
A guide for managing patients with stage I NSCLC: deciding between lobectomy, segmentectomy, wedge, SBRT and ablation—part 2: systematic review of evidence regarding resection extent in generally healthy patients
Introduction
Treatment options for clinical stage I (cI) non-small cell lung cancer (NSCLC) have evolved. Smaller tumors are being detected; average patient age is increasing, as is the number with co-morbidities. We need to match the treatment to the patient and tumor, avoiding both overtreatment and undertreatment.
Decision-making regarding stage I NSCLC is complex. Many short- and long-term outcomes are relevant. We aim to practice evidence-based medicine (EBM), but the available evidence is suboptimal and confusing. Multiple factors influence treatment selection and independently the prognosis, and evidence often only partially applies to an individual patient. Although clinicians are used to weighing various considerations and complex decision-making, better definition of the evidence regarding management of cI NSCLC is needed, including sources of uncertainty, and nuances of patients, tumors and settings that affect applicability.
We assessed the evidence regarding cI NSCLC, critically addressing confounders and limitations, to provide clarity and confidence in applicability in various circumstances. Furthermore, we developed a concise format that enhances application to individual patients. The project consists of 4 publications: Part 1 concisely summarizes the evidence and provides a framework to guide clinical decision-making (1), Part 2 (this paper) reviews evidence regarding surgery in generally healthy patients, Part 3 addresses surgery in specific patients and tumors (2), Part 4 focuses on evidence regarding SBRT and ablation (3).
Methods
General approach
The approach involved being as inclusive and as critical as possible, with attention to nuances about settings and characteristics of the available evidence to understand limitations and applicability. A detailed description of the approach is provided in the methods section of Part 1 (1). Briefly, the subject is stage cIA NSCLC (using the 8th edition nomenclature throughout); interventions include lobectomy, segmentectomy, wedge resection, SBRT and ablation. The most relevant outcomes were chosen a priori: short-term treatment-related mortality, toxicity/morbidity, pain, quality-of-life (QOL) and long-term overall survival (OS), lung cancer specific survival (LCSS), freedom from recurrence (FFR), functional status and QOL.
Because few randomized controlled trials (RCTs) are available, we relied heavily on non-randomized comparisons (NRCs) that adjusted for confounding factors (i.e., factors independently influencing treatment selection and outcomes). We critically evaluated how well confounders were accounted for to assess the confidence that observed results reflect the intervention in question. Finally, we explored sources of ambiguity to promote understanding uncertainties and limitations of applicability.
Clinical decision-making requires weighing multiple considerations for an individual. This involves balancing not only many outcomes but many aspects of each—e.g., the strength of the evidence, the magnitude of the impact, uncertainty and how well this applies to an individual. In the Part 1 paper we provide a framework to manage this complexity—allowing clinicians to identify and focus on issues with the most impact in a particular setting for a patient. Here we develop the foundation, presenting the data in a manner that can at-a-glance provide an aggregate view of an outcome as well as the nuances and uncertainties of the data. A definition of what can be reasonably considered clinically meaningful facilitates assessing the impact of differences (described elsewhere; see Tab. S1-1 of Part 1) (1).
Evidence assessment
Literature search and study selection
We systematically searched English literature from 2000–2021; details are provided elsewhere (see app. 1-2 of Part 1) (1). Selected studies provided evidence relevant to the topic, focusing on RCTs and adjusted NRCs. For major outcomes we included all RCTs, and NRCs that adjusted for confounding and had ≥50 patients per arm. Each evidence table lists specific inclusion and exclusion criteria.
Study assessment
NRCs were assessed for confounding (bias) in order to appropriately interpret findings. The assessment of NRCs is summarized below (details provided in Appendix 2-1).
Potential confounders
A comprehensive list of potential confounders was identified a priori from known prognostic factors, patterns of care and treatment discrepancies. These included non-medical patient-related factors (e.g., age, sex, race, education, socioeconomic, marital status), medical patient-related factors [e.g., comorbidities, comorbidity severity, performance status (PS)], discrepancies in stage classification [e.g., node assessment, positron emission tomography (PET) use], time period (treatments skewed towards different periods), facility factors (treatments skewed towards different facility types), treatment quality (e.g., margin adequacy, experience, technical aspects), favorable tumor selection [e.g., smaller, ground glass (GG), indolent tumors, conversion to lobectomy if upstaging suspected/encountered].
Methods of multivariable adjustment
Multivariable regression models the relationship between multiple covariates and an outcome. Simultaneous adjustment for multiple confounders requires a substantial sample size—generally ~10 events (e.g., deaths) for each covariate. Propensity scoring models the relationship between confounders and treatment assignment, collapsing all confounders into a single propensity score. While theoretically advantageous when there are many confounders and few events, whether propensity or multivariable methods more accurately estimate treatment effect is unclear (4,5). Several propensity adjustment methods exist (propensity score adjustment, matching, inverse weighting); performance of each depends on characteristics of the data and question at hand (4-6).
Assessment of confidence study results reflect the treatment of interest
Relevant NRCs were assessed using a general tool to assess overall risk of bias (7). Additionally, we developed an assessment specific to stage I lung cancer, based on the a priori list of potential confounders (details in Appendix 2-1). Two reviewers rated each domain in each study and intervention, assigning an overall degree of confidence that outcomes reflect the treatment intervention; discrepancies were resolved by discussion. The independent assessments were largely consistent (and similar to the general tool rating), providing confidence in the process. The evidence tables include the consensus ratings for residual confounding.
Aggregation of studies
A quantitative meta-analysis is deemed inappropriate because of frequent residual confounding in various domains with variable severity. It is more useful to aggregate the studies in a manner that highlights similarities and differences, with ordering that allows patterns to emerge. This facilitates an overall qualitative impression that is more conducive to guiding clinical decision-making.
To achieve this, we have thoughtfully constructed tables. Color coding rapidly provides an overall impression (despite inclusion of levels of details if close scrutiny is needed). This essentially layers the concept of a heat map onto a traditional table. We explored various ways of ordering table entries, eventually settling on what was most revealing regarding the presence/absence of an association. The table structure is noted as a subtitle. We believe that visual representation of the outcomes, uncertainties and effect modifiers provides a summary that enhances point-of-care clinical judgment.
Results
Short-term outcomes
Treatment related mortality
Several RCTs reveal no difference in mortality by resection extent in healthy patients. The Lung Cancer Study Group (LCSG821) trial, conducted in the 1980s, reported no significant mortality difference between sublobar resection (2/3rd segmentectomy) and lobectomy via thoracotomy (1% vs. 2% respectively) (8). In a US-based RCT (CALGB140503, 2007–17) 90-day mortality was not statistically different for sublobar resection vs. lobectomy (1.2% vs. 1.7%; 80% VATS resection, 60% wedge among sublobar resection) (9). No mortality occurred for either segmentectomy or lobectomy in a large Japanese RCT (JCOG0802, 2009–14, n=1,106) (10) and a smaller European RCT (n=108) (11).
Studies of perioperative mortality with adjustment for confounders (Table S2-1) (12-18) have frequently reported minimally lower mortality after lesser resection, but the magnitude of the difference is not clinically meaningful. A difference of >1% was only noted in one study (wedge resection vs. lobectomy) in subgroups of thoracotomy and patients with a forced expiratory volume in 1 second (FEV1) of <60% (12).
Similar (unadjusted) mortality for lesser resection and lobectomy is reported in large database studies (e.g., 30-day mortality of 1.51%, 1.55% and 1.6%, P=0.87 for wedge resection, segmentectomy and lobectomy in an NCDB study [2003–11] (16); 90-day mortality 3.7% and 4% for sublobar resection and lobectomy in a SEER-Medicare study [2003–9] (19); 90-day mortality of 0.5%, 0.7% and 1.2% for wedge, segment and lobectomy, respectively, in a 2010 Japanese national study) (20). However, an Australian study reported unadjusted 90-day mortality of 4.5% and 2.6% for sublobar resection and lobectomy, respectively [2008–14] (21).
Treatment-related morbidity
Treatment-related morbidity is similar in large RCTs between sublobar resection and lobectomy in healthy patients (any morbidity, 51% vs. 54% CALGB, 51% vs. 48% JCOG0802; grade ≥3 14% vs. 15% CALGB, 4.5% vs. 4.9% JCOG0802, each study using different grading definitions; and grade ≥3 pulmonary complications, 7% vs. 10% CALGB, 2.4% vs. 1.8% JCOG0802, respectively) (9,10). A nonsignificant trend towards lower grade ≥3 complications in wedge vs. segmentectomy was seen in the CALGB study (11% vs. 19%, P=0.13) (9). The small European RCT also found no significant difference in overall 90-day morbidity (17% segmentectomy vs. 26% lobectomy, P= NS) (11).
Adjusted NRCs suggest slightly lower grade ≥3 complications after sublobar resection (Table S2-1, borderline clinically significant). The 90-day unadjusted grade ≥3 complication rate was low in the 2010 Japanese national experience (4.4% wedge, 7.1% segmentectomy, 8.7% lobectomy) (20).
Short-term pain, QOL
Few QOL studies have parsed results to sublobar resection, so extrapolation from general studies is required. Presumably most symptoms are incision-related—thus largely driven by the approach (VATS vs. open); resection extent can be mainly expected to impact dyspnea.
A prospective study shows that symptoms after lung resection mostly resolve within several months (Figure 1A,1B) (22). Similarly, QOL studies report the initial impairment in many domains is improved by 3–6 months (see subsequent QOL section)—especially after VATS resection. The impact of sublobar resection is unclear (studies are confounded by varying VATS use).

Prospective study of patient reported outcomes in patients undergoing lobectomy at MD Anderson (stage I, II NSCLC, 2004–08, n=60, 48% VATS). (A) Time course of the 5 most severe symptoms; 11-point scale from 0 (not present) to 10 (as bad as you can imagine). (B) Time to return to mild pain at 2 contiguous measurements. Reproduced with permission from Fagundes et al. (22). VATS, video-assisted thoracoscopic surgery.
