, Zhao TT, Xu HM, et al. The role of EGFR mutation as a prognostic factor in survival after diagnosis of brain metastasis in non-small cell lung cancer: a systematic review and meta-analysis. BMC Cancer 2019;19:145. [Crossref] [PubMed]
- Numata T, Endo T, Yanai H, et al. Serum CEA and CYFRA Levels in ALK-rearranged NSCLC Patients: Correlation With Distant Metastasis. In Vivo 2020;34:2095-100. [Crossref] [PubMed]
- Dai H, Liu J, Liang L, et al. Increased lung cancer risk in patients with interstitial lung disease and elevated CEA and CA125 serum tumour markers. Respirology 2014;19:707-13. [Crossref] [PubMed]
- Bi H, Yin L, Fang W, et al. Association of CEA, NSE, CYFRA 21-1, SCC-Ag, and ProGRP with Clinicopathological Characteristics and Chemotherapeutic Outcomes of Lung Cancer. Lab Med 2022;lmac122. Epub ahead of print. [Crossref] [PubMed]
- Sperduto PW, Yang TJ, Beal K, et al. Estimating Survival in Patients With Lung Cancer and Brain Metastases: An Update of the Graded Prognostic Assessment for Lung Cancer Using Molecular Markers (Lung-molGPA). JAMA Oncol 2017;3:827-31. [Crossref] [PubMed]
- Sperduto PW, Chao ST, Sneed PK, et al. Diagnosis-specific prognostic factors, indexes, and treatment outcomes for patients with newly diagnosed brain metastases: a multi-institutional analysis of 4,259 patients. Int J Radiat Oncol Biol Phys 2010;77:655-61. [Crossref] [PubMed]
- Nieder C, Andratschke NH, Geinitz H, et al. Diagnosis-specific graded prognostic assessment score is valid in patients with brain metastases treated in routine clinical practice in two European countries. Med Sci Monit 2012;18:CR450-5. [Crossref] [PubMed]
- Zhao X, Wang M. Clinical utility of serum tumor markers in lung cancer. Zhongguo Fei Ai Za Zhi 2011;14:286-91. [PubMed]
- Miao P, Sheng S, Sun X, et al. Lactate dehydrogenase A in cancer: a promising target for diagnosis and therapy. IUBMB Life 2013;65:904-10. [Crossref] [PubMed]
- Holm E, Hagmüller E, Staedt U, et al. Substrate balances across colonic carcinomas in humans. Cancer Res 1995;55:1373-8. [PubMed]
- Azuma M, Shi M, Danenberg KD, et al. Serum lactate dehydrogenase levels and glycolysis significantly correlate with tumor VEGFA and VEGFR expression in metastatic CRC patients. Pharmacogenomics 2007;8:1705-13. [Crossref] [PubMed]
- Liu D, Wang D, Wu C, et al. Prognostic significance of serum lactate dehydrogenase in patients with breast cancer: a meta-analysis. Cancer Manag Res 2019;11:3611-9. [Crossref] [PubMed]
- Wang H, Wang MS, Zhou YH, et al. Prognostic Values of LDH and CRP in Cervical Cancer. Onco Targets Ther 2020;13:1255-63. [Crossref] [PubMed]
- Zhang X, Guo M, Fan J, et al. Prognostic significance of serum LDH in small cell lung cancer: A systematic review with meta-analysis. Cancer Biomark 2016;16:415-23. [Crossref] [PubMed]
- Namikawa T, Ishida N, Tsuda S, et al. Prognostic significance of serum alkaline phosphatase and lactate dehydrogenase levels in patients with unresectable advanced gastric cancer. Gastric Cancer 2019;22:684-91. [Crossref] [PubMed]
- Ding P, VanderWeele TJ. Sensitivity Analysis Without Assumptions. Epidemiology 2016;27:368-77. [Crossref] [PubMed]
- Ding P, Vanderweele TJ. Sharp sensitivity bounds for mediation under unmeasured mediator-outcome confounding. Biometrika 2016;103:483-90. [Crossref] [PubMed]
- VanderWeele TJ, Ding P. Sensitivity Analysis in Observational Research: Introducing the E-Value. Ann Intern Med 2017;167:268-74. [Crossref] [PubMed]