A small RCT reported on QOL over 12 months (2013–17, n=108, closed after accruing 19% of the target) (11). Global QOL was significantly decreased at discharge and 6 weeks, returning to baseline by 3 months, with no difference between arms (segmentectomy vs. lobectomy). Interpretation is hampered because VATS was used for 23% of segmentectomies and 43% of lobectomies (P<0.03); furthermore, 44% of segmentectomies were arguably “lobe-like” (i.e., left upper trisegmentectomy, lingulectomy, or basilar quadri-segmentectomy). Pain outcomes were similar for segmentectomy vs. lobectomy throughout, but worse than baseline in both arms even at 12 months. Dyspnea was worse than baseline throughout the follow-up year (somewhat less after segmentectomy than lobectomy) (11).
Many studies of lobectomy (including RCTs, adjusted NRCs) report better outcomes with VATS vs. thoracotomy [including lower operative mortality, fewer complications, shorter hospital length of stay (LOS) and less pain] (23). A recent RCT of lobectomy by VATS vs. anterolateral thoracotomy found less pain and less QOL reduction in the VATS arm; the QOL impact resolved in most patients by 6 (VATS) to 12 weeks (thoracotomy) (24).
VATS is also beneficial in sublobar resections. An extensively adjusted NRC found fewer complications with VATS (rated as “very high” confidence that outcomes reflect VATS vs. open approach to segmentectomy) (25). A retrospective comparison of VATS vs. open segmentectomy found fewer pulmonary complications and shorter LOS after VATS (n=193, 2000-13, mostly healthy, lobectomy eligible patients) (26). Another retrospective comparison of VATS vs. open segmentectomy (n=104 vs. 121) found that VATS was associated with fewer pulmonary complications (15% vs. 30%, P=0.012), shorter LOS (5 vs. 7 days, P<0.001), and statistically non-significant differences in overall complications (26% vs. 34%), major complications (6% vs. 12%) and operative mortality (0 vs. 1.7%), respectively (27).
Nomori et al. assessed pain, comparing segmentectomy via thoracotomy, segmentectomy via hybrid-VATS (VATS camera with mini-thoracotomy) and lobectomy via complete VATS (n=220, 2012-15) (28). Short-term pain was less after VATS/hybrid-VATS than thoracotomy, but similar for hybrid-VATS segmentectomy or VATS lobectomy. By 3 months pain had resolved equally in all groups, with <5% requiring any analgesics (28).
Nuances and sources of ambiguity
The type of segmentectomy may play a role: multivariable analysis of a prospective study observed more grade ≥2 pulmonary complications following complex vs. simple segmentectomy (7.7% vs. 6.1%) (10). Complex segmentectomy was defined as requiring division of >1 intersegmental plane. However, another study found no difference in morbidity or mortality following complex (n=117) or simple (n=92) VATS segmentectomy (29).
Long-term outcomes
Survival
The LCSG821 RCT enrolled cN0 lung cancers ≤3 cm on the basis of CXR and not visible on (primarily rigid) bronchoscopy from 1982–88 (8,30,31). After intraoperative confirmation of T1N0 (frozen section of segmental, lobar, hilar, and mediastinal nodes)—patients were randomized to sublobar resection (67% segmentectomy) vs. lobectomy. A ≥2 cm margin was required; these tumors were undoubtedly primarily solid and resected via thoracotomy. In the final corrected analysis sublobar resection was associated with lower 5-year OS (56% vs. 73%; P=0.06), worse FFR (63% vs. 78%; P=0.04), and higher locoregional recurrence (5.4% vs. 1.9% per person per year, P=0.009) (30,31). However, present-day applicability of this evidence is questionable.
There are 2 major contemporary RCTs (Figure 2) (8-11,32-35). The CALGB140503 trial (9) randomized 697 patients with peripheral (outer 1/3), mostly solid tumors, ≤2 cm (total size) to sublobar resection (60% wedge) vs. lobectomy—mature results are awaited. The JCOG0802 trial (10,34) randomized 1,106 patients with peripheral (outer 1/3), part-solid tumors [88% with >0.5 consolidation/tumor ratio (CTR)], ≤2 cm (total size) to segmentectomy vs. lobectomy. A margin of ≥2 cm or a margin/tumor ratio ≥1 was required in both trials.
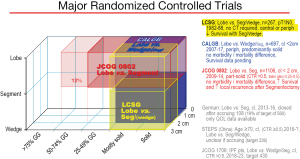
Graphic depiction of the 3 major randomized controlled trials. The x axis depicts the type of tumors included relative to proportion of solid/ground glass component, the z axis depicts tumor size, the y axis the resection extent. Three additional RCTs (German, STEPS and JCOG1706) are listed which have limited accrual. References: LCSG (8), CALGB (9), JCOG0802 (10), German (11), STEPS (32), JCOG1706 (33). CALGB, Cancer and Leukemia Group B; CTR, consolidation/tumor ratio; GG, ground glass appearance; IPF pts, Idiopathic pulmonary fibrosis patients; JCOG, Japan Cancer Oncology Group; LCSG, Lung Cancer Study Group; Lobe, lobectomy; Periph, peripheral; QOL, quality of life; Seg, segmentectomy; SL, sublobar; STEPS, Surgical Treatment of Elderly Patients.
Long-term results of the JCOG0802 trial have been published (35), with similar results after segmentectomy vs. lobectomy. These results are discussed elsewhere (2) because this study involves part-solid tumors.
Adjusted NRCs of segmentectomy or wedge vs. lobectomy in apparently healthy patients are shown in Table 1 (16,36-52), Table 2 (16,36,42,47,48,50,53-62), Table 3 (36,47,48,50,63-66) and Figures S2-1,S2-2,S2-3. Interpretation is challenging because of frequent limited accounting for confounders. Nevertheless, in aggregate, several observations can be made. First, the number of studies is impressive, and how inadequately most studies accounted for confounding factors. Second, the hazard ratios (HRs) for OS favor lobectomy (with few exceptions); while this could be due to confounders, the similar HRs for LCSS largely eliminates greater comorbidities among sublobar resection patients as an explanation. Third, statistically significant differences are seen in most studies involving wedge/sublobar resection vs. lobectomy, and in ~1/3rd of studies involving segmentectomy vs. lobectomy or wedge vs. segment resection. There are no clear additional correlations—results do not seem to track with particular sources of confounding, larger studies, stage, time period or data source.
Table 1
Ordered by resection extent, degree of confidence that results reflect the effect of the treatment, stage
Table 2
Ordered by resection extent, degree of confidence that results reflect the effect of the treatment, stage
Table 3
Ordered by resection extent, degree of confidence that results reflect the effect of the treatment, stage
Several studies (Khullar, Eguchi, Razi) (16,52,53) are categorized as providing high confidence that outcomes are attributable to the resection extent. Two of these found better adjusted OS and LCSS after lobectomy. Figure 3 shows OS of propensity matched cohorts from the Khullar et al. study, which involved extensive matching with several additional analyses (size subsets, margin status, facility type, number of nodes assessed intraoperatively) (16).
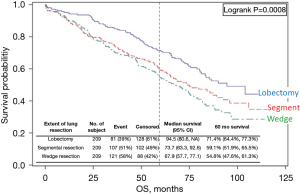
Comparison of resection extent in the National Cancer Database of cIA1,2 NSCLC [2003–6]. This study matched for 14 prognostic factors and performed multiple sensitivity tests; it is assessed to have a low level of residual confounding. Reproduced with permission from Khullar et al. (16). OS, overall survival.
On the other hand, Razi et al. found no difference in OS for the subset of cIA patients in whom unsuspected pN1 or pN2 nodes were found (52). This study involved extensive adjustment for confounders, including details of the node assessment and use of adjuvant chemotherapy (which was associated with better OS) (52). Possible reasons for the similar outcomes include that there is no inherent difference between segmentectomy and lobectomy, that any impact of resection extent is overshadowed by that of node involvement, or that a benefit to lobectomy stems from more accurate node assessment and adjuvant chemotherapy (despite being adjusted for). The latter hypothesis is supported by some studies (i.e., similar outcomes with sublobar resection vs. lobectomy when a similar nodal assessment was performed) (61,67,68). However, among adjusted studies overall there is no consistent correlation between long-term outcome differences and adjustment for either adjuvant therapy or extent of node assessment.
Many authors have reported systematic reviews and meta-analyses of non-randomized studies comparing lesser resection to lobectomy (69-75). However, no degree of systematic search rigor or meta-analytic proficiency in amalgamating reported results can overcome residual confounding in the source data. In fact, by combining studies the meta-analytic process obscures the weaknesses of each study. Thus, because of unaccounted (and obscured) confounders, drawing conclusions from meta-analyses of non-randomized studies is problematic.
Recurrence
Recurrence is a concern, especially because the LCSG821 RCT found a higher local recurrence rate after sublobar resection (8,31). However, assessment of this outcome is impacted by multiple factors (e.g., competing causes of death, length of follow-up, staging accuracy, tumor biology). The cleanest measure is FFR (or cumulative-incidence-of-recurrence). Recurrence-free or disease-free survival (RFS/DFS) is muddy because it mingles recurrence with competing causes of death. Simple comparison of the number (or type) of observed recurrences in cohorts is frequently reported but hard to interpret (no accounting for confounding factors or follow-up duration).
Few adjusted NRCs report recurrence by resection extent (Table 4) (39,43,45,46,53,57,60,64,76-80). The available evidence is unclear whether lesser resection increases recurrence risk. The confidence that confounders are accounted for is low. Variability in the incidence of recurrence is only partially potentially explained by tumor stage or follow-up duration. Most studies found a non-significant trend towards a higher recurrence rate after sublobar resection, rarely the opposite trend. Rates of locoregional recurrence are generally low (the outcome most likely affected by resection extent).
Table 4
Ordered by resection extent, degree of confidence that results reflect the effect of the treatment, stage
Pulmonary function tests (PFTs)
The impact of resection on PFTs serves as a surrogate for functional capacity (which hasn’t been studied). Segmentectomy doesn’t confer a meaningful benefit over lobectomy in healthy patients; studies reporting FEV1 ≥6 months postoperatively are shown in Table 5 (changes in diffusion capacity are seldom reported) (8,29,35,51,81-96) (it takes ~6 months following surgery for PFTs to reach a plateau; less after VATS resection) (95,97-99).
Table 5
Ordered by single/multi-segmentectomy, VATS/open approach, years of accrual
Lobectomy causes a ~14% long-term decrease in FEV1. Segmentectomy results in an FEV1 decrease of ~12% in studies involving many multi-segment resections (e.g., left upper tri-segmentectomy) and a decrease of ~5% in studies involving primarily single segment resections. Such decreases are not in a clinically relevant range for healthy patients. Indeed, exercise capacity is reported unchanged despite the FEV1 decrease (83,91). Available data shows an FEV1 decrease of 2–8% after wedge resection (89,95,100,101). The long-term impact of resection on FEV1 does not correlate with the time period or the approach (VATS/open).
Long-term QOL
In Table 6 (102-117) and Table 7 (11,24,118-130) postoperative QOL results are depicted reflecting no change, or small, moderate or large changes vs. baseline by generally accepted thresholds for clinically meaningful differences (128,131-136). Table 6 is mostly yellow (i.e., no change); these studies used the SF-36 tool (why this tool appears less sensitive is unclear; little change remains when using lower proposed thresholds for clinically meaningful differences). In Table 6 and Table 7, there is diminishing QOL impairment towards the right (i.e., increasing interval from surgery) and increasing impairment moving downward. The vertical gradient reflects increased VATS near the top and more extensive resections (e.g., pneumonectomy) towards the bottom (also generally older studies).
Table 6
Ordered by treatment approach, extent of resection
Table 7
Ordered by treatment approach, extent of resection
What conclusions can be drawn? The SF-36 tool seems less useful. VATS is associated with less QOL impairment vs. baseline, and this has mostly resolved by 6 months (except dyspnea). Whether sublobar resection has an impact is less clear—studies are limited and confounded by the use of VATS. Open lobectomy is associated with long-term QOL decreases in many domains. Older studies tend to show larger and more frequent QOL impairment, but often include larger resections.
The average doesn’t necessarily reflect an individual’s experience. Another measure is the proportion of patients that have improved, unchanged or worse QOL after surgery. Six months after thoracotomy, one study reported that 30–50% of patients experience meaningfully worse QOL vs. baseline (SF-36 instrument, included 9% pneumonectomy) (137). In another study, long-term QOL after thoracotomy was meaningfully worse in ~10–40% and improved in a similar proportion in various domains of the EORTC C-30 instrument in patients without recurrence (129). These authors reported that long-term symptoms were absent or meaningfully improved in ~60% and worse in ~10–20%—with the exception of dyspnea which was worse in ~40% vs. baseline. A prospective study involving primarily minimally invasive resections found that 20–40% were meaningfully worse and a similar proportion improved at 6 and 12 months in multiple EORTC domains (120). No data is available whether these proportions are influenced by sublobar resection.
Various predictors of worse QOL have been noted, mostly in single studies and measures of physical functioning. Worse long-term QOL has been associated with age (137) smoking (138), adjuvant chemotherapy (137), recurrence (129), higher baseline QOL (139), thoracotomy (vs. VATS) (111) and larger resection (i.e., pneumonectomy or lower ppoFEV1) (137,139). One study noted a non-significant trend to less impact on QOL with sublobar resection vs. lobectomy (137); another found physical QOL at ~11 months was unchanged after limited resection but decreased after lobectomy (likely confounded by use of VATS) (108,111). Conversely, variables that don’t correlate with QOL changes include gender (112,140), comorbidities, occurrence of postoperative complications, and stage (137). A case-matched study found no association between the presence of COPD and postoperative QOL (114).
Two recent small RCTs deserve mention. A RCT of lobectomy (VATS vs. open) found a transient QOL impairment with return to baseline or higher; the return was faster after VATS (6 vs. 12 weeks) (24). A small RCT of segmentectomy vs. lobectomy found that global QOL returned to baseline by 3 months in both arms (11). Interpretation is difficult, however, because of the study size (n=108) and higher VATS use in the lobectomy arm (11).
Chronic pain
The incidence of chronic pain is reported variably. The impact of sublobar resection is unclear, confounded by VATS use. No differences were found in one study of 220 patients undergoing either VATS lobectomy, segmentectomy via mini-thoracotomy, or segmentectomy via thoracotomy with rib-spreading [2012–5]. At 1 month ~25% in each group were taking analgesics (of any kind), and by 3 months it was ≤5% (28). Moderate to severe pain persisted in 5–10% of patients at 1 year in a RCT of VATS vs. open lobectomy but was approximately half as frequent after VATS (24). In Table 6 and Table 7, pain at ≥6 months postoperatively is noted frequently after thoracotomy but infrequently after VATS.
Several studies addressing chronic pain report pain ≥1 year postoperatively in 30–60% of patients after thoracotomy (141-144) and 20–25% after VATS (141,144). The incidence of taking analgesics is much less (5% after VATS and 20% after thoracotomy) (141,142). Chronic pain has been associated with preoperative narcotic use, the intensity of early postoperative pain and intercostal nerve trauma (145).
The discrepancy between studies investigating QOL and chronic pain is probably due to semantic differences. An earlier review of chronic post-thoracotomy pain found that 50% had some discomfort/pain, ~10% used occasional narcotics, and <5% required more involved treatment (146). Taking this and the more recent studies on QOL and pain together, it appears these rates are still seen after thoracotomy, but approximately half as frequent after VATS.
Nuances and sources of ambiguity
Impact of resection margin
Guidelines recommend a resection margin of ≥2 cm (from tumor edge to cut lung parenchyma) or a margin to tumor size (M/T) ratio of ≥1) (147,148). Clinical practice, however, requires quantification of the risk of a narrow margin so it can be weighed against issues associated with additional resection. The ideal measure is actuarial locoregional recurrence (survival is muddied by unrelated deaths).
Variability in studies of margin distance and M/T ratio (Tables 8,9) (53,149-164) likely reflects multiple factors—e.g., adjustment for confounders, proportion of unfit patients or favorable tumors, follow-up duration, resection extent (average margin 15 mm for segment vs. 8 mm for wedge in a prospective study) (165). The data loosely suggest an inflection point around 1 cm, with ~25% recurrence with <1 cm margins. Why Maurizi et al. found no difference is unclear (150). The data regarding M/T ratio loosely suggests a locoregional recurrence rate of ~20% for M/T <1 vs. ~10% for ≥1. Margin distance appears to have little impact in primarily GG tumors (152,156).
Table 8
Ordered by outcome, proportion of low-risk tumors, stage
Table 9
Ordered by outcome, proportion of low-risk tumors
Most studies have reported whole tumor size. Those reporting invasive size suggest the M/T (invasive) ratio is important (53,162). The discrepancy between the surgeon’s and pathologist’s margin assessment is another issue (not quantitatively defined). The pathologist typically removes the staple line, and measures the deflated, fixed lung. Studies mostly report the pathologic margin. Surgeons should aim for a surgical margin well beyond a M/T ratio of 1.
In conclusion, for solid tumors evidence loosely suggests a local recurrence rate of ~20–25% for a M/T ratio <1 or a margin <1 cm vs. ~10% for larger margins (recognizing that the pathologic measurement is likely ~3–5 mm less than the surgical assessment).
Impact of STAS
The term “spread through air spaces” (STAS) refers to a microscopic observation of tumor cells adjacent to a lung cancer; the median distance is 1–1.5 mm, but distances of 8–10 mm have been observed (166-169). STAS occurs in essentially all lung cancer types (adenocarcinoma, squamous, small cell, carcinoid, pleomorphic etc.) (169). The reported incidence is quite variable (15–80%) for each tumor type. STAS is rarely observed in adenocarcinoma in situ, minimally invasive adenocarcinoma or pure GG tumors (156,170-174) with some exceptions (29% STAS+ in pure GG, 34% among preinvasive tumors in one study) (175).
STAS is widely associated with worse long-term outcomes (169,176)—but also associated with multiple negative prognostic factors, e.g., aggressive adenocarcinoma subtypes (e.g., solid, micropapillary) (166,167,174,177-181), higher stage (174,175,180,182,183), larger tumors (169,174,175,180-183), and a greater solid component on imaging (172,175,181). No consistent correlation of STAS with genetic characteristics has emerged (169).
In most studies STAS portends worse RFS and higher recurrence rates after sublobar resection (Tables 10,11) (156,166-168,170,173,174,178,181-186). This is generally maintained after multivariable adjustment (only limited confounders accounted for). There is less data after lobectomy—STAS portends worse RFS but this is generally not maintained after multivariable adjustment. STAS is associated with a higher distant recurrence rate after sublobar resection in some studies (181,184) but not in others (170,174,186). A greater proportion of favorable tumors doesn’t mitigate the negative prognostic impact of STAS.
Table 10
Ordered by outcome and estimated proportion of favorable tumors
A simplistic assumption is that STAS represents a mechanism by which metastasis occurs. This creates a focus on intraoperative detection (frozen-section sensitivity), resection extent and defining a safe margin. However, decades of evidence demonstrate that metastasis is determined by complex cellular transformations, signaling and host-tumor interactions (187-189). STAS may reflect microenvironment evidence of these processes. In other cancers microenvironment evidence of immune recognition of cancer cells and activation of tumor-host interaction predicts long-term outcomes (190). This mental construct suggests that surgical interventions would not affect the impact of STAS.
The available data is inconclusive whether a negative prognostic impact of STAS can be altered by a more extensive resection. Few studies have addressed this with conflicting results (Table 11) (53,178,185). In an extensively adjusted retrospective analysis Eguchi et al. found that if STAS is present, lobectomy is associated with better RFS and fewer recurrences than sublobar resection (53). Eguchi et al. also observed that recurrences after sublobar resection in STAS + tumors were associated with an M/T ratio of <1 (this margin/STAS analysis was unadjusted for any confounders) (53). The observation invited speculation that a wider margin might mitigate the negative prognostic impact of STAS. Another unadjusted analysis of sublobar resection found that STAS was associated with a similar increase in loco-regional recurrence for M/T ≥1 as for M/T <1 (174).
Table 11
Ordered by outcome and estimated proportion of favorable tumors
Single vs. multi-segmentectomy
A right upper lobectomy is arguably the same as a left upper tri-segmentectomy, and a right middle lobectomy the same as lingulectomy. In database studies the proportion of such “lobe-like” segmentectomies is unavailable. In single-institution series, the proportion is 20–40% (43,46,51,191,192), and 30–55% of segmentectomies involve ≥3 segments (43,46,51,191,192). Studies involving many multi-segmentectomies found no OS or LCSS difference between segmentectomy vs. lobectomy (43,46,51).