- Petrelli F, Cabiddu M, Coinu A, et al. Prognostic role of lactate dehydrogenase in solid tumors: a systematic review and meta-analysis of 76 studies. Acta Oncol 2015;54:961-70. [Crossref] [PubMed]
- Crane CA, Austgen K, Haberthur K, et al. Immune evasion mediated by tumor-derived lactate dehydrogenase induction of NKG2D ligands on myeloid cells in glioblastoma patients. Proc Natl Acad Sci U S A 2014;111:12823-8. [Crossref] [PubMed]
- Fischer K, Hoffmann P, Voelkl S, et al. Inhibitory effect of tumor cell-derived lactic acid on human T cells. Blood 2007;109:3812-9. [Crossref] [PubMed]
- Peng L, Wang Y, Liu F, et al. Peripheral blood markers predictive of outcome and immune-related adverse events in advanced non-small cell lung cancer treated with PD-1 inhibitors. Cancer Immunol Immunother 2020;69:1813-22. [Crossref] [PubMed]
- Koukourakis MI, Pitiakoudis M, Giatromanolaki A, et al. Oxygen and glucose consumption in gastrointestinal adenocarcinomas: correlation with markers of hypoxia, acidity and anaerobic glycolysis. Cancer Sci 2006;97:1056-60. [Crossref] [PubMed]
- Tjokrowidjaja A, Lord SJ, John T, et al. Pre- and on-treatment lactate dehydrogenase as a prognostic and predictive biomarker in advanced non-small cell lung cancer. Cancer 2022;128:1574-83. [Crossref] [PubMed]
- de Jong C, Deneer VHM, Kelder JC, et al. Association between serum biomarkers CEA and LDH and response in advanced non-small cell lung cancer patients treated with platinum-based chemotherapy. Thorac Cancer 2020;11:1790-800. [Crossref] [PubMed]
- Balboni A, Govoni M, Rossi V, et al. Lactate dehydrogenase inhibition affects homologous recombination repair independently of cell metabolic asset; implications for anticancer treatment. Biochim Biophys Acta Gen Subj 2021;1865:129760. [Crossref] [PubMed]
- Xie H, Hanai J, Ren JG, et al. Targeting lactate dehydrogenase--a inhibits tumorigenesis and tumor progression in mouse models of lung cancer and impacts tumor-initiating cells. Cell Metab 2014;19:795-809. [Crossref] [PubMed]
- Farabegoli F, Vettraino M, Manerba M, et al. Galloflavin, a new lactate dehydrogenase inhibitor, induces the death of human breast cancer cells with different glycolytic attitude by affecting distinct signaling pathways. Eur J Pharm Sci 2012;47:729-38. [Crossref] [PubMed]
- Cassim S, Raymond VA, Dehbidi-Assadzadeh L, et al. Metabolic reprogramming enables hepatocarcinoma cells to efficiently adapt and survive to a nutrient-restricted microenvironment. Cell Cycle 2018;17:903-16. [Crossref] [PubMed]
- Han X, Sheng X, Jones HM, et al. Evaluation of the anti-tumor effects of lactate dehydrogenase inhibitor galloflavin in endometrial cancer cells. J Hematol Oncol 2015;8:2. [Crossref] [PubMed]
- Moir JAG, Long A, Haugk B, et al. Therapeutic Strategies Toward Lactate Dehydrogenase Within the Tumor Microenvironment of Pancreatic Cancer. Pancreas 2020;49:1364-71. [Crossref] [PubMed]
- Li X, Lu W, Hu Y, et al. Effective inhibition of nasopharyngeal carcinoma in vitro and in vivo by targeting glycolysis with oxamate. Int J Oncol 2013;43:1710-8. [Crossref] [PubMed]
- Vettraino M, Manerba M, Govoni M, et al. Galloflavin suppresses lactate dehydrogenase activity and causes MYC downregulation in Burkitt lymphoma cells through NAD/NADH-dependent inhibition of sirtuin-1. Anticancer Drugs 2013;24:862-70. [Crossref] [PubMed]
(English Language Editor: A. Kassem)