Anatomic location
Whether the tumor size and anatomic location confidently permit an adequate margin is important in deciding the resection extent in an individual patient. Wedge resection is only feasible for tumors in the outer third of the lung (from the pleural space to the hilum). Achieving an adequate margin is difficult even for segmentectomy when tumors are central or near an intersegmental boundary. A simulation model estimated that ~25–33% of 1–2 cm tumors would be amenable to segmentectomy (defined as ≥2 cm from an intersegmental plane); for bi-segmentectomy ~50% would meet this criterion (assuming uniform tumor distribution throughout the lungs) (193).
Summary of outcomes in healthy patients
In healthy patients contemporary RCTs demonstrate equivalent perioperative mortality for segmentectomy or wedge vs. lobectomy (1–4% 90-day mortality). The incidence of major complications is also low (5–15% grade ≥3) and not improved by sublobar resection. A significant benefit to VATS over thoracotomy has been demonstrated extensively for lobectomy; this also appears true for segmentectomy. Pain and impaired QOL is generally resolved by 3 months after VATS resection.
Adjusted NRCs with high confidence that results reflect the treatment demonstrate worse OS for segmentectomy or wedge resection than lobectomy. Multiple additional NRCs with greater residual confounding mostly favor lobectomy; statistical significance is fairly consistent for OS and LCSS for wedge but less so for segmentectomy vs. lobectomy. While we await mature results from RCTs, the aggregate evidence indicates meaningfully worse long-term outcomes after segmentectomy or wedge resection than lobectomy in healthy patients with cI NSCLC.
VATS resection has little long-term impact on QOL, but open resection results in persistently worse QOL. A QOL benefit to sublobar resection is unclear due to confounding by VATS/open approach. Sublobar resection may attenuate an increase in dyspnea that is commonly noted after lobectomy. However, PFTs demonstrate no meaningful advantage for segmentectomy over lobectomy in healthy patients, particularly when including multi-segmentectomies.
Evidence suggests no meaningful difference in short-, intermediate- or long-term outcomes for a “lobe-like” multi-segmentectomy vs. lobectomy. The risk of an inadequate margin given an individual tumor’s anatomic location is an important consideration. Locoregional recurrence rates of ~20–25% for margins of <1 cm or a margin/tumor ratio of <1 are half as frequent with larger margins for solid tumors; margin appears to have less impact in primarily GG tumors. Worse long-term outcomes are reported when STAS is present (especially after sublobar resection); this is confounded because STAS is associated with many negative prognostic factors. It is unclear whether the impact of STAS can be mitigated by converting to a lobectomy.
Short-term and long-term outcomes for segmentectomy or wedge resection vs. lobectomy are summarized in Table S2-2. A benefit or detriment is qualitatively depicted relative to clinically meaningful differences, together with the confidence in and consistency of the evidence. This provides a succinct summary that can inform judgment for individual patients, as discussed in the Part 1 paper (1).
Conclusions
Choosing which type of resection is best for a particular patient demands balancing various factors and outcomes. This analysis of the relevant evidence in generally healthy patients provides a foundation for a framework to facilitate individualized decision-making across the spectrum of lung cancer patients.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Journal of Thoracic Disease for the series “A Guide for Managing Patients with Stage I NSCLC: Deciding between Lobectomy, Segmentectomy, Wedge, SBRT and Ablation”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1824/coif). The series “A Guide for Managing Patients with Stage I NSCLC: Deciding between Lobectomy, Segmentectomy, Wedge, SBRT and Ablation” was commissioned by the editorial office without any funding or sponsorship. FCD served as the unpaid Guest Editor of the series. HSP serves as an unpaid editorial board member of Journal of Thoracic Disease. HSP reports research funding from RefleXion Medical; consulting fees from AstraZeneca; honoraria and speaking fees from Bristol Myers Squibb; and advisory board fees from Galera Therapeutics; all unrelated to current work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Detterbeck FC, Blasberg JD, Woodard GA, et al. A guide for managing patients with stage I NSCLC: deciding between lobectomy, segmentectomy, wedge, SBRT and ablation—part 1: a guide to decision-making. J Thorac Dis 2022; [Crossref]
- Bade BC, Blasberg JD, Mase VJ Jr, et al. A guide for managing patients with stage I NSCLC: deciding between lobectomy, segmentectomy, wedge, SBRT and ablation—part 3: systematic review of evidence regarding surgery in compromised patients or specific tumors. J Thorac Dis 2022; [Crossref]
- Park HS, Detterbeck FC, Madoff DC, et al. A guide for managing patients with stage I NSCLC: deciding between lobectomy, segmentectomy, wedge, SBRT and ablation—part 4: systematic review of evidence involving SBRT and ablation. J Thorac Dis 2022; [Crossref]
- Elze MC, Gregson J, Baber U, et al. Comparison of Propensity Score Methods and Covariate Adjustment: Evaluation in 4 Cardiovascular Studies. J Am Coll Cardiol 2017;69:345-57. [Crossref] [PubMed]
- Hade EM, Lu B. Bias associated with using the estimated propensity score as a regression covariate. Stat Med 2014;33:74-87. [Crossref] [PubMed]
- Benedetto U, Head SJ, Angelini GD, et al. Statistical primer: propensity score matching and its alternatives. Eur J Cardiothorac Surg 2018;53:1112-7. [Crossref] [PubMed]
- Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919. [Crossref] [PubMed]
- Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615-22; discussion 622-3. [Crossref] [PubMed]
- Altorki NK, Wang X, Wigle D, et al. Perioperative mortality and morbidity after sublobar versus lobar resection for early-stage non-small-cell lung cancer: post-hoc analysis of an international, randomised, phase 3 trial (CALGB/Alliance 140503). Lancet Respir Med 2018;6:915-24. [Crossref] [PubMed]
- Suzuki K, Saji H, Aokage K, et al. Comparison of pulmonary segmentectomy and lobectomy: Safety results of a randomized trial. J Thorac Cardiovasc Surg 2019;158:895-907. [Crossref] [PubMed]
- Stamatis G, Leschber G, Schwarz B, et al. Perioperative course and quality of life in a prospective randomized multicenter phase III trial, comparing standard lobectomy versus anatomical segmentectomy in patients with non-small cell lung cancer up to 2 cm, stage IA (7th edition of TNM staging system). Lung Cancer 2019;138:19-26.
- Linden PA, D'Amico TA, Perry Y, et al. Quantifying the safety benefits of wedge resection: a society of thoracic surgery database propensity-matched analysis. Ann Thorac Surg 2014;98:1705-11; discussion 1711-2. [Crossref] [PubMed]
- Stokes WA, Bronsert MR, Meguid RA, et al. Post-Treatment Mortality After Surgery and Stereotactic Body Radiotherapy for Early-Stage Non-Small-Cell Lung Cancer. J Clin Oncol 2018;36:642-51. [Crossref] [PubMed]
- Zhang Z, Feng H, Zhao H, et al. Sublobar resection is associated with better perioperative outcomes in elderly patients with clinical stage I non-small cell lung cancer: a multicenter retrospective cohort study. J Thorac Dis 2019;11:1838-48. [Crossref] [PubMed]
- Bédat B, Abdelnour-Berchtold E, Perneger T, et al. Comparison of postoperative complications between segmentectomy and lobectomy by video-assisted thoracic surgery: a multicenter study. J Cardiothorac Surg 2019;14:189. [Crossref] [PubMed]
- Khullar OV, Liu Y, Gillespie T, et al. Survival After Sublobar Resection versus Lobectomy for Clinical Stage IA Lung Cancer: An Analysis from the National Cancer Data Base. J Thorac Oncol 2015;10:1625-33. [Crossref] [PubMed]
- Tsutani Y, Tsubokawa N, Ito M, et al. Postoperative complications and prognosis after lobar resection versus sublobar resection in elderly patients with clinical Stage I non-small-cell lung cancer. Eur J Cardiothorac Surg 2018;53:366-71. [Crossref] [PubMed]
- Husain ZA, Kim AW, Yu JB, et al. Defining the High-Risk Population for Mortality After Resection of Early Stage NSCLC. Clin Lung Cancer 2015;16:e183-7. [Crossref] [PubMed]
- Shirvani SM, Jiang J, Chang JY, et al. Lobectomy, sublobar resection, and stereotactic ablative radiotherapy for early-stage non-small cell lung cancers in the elderly. JAMA Surg 2014;149:1244-53. [Crossref] [PubMed]
- Okami J, Shintani Y, Okumura M, et al. Demographics, Safety and Quality, and Prognostic Information in Both the Seventh and Eighth Editions of the TNM Classification in 18,973 Surgical Cases of the Japanese Joint Committee of Lung Cancer Registry Database in 2010. J Thorac Oncol 2019;14:212-22.
- Thai AA, Stuart E, Te Marvelde L, et al. Hospital lung surgery volume and patient outcomes. Lung Cancer 2019;129:22-7. [Crossref] [PubMed]
- Fagundes CP, Shi Q, Vaporciyan AA, et al. Symptom recovery after thoracic surgery: Measuring patient-reported outcomes with the MD Anderson Symptom Inventory. J Thorac Cardiovasc Surg 2015;150:613-9.e2. [Crossref] [PubMed]
- Detterbeck F, Antonicelli A, Okada M. Results of Video-Assisted Techniques for Resection of Lung Cancer. In: Pass H, Ball D, Scagliotti G. editors. Thoracic Oncology: The IASLC Multidisciplinary Approach (2nd Edition): IASLC, 2018.
- Bendixen M, Jørgensen OD, Kronborg C, et al. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: a randomised controlled trial. Lancet Oncol 2016;17:836-44. [Crossref] [PubMed]
- Smith CB, Kale M, Mhango G, et al. Comparative outcomes of elderly stage I lung cancer patients treated with segmentectomy via video-assisted thoracoscopic surgery versus open resection. J Thorac Oncol 2014;9:383-9. [Crossref] [PubMed]
- Ghaly G, Kamel M, Nasar A, et al. Video-Assisted Thoracoscopic Surgery Is a Safe and Effective Alternative to Thoracotomy for Anatomical Segmentectomy in Patients With Clinical Stage I Non-Small Cell Lung Cancer. Ann Thorac Surg 2016;101:465-72; discussion 472. [Crossref] [PubMed]
- Schuchert MJ, Pettiford BL, Pennathur A, et al. Anatomic segmentectomy for stage I non-small-cell lung cancer: comparison of video-assisted thoracic surgery versus open approach. J Thorac Cardiovasc Surg 2009;138:1318-25.e1. [Crossref] [PubMed]
- Nomori H, Cong Y, Sugimura H. Limited thoracotomy for segmentectomy: a comparison of postoperative pain with thoracoscopic lobectomy. Surg Today 2016;46:1243-8. [Crossref] [PubMed]
- Handa Y, Tsutani Y, Mimae T, et al. Surgical Outcomes of Complex Versus Simple Segmentectomy for Stage I Non-Small Cell Lung Cancer. Ann Thorac Surg 2019;107:1032-9. [Crossref] [PubMed]
- Detterbeck FC. Lobectomy versus limited resection in T1N0 lung cancer. Ann Thorac Surg 2013;96:742-4. [Crossref] [PubMed]
- Lederle FA. Lobectomy versus limited resection in T1 N0 lung cancer. Ann Thorac Surg 1996;62:1249-50. [Crossref] [PubMed]
- Yang F, Sui X, Chen X, et al. Sublobar resection versus lobectomy in Surgical Treatment of Elderly Patients with early-stage non-small cell lung cancer (STEPS): study protocol for a randomized controlled trial. Trials 2016;17:191. [Crossref] [PubMed]
- Tanaka K, Tsutani Y, Wakabayashi M, et al. Sublobar resection versus lobectomy for patients with resectable stage I non-small cell lung cancer with idiopathic pulmonary fibrosis: a phase III study evaluating survival (JCOG1708, SURPRISE). Jpn J Clin Oncol 2020;50:1076-9. [Crossref] [PubMed]
- Asamura H, Okada M, Saji H, et al. Randomized Trial of Segmentectomy Compared to Lobectomy in Small-Sized Peripheral Non-Small Cell Lung Cancer. AATS Virtual 2021.
- Saji H, Okada M, Tsuboi M, et al. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): a multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet 2022;399:1607-17. [Crossref] [PubMed]
- Cao J, Yuan P, Wang Y, et al. Survival Rates After Lobectomy, Segmentectomy, and Wedge Resection for Non-Small Cell Lung Cancer. Ann Thorac Surg 2018;105:1483-91. [Crossref] [PubMed]
- Onaitis MW, Furnary AP, Kosinski AS, et al. Equivalent Survival Between Lobectomy and Segmentectomy for Clinical Stage IA Lung Cancer. Ann Thorac Surg 2020;110:1882-91. [Crossref] [PubMed]
- Li F, Zhao Y, Yuan L, et al. Oncologic outcomes of segmentectomy vs lobectomy in pathologic stage IA (≤2 cm) invasive lung adenocarcinoma: A population-based study. J Surg Oncol 2020;121:1132-9. [Crossref] [PubMed]
- Koike T, Kitahara A, Sato S, et al. Lobectomy Versus Segmentectomy in Radiologically Pure Solid Small-Sized Non-Small Cell Lung Cancer. Ann Thorac Surg 2016;101:1354-60. [Crossref] [PubMed]
- Zhao ZR, Situ DR, Lau RWH, et al. Comparison of Segmentectomy and Lobectomy in Stage IA Adenocarcinomas. J Thorac Oncol 2017;12:890-6. [Crossref] [PubMed]
- Moon MH, Moon YK, Moon SW. Segmentectomy versus lobectomy in early non-small cell lung cancer of 2 cm or less in size: A population-based study. Respirology 2018;23:695-703. [Crossref] [PubMed]
- Yendamuri S, Sharma R, Demmy M, et al. Temporal trends in outcomes following sublobar and lobar resections for small (≤ 2 cm) non-small cell lung cancers--a Surveillance Epidemiology End Results database analysis. J Surg Res 2013;183:27-32. [Crossref] [PubMed]
- Yamashita S, Tokuishi K, Anami K, et al. Thoracoscopic segmentectomy for T1 classification of non-small cell lung cancer: a single center experience. Eur J Cardiothorac Surg 2012;42:83-8. [Crossref] [PubMed]
- Qu X, Wang K, Zhang T, et al. Long-term outcomes of stage I NSCLC (≤3 cm) patients following segmentectomy are equivalent to lobectomy under analogous extent of lymph node removal: a PSM based analysis. J Thorac Dis 2017;9:4561-73. [Crossref] [PubMed]
- Chan EG, Chan PG, Mazur SN, et al. Outcomes with segmentectomy versus lobectomy in patients with clinical T1cN0M0 non-small cell lung cancer. J Thorac Cardiovasc Surg 2021;161:1639-1648.e2. [Crossref] [PubMed]
- Landreneau RJ, Normolle DP, Christie NA, et al. Recurrence and survival outcomes after anatomic segmentectomy versus lobectomy for clinical stage I non-small-cell lung cancer: a propensity-matched analysis. J Clin Oncol 2014;32:2449-55. [Crossref] [PubMed]
- Fan X, Liang Y, Bai Y, et al. Conditional survival rate estimates of lobectomy, segmentectomy and wedge resection for stage IA1 non-small cell lung cancer: A population-based study. Oncol Lett 2020;20:1607-18. [Crossref] [PubMed]
- Dai C, Shen J, Ren Y, et al. Choice of Surgical Procedure for Patients With Non-Small-Cell Lung Cancer ≤ 1 cm or > 1 to 2 cm Among Lobectomy, Segmentectomy, and Wedge Resection: A Population-Based Study. J Clin Oncol 2016;34:3175-82. [Crossref] [PubMed]
- Whitson BA, Groth SS, Andrade RS, et al. Survival after lobectomy versus segmentectomy for stage I non-small cell lung cancer: a population-based analysis. Ann Thorac Surg 2011;92:1943-50. [Crossref] [PubMed]
- Dziedzic R, Zurek W, Marjanski T, et al. Stage I non-small-cell lung cancer: long-term results of lobectomy versus sublobar resection from the Polish National Lung Cancer Registry. Eur J Cardiothorac Surg 2017;52:363-9. [Crossref] [PubMed]
- Hwang Y, Kang CH, Kim HS, et al. Comparison of thoracoscopic segmentectomy and thoracoscopic lobectomy on the patients with non-small cell lung cancer: a propensity score matching study. Eur J Cardiothorac Surg 2015;48:273-8. [Crossref] [PubMed]
- Razi SS, Nguyen D, Villamizar N. Lobectomy does not confer survival advantage over segmentectomy for non-small cell lung cancer with unsuspected nodal disease. J Thorac Cardiovasc Surg 2020;159:2469-2483.e4. [Crossref] [PubMed]
- Eguchi T, Kameda K, Lu S, et al. Lobectomy Is Associated with Better Outcomes than Sublobar Resection in Spread through Air Spaces (STAS)-Positive T1 Lung Adenocarcinoma: A Propensity Score-Matched Analysis. J Thorac Oncol 2019;14:87-98. [Crossref] [PubMed]
- Yu Y, Huang R, Wang P, et al. Sublobectomy versus lobectomy for long-term survival outcomes of early-stage non-small cell lung cancer with a tumor size ≤2 cm accompanied by visceral pleural invasion: a SEER population-based study. J Thorac Dis 2020;12:592-604. [Crossref] [PubMed]
- Eguchi T, Bains S, Lee MC, et al. Impact of Increasing Age on Cause-Specific Mortality and Morbidity in Patients With Stage I Non-Small-Cell Lung Cancer: A Competing Risks Analysis. J Clin Oncol 2017;35:281-90. [Crossref] [PubMed]
- Liang Y, Fan X, Bai Y, et al. Conditional survival analysis of four treatment strategies for patients with stage I non-small cell lung cancer. Oncol Lett 2019;18:1089-98. [Crossref] [PubMed]
- Dolan DP, White A, Mazzola E, et al. Outcomes of superior segmentectomy versus lower lobectomy for superior segment Stage I non-small-cell lung cancer are equivalent: An analysis of 196 patients at a single, high volume institution. J Surg Oncol 2021;123:570-8. [Crossref] [PubMed]
- Boyer MJ, Williams CD, Harpole DH, et al. Improved Survival of Stage I Non-Small Cell Lung Cancer: A VA Central Cancer Registry Analysis. J Thorac Oncol 2017;12:1814-23. [Crossref] [PubMed]
- Speicher PJ, Gu L, Gulack BC, et al. Sublobar Resection for Clinical Stage IA Non-small-cell Lung Cancer in the United States. Clin Lung Cancer 2016;17:47-55. [Crossref] [PubMed]
- Subramanian M, McMurry T, Meyers BF, et al. Long-Term Results for Clinical Stage IA Lung Cancer: Comparing Lobectomy and Sublobar Resection. Ann Thorac Surg 2018;106:375-81. [Crossref] [PubMed]
- Cox ML, Yang CJ, Speicher PJ, et al. The Role of Extent of Surgical Resection and Lymph Node Assessment for Clinical Stage I Pulmonary Lepidic Adenocarcinoma: An Analysis of 1991 Patients. J Thorac Oncol 2017;12:689-96. [Crossref] [PubMed]
- Nakamura H, Taniguchi Y, Miwa K, et al. Comparison of the surgical outcomes of thoracoscopic lobectomy, segmentectomy, and wedge resection for clinical stage I non-small cell lung cancer. Thorac Cardiovasc Surg 2011;59:137-41. [Crossref] [PubMed]
- Smith CB, Swanson SJ, Mhango G, et al. Survival after segmentectomy and wedge resection in stage I non-small-cell lung cancer. J Thorac Oncol 2013;8:73-8. [Crossref] [PubMed]
- Koike T, Koike T, Yoshiya K, et al. Risk factor analysis of locoregional recurrence after sublobar resection in patients with clinical stage IA non-small cell lung cancer. J Thorac Cardiovasc Surg 2013;146:372-8. [Crossref] [PubMed]
- Zhang Y, Sun Y, Chen H. A propensity score matching analysis of survival following segmentectomy or wedge resection in early-stage lung invasive adenocarcinoma or squamous cell carcinoma. Oncotarget 2016;7:13880-5. [Crossref] [PubMed]
- Zhao M, Lu T, Huang Y, et al. Survival and Long-Term Cause-Specific Mortality Associated With Stage IA Lung Adenocarcinoma After Wedge Resection vs. Segmentectomy: A Population-Based Propensity Score Matching and Competing Risk Analysis. Front Oncol 2019;9:593. [Crossref] [PubMed]
- Stiles BM, Mao J, Harrison S, et al. Extent of lymphadenectomy is associated with oncological efficacy of sublobar resection for lung cancer ≤2 cm. J Thorac Cardiovasc Surg 2019;157:2454-2465.e1. [Crossref] [PubMed]
- Zhang B, Liu R, Ren D, et al. Comparison of Lobectomy and Sublobar Resection for Stage IA Elderly NSCLC Patients (≥70 Years): A Population-Based Propensity Score Matching's Study. Front Oncol 2021;11:610638. [Crossref] [PubMed]
- Cao C, Gupta S, Chandrakumar D, et al. Meta-analysis of intentional sublobar resections versus lobectomy for early stage non-small cell lung cancer. Ann Cardiothorac Surg 2014;3:134-41. [PubMed]
- Cao C, Chandrakumar D, Gupta S, et al. Could less be more?-A systematic review and meta-analysis of sublobar resections versus lobectomy for non-small cell lung cancer according to patient selection. Lung Cancer 2015;89:121-32. [Crossref] [PubMed]
- Bao F, Ye P, Yang Y, et al. Segmentectomy or lobectomy for early stage lung cancer: a meta-analysis. Eur J Cardiothorac Surg 2014;46:1-7. [Crossref] [PubMed]
- Bedetti B, Bertolaccini L, Rocco R, et al. Segmentectomy versus lobectomy for stage I non-small cell lung cancer: a systematic review and meta-analysis. J Thorac Dis 2017;9:1615-23. [Crossref] [PubMed]
- Guo J, Liu Y, Tian X, et al. Less is more in solid-dominant lung cancer? Sublobar resection versus lobectomy for solid-dominant stage IA non-small-cell lung cancer: A meta-analysis study. Mol Clin Oncol 2019;11:465-73. [Crossref] [PubMed]
- Hennon M, Landreneau RJ. Role of Segmentectomy in Treatment of Early-Stage Non-Small Cell Lung Cancer. Ann Surg Oncol 2018;25:59-63. [Crossref] [PubMed]
- Rao S, Ye L, Min L, et al. Meta-analysis of segmentectomy versus lobectomy for radiologically pure solid or solid-dominant stage IA non-small cell lung cancer. J Cardiothorac Surg 2019;14:197. [Crossref] [PubMed]
- Huang CS, Hsu PK, Chen CK, et al. Surgeons' preference sublobar resection for stage I NSCLC less than 3 cm. Thorac Cancer 2020;11:907-17. [Crossref] [PubMed]
- Kamigaichi A, Tsutani Y, Mimae T, et al. Prognosis of segmentectomy and lobectomy for radiologically aggressive small-sized lung cancer. Eur J Cardiothorac Surg 2020;58:1245-53. [Crossref] [PubMed]
- El-Sherif A, Gooding WE, Santos R, et al. Outcomes of sublobar resection versus lobectomy for stage I non-small cell lung cancer: a 13-year analysis. Ann Thorac Surg 2006;82:408-15; discussion 415-6. [Crossref] [PubMed]
- Tsutani Y, Handa Y, Shimada Y, et al. Comparison of cancer control between segmentectomy and wedge resection in patients with clinical stage IA non-small cell lung cancer. J Thorac Cardiovasc Surg 2021;162:1244-1252.e1. [Crossref] [PubMed]
- Altorki NK, Kamel MK, Narula N, et al. Anatomical Segmentectomy and Wedge Resections Are Associated with Comparable Outcomes for Patients with Small cT1N0 Non-Small Cell Lung Cancer. J Thorac Oncol 2016;11:1984-92. [Crossref] [PubMed]
- Yoshikawa K, Tsubota N, Kodama K, et al. Prospective study of extended segmentectomy for small lung tumors: the final report. Ann Thorac Surg 2002;73:1055-8; discussion 1058-9. [Crossref] [PubMed]
- Takizawa T, Haga M, Yagi N, et al. Pulmonary function after segmentectomy for small peripheral carcinoma of the lung. J Thorac Cardiovasc Surg 1999;118:536-41. [Crossref] [PubMed]
- Harada H, Okada M, Sakamoto T, et al. Functional advantage after radical segmentectomy versus lobectomy for lung cancer. Ann Thorac Surg 2005;80:2041-5. [Crossref] [PubMed]
- Kashiwabara K, Sasaki J, Mori T, et al. Relationship between functional preservation after segmentectomy and volume-reduction effects after lobectomy in stage I non-small cell lung cancer patients with emphysema. J Thorac Oncol 2009;4:1111-6. [Crossref] [PubMed]
- Yoshimoto K, Nomori H, Mori T, et al. Quantification of the impact of segmentectomy on pulmonary function by perfusion single-photon-emission computed tomography and multidetector computed tomography. J Thorac Cardiovasc Surg 2009;137:1200-5. [Crossref] [PubMed]
- Saito H, Nakagawa T, Ito M, et al. Pulmonary function after lobectomy versus segmentectomy in patients with stage I non-small cell lung cancer. World J Surg 2014;38:2025-31. [Crossref] [PubMed]
- Nomori H, Cong Y, Sugimura H. Systemic and regional pulmonary function after segmentectomy. J Thorac Cardiovasc Surg 2016;152:747-53. [Crossref] [PubMed]
- Suzuki H, Morimoto J, Mizobuchi T, et al. Does segmentectomy really preserve the pulmonary function better than lobectomy for patients with early-stage lung cancer? Surg Today 2017;47:463-9. [Crossref] [PubMed]
- Gu Z, Wang H, Mao T, et al. Pulmonary function changes after different extent of pulmonary resection under video-assisted thoracic surgery. J Thorac Dis 2018;10:2331-7. [Crossref] [PubMed]
- Tane S, Nishio W, Fujibayashi Y, et al. Thoracoscopic left S1 + 2 segmentectomy as a good resolution for preserving pulmonary function. Interact Cardiovasc Thorac Surg 2020;31:331-8. [Crossref] [PubMed]
- Keenan RJ, Landreneau RJ, Maley RH Jr, et al. Segmental resection spares pulmonary function in patients with stage I lung cancer. Ann Thorac Surg 2004;78:228-33; discussion 228-33. [Crossref] [PubMed]
- Nomori H, Mori T, Ikeda K, et al. Segmentectomy for selected cT1N0M0 non-small cell lung cancer: a prospective study at a single institute. J Thorac Cardiovasc Surg 2012;144:87-93. [Crossref] [PubMed]
- Nomori H, Shiraishi A, Cong Y, et al. Differences in postoperative changes in pulmonary functions following segmentectomy compared with lobectomy. Eur J Cardiothorac Surg 2018;53:640-7. [Crossref] [PubMed]
- Macke RA, Schuchert MJ, Odell DD, et al. Parenchymal preserving anatomic resections result in less pulmonary function loss in patients with Stage I non-small cell lung cancer. J Cardiothorac Surg 2015;10:49. [Crossref] [PubMed]
- Kobayashi N, Kobayashi K, Kikuchi S, et al. Long-term pulmonary function after surgery for lung cancer. Interact Cardiovasc Thorac Surg 2017;24:727-32. [Crossref] [PubMed]
- Helminen O, Valo J, Andersen H, et al. Thoracoscopic segmentectomy with simple routine bronchoscopic inflation for intersegmental plane identification: short and mid-term outcomes compared with lobectomy. J Thorac Dis 2020;12:3073-84. [Crossref] [PubMed]
- Nagamatsu Y, Maeshiro K, Kimura NY, et al. Long-term recovery of exercise capacity and pulmonary function after lobectomy. J Thorac Cardiovasc Surg 2007;134:1273-8. [Crossref] [PubMed]
- Nakata M, Saeki H, Yokoyama N, et al. Pulmonary function after lobectomy: video-assisted thoracic surgery versus thoracotomy. Ann Thorac Surg 2000;70:938-41. [Crossref] [PubMed]
- Charloux A, Quoix E. Lung segmentectomy: does it offer a real functional benefit over lobectomy?. Eur Respir Rev 2017;26:170079. [Crossref] [PubMed]
- Kim SJ, Lee YJ, Park JS, et al. Changes in pulmonary function in lung cancer patients after video-assisted thoracic surgery. Ann Thorac Surg 2015;99:210-7. [Crossref] [PubMed]
- Suzuki K, Watanabe SI, Wakabayashi M, et al. A single-arm study of sublobar resection for ground-glass opacity dominant peripheral lung cancer. J Thorac Cardiovasc Surg 2022;163:289-301.e2. [Crossref] [PubMed]
- Février E, Yip R, Becker BJ, et al. Change in quality of life of stage IA lung cancer patients after sublobar resection and lobectomy. J Thorac Dis 2020;12:3488-99. [Crossref] [PubMed]
- Handy JR Jr, Asaph JW, Douville EC, et al. Does video-assisted thoracoscopic lobectomy for lung cancer provide improved functional outcomes compared with open lobectomy? Eur J Cardiothorac Surg 2010;37:451-5. [PubMed]
- Rizk NP, Ghanie A, Hsu M, et al. A prospective trial comparing pain and quality of life measures after anatomic lung resection using thoracoscopy or thoracotomy. Ann Thorac Surg 2014;98:1160-6. [Crossref] [PubMed]
- Khullar OV, Rajaei MH, Force SD, et al. Pilot Study to Integrate Patient Reported Outcomes After Lung Cancer Operations Into The Society of Thoracic Surgeons Database. Ann Thorac Surg 2017;104:245-53. [Crossref] [PubMed]
- Anami K, Horie J, Hirayama Y, et al. Changes in exercise tolerance and quality of life are unrelated in lung cancer survivors who undergo video-assisted thoracic surgery. J Phys Ther Sci 2018;30:467-73. [Crossref] [PubMed]
- Fernando HC, Landreneau RJ, Mandrekar SJ, et al. Analysis of longitudinal quality-of-life data in high-risk operable patients with lung cancer: results from the ACOSOG Z4032 (Alliance) multicenter randomized trial. J Thorac Cardiovasc Surg 2015;149:718-25; discussion 725-6. [Crossref] [PubMed]
- Schwartz RM, Yip R, Olkin I, et al. Impact of surgery for stage IA non-small-cell lung cancer on patient quality of life. J Community Support Oncol 2016;14:37-44. [Crossref] [PubMed]
- Schwartz RM, Alpert N, Rosenzweig K, et al. Changes in quality of life after surgery or radiotherapy in early-stage lung cancer. J Thorac Dis 2019;11:154-61. [Crossref] [PubMed]
- Sarna L, Cooley ME, Brown JK, et al. Women with lung cancer: quality of life after thoracotomy: a 6-month prospective study. Cancer Nurs 2010;33:85-92. [Crossref] [PubMed]
- Schwartz RM, Yip R, Flores RM, et al. The impact of resection method and patient factors on quality of life among stage IA non-small cell lung cancer surgical patients. J Surg Oncol 2017;115:173-80. [Crossref] [PubMed]
- Möller A, Sartipy U. Long-term health-related quality of life following surgery for lung cancer. Eur J Cardiothorac Surg 2012;41:362-7. [Crossref] [PubMed]
- Möller A, Sartipy U. Changes in quality of life after lung surgery in old and young patients: are they similar? World J Surg 2010;34:684-91. [Crossref] [PubMed]
- Pompili C, Brunelli A, Refai M, et al. Does chronic obstructive pulmonary disease affect postoperative quality of life in patients undergoing lobectomy for lung cancer? A case-matched study. Eur J Cardiothorac Surg 2010;37:525-30. [Crossref] [PubMed]
- Salati M, Brunelli A, Xiumè F, et al. Quality of life in the elderly after major lung resection for lung cancer. Interact Cardiovasc Thorac Surg 2009;8:79-83. [Crossref] [PubMed]
- Brunelli A, Socci L, Refai M, et al. Quality of life before and after major lung resection for lung cancer: a prospective follow-up analysis. Ann Thorac Surg 2007;84:410-6. [Crossref] [PubMed]
- Handy JR Jr, Asaph JW, Skokan L, et al. What happens to patients undergoing lung cancer surgery? Outcomes and quality of life before and after surgery. Chest 2002;122:21-30. [Crossref] [PubMed]
- Xu GW, Xie MR, Wu HR, et al. A prospective study examining the impact of uniportal video-assisted thoracic surgery on the short-term quality of life in patients with lung cancer. Thorac Cancer 2020;11:612-8. [Crossref] [PubMed]
- Pompili C, Koller M, Velikova G, et al. EORTC QLQ-C30 summary score reliably detects changes in QoL three months after anatomic lung resection for Non-Small Cell Lung Cancer (NSCLC). Lung Cancer 2018;123:149-54. [Crossref] [PubMed]
- Nugent SM, Golden SE, Hooker ER, et al. Longitudinal Health-related Quality of Life among Individuals Considering Treatment for Stage I Non-Small-Cell Lung Cancer. Ann Am Thorac Soc 2020;17:988-97. [Crossref] [PubMed]
- Avery KNL, Blazeby JM, Chalmers KA, et al. Impact on Health-Related Quality of Life of Video-Assisted Thoracoscopic Surgery for Lung Cancer. Ann Surg Oncol 2020;27:1259-71. [Crossref] [PubMed]
- Burfeind WR Jr, Tong BC, O'Branski E, et al. Quality of life outcomes are equivalent after lobectomy in the elderly. J Thorac Cardiovasc Surg 2008;136:597-604. [Crossref] [PubMed]
- Balduyck B, Hendriks J, Lauwers P, et al. Quality of life evolution after lung cancer surgery: a prospective study in 100 patients. Lung Cancer 2007;56:423-31. [Crossref] [PubMed]
- Alberts L, Wolff HB, Kastelijn EA, et al. Patient-reported Outcomes After the Treatment of Early Stage Non-small-cell Lung Cancer With Stereotactic Body Radiotherapy Compared With Surgery. Clin Lung Cancer 2019;20:370-377.e3. [Crossref] [PubMed]
- Balduyck B, Hendriks J, Lauwers P, et al. Quality of life evolution after lung cancer surgery in septuagenarians: a prospective study. Eur J Cardiothorac Surg 2009;35:1070-5; discussion 1075. [Crossref] [PubMed]
- Schulte T, Schniewind B, Dohrmann P, et al. The extent of lung parenchyma resection significantly impacts long-term quality of life in patients with non-small cell lung cancer. Chest 2009;135:322-9. [Crossref] [PubMed]
- Schulte T, Schniewind B, Walter J, et al. Age-related impairment of quality of life after lung resection for non-small cell lung cancer. Lung Cancer 2010;68:115-20. [Crossref] [PubMed]
- Ilonen IK, Räsänen JV, Knuuttila A, et al. Quality of life following lobectomy or bilobectomy for non-small cell lung cancer, a two-year prospective follow-up study. Lung Cancer 2010;70:347-51. [Crossref] [PubMed]
- Kenny PM, King MT, Viney RC, et al. Quality of life and survival in the 2 years after surgery for non small-cell lung cancer. J Clin Oncol 2008;26:233-41. [Crossref] [PubMed]
- Balduyck B, Hendriks J, Lauwers P, et al. Quality of life after lung cancer surgery: a prospective pilot study comparing bronchial sleeve lobectomy with pneumonectomy. J Thorac Oncol 2008;3:604-8. [Crossref] [PubMed]
- Wyrwich KW, Fihn SD, Tierney WM, et al. Clinically important changes in health-related quality of life for patients with chronic obstructive pulmonary disease: an expert consensus panel report. J Gen Intern Med 2003;18:196-202. [Crossref] [PubMed]
- Wyrwich KW, Metz SM, Kroenke K, et al. Measuring patient and clinician perspectives to evaluate change in health-related quality of life among patients with chronic obstructive pulmonary disease. J Gen Intern Med 2007;22:161-70. [Crossref] [PubMed]
- Maringwa JT, Quinten C, King M, et al. Minimal important differences for interpreting health-related quality of life scores from the EORTC QLQ-C30 in lung cancer patients participating in randomized controlled trials. Support Care Cancer 2011;19:1753-60. [Crossref] [PubMed]
- Yost KJ, Eton DT, Garcia SF, et al. Minimally important differences were estimated for six Patient-Reported Outcomes Measurement Information System-Cancer scales in advanced-stage cancer patients. J Clin Epidemiol 2011;64:507-16. [Crossref] [PubMed]
- Osoba D, Rodrigues G, Myles J, et al. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol 1998;16:139-44. [Crossref] [PubMed]
- Sloan JA. Assessing the minimally clinically significant difference: scientific considerations, challenges and solutions. COPD 2005;2:57-62. [Crossref] [PubMed]
- Möller A, Sartipy U. Predictors of postoperative quality of life after surgery for lung cancer. J Thorac Oncol 2012;7:406-11. [Crossref] [PubMed]
- Balduyck B, Sardari Nia P, Cogen A, et al. The effect of smoking cessation on quality of life after lung cancer surgery. Eur J Cardiothorac Surg 2011;40:1432-7; discussion 1437-8. [Crossref] [PubMed]
- Pompili C, Brunelli A, Xiumé F, et al. Predictors of postoperative decline in quality of life after major lung resections. Eur J Cardiothorac Surg 2011;39:732-7. [Crossref] [PubMed]
- Sartipy U. Influence of gender on quality of life after lung surgery. Eur J Cardiothorac Surg 2010;37:802-6. [Crossref] [PubMed]
- Landreneau RJ, Mack MJ, Hazelrigg SR, et al. Prevalence of chronic pain after pulmonary resection by thoracotomy or video-assisted thoracic surgery. J Thorac Cardiovasc Surg 1994;107:1079-85; discussion 1085-6. [Crossref] [PubMed]
- Maguire MF, Ravenscroft A, Beggs D, et al. A questionnaire study investigating the prevalence of the neuropathic component of chronic pain after thoracic surgery. Eur J Cardiothorac Surg 2006;29:800-5. [Crossref] [PubMed]
- Bayman EO, Brennan TJ. Incidence and severity of chronic pain at 3 and 6 months after thoracotomy: meta-analysis. J Pain 2014;15:887-97. [Crossref] [PubMed]
- Kwon ST, Zhao L, Reddy RM, et al. Evaluation of acute and chronic pain outcomes after robotic, video-assisted thoracoscopic surgery, or open anatomic pulmonary resection. J Thorac Cardiovasc Surg 2017;154:652-659.e1. [Crossref] [PubMed]
- Brown LM, Kratz A, Verba S, et al. Pain and Opioid Use After Thoracic Surgery: Where We Are and Where We Need To Go. Ann Thorac Surg 2020;109:1638-45. [Crossref] [PubMed]
- Kiser AC, Detterbeck FC. General aspects of surgical treatment. In: Detterbeck FC, Rivera MP, Socinski MA, et al. editors. Diagnosis and Treatment of Lung Cancer: An Evidence-Based Guide for the Practicing Clinician. Philadelphia, PA: W.B. Saunders, 2001:133-47.
- Howington JA, Blum MG, Chang AC, et al. Treatment of Stage I and II Non-small Cell Lung Cancer. Chest 2013;143:e278S-e313S. [Crossref] [PubMed]
- National Comprehensive CN. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) Non-Small Cell Lung Cancer Version 7.2019 - August 30, 2019 2019.
- Mohiuddin K, Haneuse S, Sofer T, et al. Relationship between margin distance and local recurrence among patients undergoing wedge resection for small (≤2 cm) non-small cell lung cancer. J Thorac Cardiovasc Surg 2014;147:1169-75; discussion 1175-7. [Crossref] [PubMed]
- Maurizi G, D'Andrilli A, Ciccone AM, et al. Margin Distance Does Not Influence Recurrence and Survival After Wedge Resection for Lung Cancer. Ann Thorac Surg 2015;100:918-24; discussion 924-5. [Crossref] [PubMed]
- Sienel W, Stremmel C, Kirschbaum A, et al. Frequency of local recurrence following segmentectomy of stage IA non-small cell lung cancer is influenced by segment localisation and width of resection margins--implications for patient selection for segmentectomy. Eur J Cardiothorac Surg 2007;31:522-7; discussion 527-8. [Crossref] [PubMed]
- Moon Y, Lee KY, Moon SW, et al. Sublobar Resection Margin Width Does Not Affect Recurrence of Clinical N0 Non-small Cell Lung Cancer Presenting as GGO-Predominant Nodule of 3 cm or Less. World J Surg 2017;41:472-9. [Crossref] [PubMed]
- Dolan D, Swanson SJ, Gill R, et al. Survival and Recurrence Following Wedge Resection Versus Lobectomy for Early-Stage Non-Small Cell Lung Cancer. Semin Thorac Cardiovasc Surg 2022;34:712-23. [Crossref] [PubMed]
- El-Sherif A, Fernando HC, Santos R, et al. Margin and local recurrence after sublobar resection of non-small cell lung cancer. Ann Surg Oncol 2007;14:2400-5. [Crossref] [PubMed]
- Wolf AS, Swanson SJ, Yip R, et al. The Impact of Margins on Outcomes After Wedge Resection for Stage I Non-Small Cell Lung Cancer. Ann Thorac Surg 2017;104:1171-8. [Crossref] [PubMed]
- Masai K, Sakurai H, Sukeda A, et al. Prognostic Impact of Margin Distance and Tumor Spread Through Air Spaces in Limited Resection for Primary Lung Cancer. J Thorac Oncol 2017;12:1788-97. [Crossref] [PubMed]
- Sawabata N, Maeda H, Matsumura A, et al. Clinical implications of the margin cytology findings and margin/tumor size ratio in patients who underwent pulmonary excision for peripheral non-small cell lung cancer. Semin Thorac Cardiovasc Surg 2022;34:712-23.
- Takahashi N, Sawabata N, Kawamura M, et al. Optimal sublobar resection for c-stage I non-small cell lung cancer: significance of margin distance to tumor size ratio and margin cytology (Supplementary analysis of KLSG-0801): complete republication. Gen Thorac Cardiovasc Surg 2019;67:690-6. [Crossref] [PubMed]
- Fernando HC, Landreneau RJ, Mandrekar SJ, et al. Impact of brachytherapy on local recurrence rates after sublobar resection: results from ACOSOG Z4032 (Alliance), a phase III randomized trial for high-risk operable non-small-cell lung cancer. J Clin Oncol 2014;32:2456-62. [Crossref] [PubMed]
- Tamura M, Matsumoto I, Tanaka Y, et al. Comparison Between Stereotactic Radiotherapy and Sublobar Resection for Non-Small Cell Lung Cancer. Ann Thorac Surg 2019;107:1544-50. [Crossref] [PubMed]
- Moon Y, Lee KY, Park JK. Margin Width of Resected Lepidic Lung Cancer Does Not Affect Recurrence After Sublobar Resection. World J Surg 2018;42:1449-57. [Crossref] [PubMed]
- Moon Y, Park JK, Lee KY. The Effect of Resection Margin Distance and Invasive Component Size on Recurrence After Sublobar Resection in Patients With Small (≤2 Cm) Lung Adenocarcinoma. World J Surg 2020;44:990-7. [Crossref] [PubMed]
- Schuchert MJ, Pettiford BL, Keeley S, et al. Anatomic segmentectomy in the treatment of stage I non-small cell lung cancer. Ann Thorac Surg 2007;84:926-32; discussion 932-3. [Crossref] [PubMed]
- Sawabata N, Ohta M, Matsumura A, et al. Optimal distance of malignant negative margin in excision of nonsmall cell lung cancer: a multicenter prospective study. Ann Thorac Surg 2004;77:415-20. [Crossref] [PubMed]
- Kent M, Landreneau R, Mandrekar S, et al. Segmentectomy versus wedge resection for non-small cell lung cancer in high-risk operable patients. Ann Thorac Surg 2013;96:1747-54; discussion 1754-5. [Crossref] [PubMed]
- Ren Y, Xie H, Dai C, et al. Prognostic Impact of Tumor Spread Through Air Spaces in Sublobar Resection for 1A Lung Adenocarcinoma Patients. Ann Surg Oncol 2019;26:1901-8. [Crossref] [PubMed]
- Kadota K, Nitadori JI, Sima CS, et al. Tumor Spread through Air Spaces is an Important Pattern of Invasion and Impacts the Frequency and Location of Recurrences after Limited Resection for Small Stage I Lung Adenocarcinomas. J Thorac Oncol 2015;10:806-14. [Crossref] [PubMed]
- Yanagawa N, Shiono S, Endo M, et al. Tumor spread through air spaces is a useful predictor of recurrence and prognosis in stage I lung squamous cell carcinoma, but not in stage II and III. Lung Cancer 2018;120:14-21. [Crossref] [PubMed]
- Jia M, Yu S, Gao H, et al. Spread Through Air Spaces (STAS) in Lung Cancer: A Multiple-Perspective and Update Review. Cancer Manag Res 2020;12:2743-52. [Crossref] [PubMed]
- Shiono S, Endo M, Suzuki K, et al. Spread through air spaces affects survival and recurrence of patients with clinical stage IA non-small cell lung cancer after wedge resection. J Thorac Dis 2020;12:2247-60. [Crossref] [PubMed]
- Vaghjiani RG, Takahashi Y, Eguchi T, et al. Tumor Spread Through Air Spaces Is a Predictor of Occult Lymph Node Metastasis in Clinical Stage IA Lung Adenocarcinoma. J Thorac Oncol 2020;15:792-802. [Crossref] [PubMed]
- Kim SK, Kim TJ, Chung MJ, et al. Lung Adenocarcinoma: CT Features Associated with Spread through Air Spaces. Radiology 2018;289:831-40. [Crossref] [PubMed]
- Uruga H, Fujii T, Fujimori S, et al. Semiquantitative Assessment of Tumor Spread through Air Spaces (STAS) in Early-Stage Lung Adenocarcinomas. J Thorac Oncol 2017;12:1046-51. [Crossref] [PubMed]
- Han YB, Kim H, Mino-Kenudson M, et al. Tumor spread through air spaces (STAS): prognostic significance of grading in non-small cell lung cancer. Mod Pathol 2021;34:549-61. [Crossref] [PubMed]
- Toyokawa G, Yamada Y, Tagawa T, et al. Computed tomography features of resected lung adenocarcinomas with spread through air spaces. J Thorac Cardiovasc Surg 2018;156:1670-1676.e4. [Crossref] [PubMed]
- Chen D, Mao Y, Wen J, et al. Tumor Spread Through Air Spaces in Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis. Ann Thorac Surg 2019;108:945-54. [Crossref] [PubMed]
- Dai C, Xie H, Su H, et al. Tumor Spread through Air Spaces Affects the Recurrence and Overall Survival in Patients with Lung Adenocarcinoma >2 to 3 cm. J Thorac Oncol 2017;12:1052-60. [Crossref] [PubMed]
- Kadota K, Kushida Y, Kagawa S, et al. Limited Resection Is Associated With a Higher Risk of Locoregional Recurrence than Lobectomy in Stage I Lung Adenocarcinoma With Tumor Spread Through Air Spaces. Am J Surg Pathol 2019;43:1033-41. [Crossref] [PubMed]
- Song T, Jiang L, Zhuo Z, et al. Impacts of thoracoscopic surgery and high grade histologic subtypes on spread through air spaces in small stage I lung adenocarcinomas. J Cancer Res Clin Oncol 2019;145:2375-82. [Crossref] [PubMed]
- Hu SY, Hsieh MS, Hsu HH, et al. Correlation of tumor spread through air spaces and clinicopathological characteristics in surgically resected lung adenocarcinomas. Lung Cancer 2018;126:189-93. [Crossref] [PubMed]
- Chae M, Jeon JH, Chung JH, et al. Prognostic significance of tumor spread through air spaces in patients with stage IA part-solid lung adenocarcinoma after sublobar resection. Lung Cancer 2021;152:21-6. [Crossref] [PubMed]
- Shiono S, Endo M, Suzuki K, et al. Spread Through Air Spaces Is a Prognostic Factor in Sublobar Resection of Non-Small Cell Lung Cancer. Ann Thorac Surg 2018;106:354-60. [Crossref] [PubMed]
- Toyokawa G, Yamada Y, Tagawa T, et al. Significance of Spread Through Air Spaces in Resected Pathological Stage I Lung Adenocarcinoma. Ann Thorac Surg 2018;105:1655-63. [Crossref] [PubMed]
- Kadota K, Kushida Y, Katsuki N, et al. Tumor Spread Through Air Spaces Is an Independent Predictor of Recurrence-free Survival in Patients With Resected Lung Squamous Cell Carcinoma. Am J Surg Pathol 2017;41:1077-86. [Crossref] [PubMed]
- Kagimoto A, Tsutani Y, Kushitani K, et al. Segmentectomy vs Lobectomy for Clinical Stage IA Lung Adenocarcinoma With Spread Through Air Spaces. Ann Thorac Surg 2021;112:935-43. [Crossref] [PubMed]
- Toyokawa G, Yamada Y, Tagawa T, et al. Significance of spread through air spaces in early-stage lung adenocarcinomas undergoing limited resection. Thorac Cancer 2018;9:1255-61. [Crossref] [PubMed]
- Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell 2011;147:275-92. [Crossref] [PubMed]
- Wan L, Pantel K, Kang Y. Tumor metastasis: moving new biological insights into the clinic. Nat Med 2013;19:1450-64. [Crossref] [PubMed]
- Weinberg RA. Mechanisms of malignant progression. Carcinogenesis 2008;29:1092-5. [Crossref] [PubMed]
- Angelova M, Mlecnik B, Vasaturo A, et al. Evolution of Metastases in Space and Time under Immune Selection. Cell 2018;175:751-765.e16. [Crossref] [PubMed]
- Nishio W, Yoshimura M, Maniwa Y, et al. Re-Assessment of Intentional Extended Segmentectomy for Clinical T1aN0 Non-Small Cell Lung Cancer. Ann Thorac Surg 2016;102:1702-10. [Crossref] [PubMed]
- Roman M, Labbouz S, Valtzoglou V, et al. Lobectomy vs. segmentectomy. A propensity score matched comparison of outcomes. Eur J Surg Oncol 2019;45:845-50. [Crossref] [PubMed]
- Ueda K, Tanaka T, Hayashi M, et al. What proportion of lung cancers can be operated by segmentectomy? A computed-tomography-based simulation. Eur J Cardiothorac Surg 2012;41:341-5. [Crossref] [PubMed]


