
Clinical and radiographic benefits of skipping C7 instrumentation in posterior cervicothoracic fusion: a retrospective analysis
Introduction
Degenerative cervical spine disease is a common radiographic diagnosis in over half of patients over the age of 55, and symptomatic disease is one of the most common indications for neurosurgical intervention (1-8). Over the past 30 years, rates of cervical spine surgery have increased dramatically with rates of cervical spine fusion increasing by 206% between 1992 and 2005 alone (1,5). Cervical spinal fusion is most commonly performed via an anterior or posterior approach. Posterior cervical fusion (PCF) is often indicated for patients with spinal stenosis, spondylosis, or degenerative disc disorders that result in radiculopathy or myelopathy; however, it can also be performed for a variety of other spinal conditions including tumor, infection, or deformity (9,10). PCF is also commonly used in conjunction with anterior cervical fusion in cervical spondylotic myelopathy patients with substantial kyphotic deformity to provide adequate stabilization following anterior decompression and fusion (11). This combined approach becomes exceedingly important in multilevel procedures as increased rates of pseudoarthrosis and adjacent segment disease are reported in long-segment cervical fusions, especially those ending distally at the C7 vertebral body (11-15). PCF offers the unique ability to extend arthrodesis beyond the cervicothoracic junction (CTJ) with minimal additional surgery thereby maintaining spinal alignment and preventing instability (11-13). While the extension of PCF into the upper thoracic spine is well documented, there remains a relative paucity of literature regarding specific procedural elements and complications associated with posterior spinal fusion (PSF) across the CTJ.
Historically, instrumentation of the CTJ has presented a major problem for treating physicians due to the unique biomechanical properties of the transition between cervical and thoracic spine. Primarily, the CTJ includes the C7 vertebra, the T1 vertebra, the disc between these two vertebrae, and associated ligaments; however, functional definitions additionally include the T2 and T3 vertebrae as they are involved in most fusion constructs of the CTJ (16). The CTJ represents a transitional region between the mobile and lordotic nature of the cervical spine to the rigid and kyphotic nature of the thoracic spine. Additionally, the size of the posterior elements changes significantly from lower cervical to upper thoracic spine. Lateral masses, commonly instrumented in PCF gradually decrease in thickness from C5-C7; however, the C7 lateral mass is in morphological transition between a cervical spine lateral mass and a transverse process of the thoracic spine (16,17). Conversely, pedicles (instrumented in both cervical and thoracic spine to achieve posterior fusion) gradually increase in size progressing from lower cervical to upper thoracic vertebral bodies (16,17). In addition to changes in pedicle height and width, the angle of the pedicle on the vertebral body changes significantly through the CTJ as well and must be taken into account upon insertion of pedicle screws (16,17). Screws placed in C7 (whether pedicle or lateral mass) lie in a different anatomical plane and necessitate either aggressive rod bending on the coronal plane, potentially weakening the rod, or the use of side connectors, which can be unwieldy and time-consuming, posing risks of increased operative time on patients and blood loss as well as affect deformity correction on the coronal plane (18). Thus, the combination of these changes produces significant difficulty in posterior fusion across the CTJ.
Pedicle screws are preferred to lateral mass screws at C7 due to the superior biomechanical profile (16,19). However, structurally, the decreased pedicle area of C7 can potentially compromise the strength of the entire construct spanning the CTJ (18). While, overall, posterior fusion of the CTJ has become increasingly safer with only 1-2% incidence of radiculopathy and extremely rare incidence of vascular injuries, the challenges of instrumentation at C7 specifically can potentially pose undue risk of hardware failure and put patients at risk of further neurological deterioration (20). Our previous study of 53 patients that underwent posterior instrumentation skipping C7 fixation showed decreased operative blood loss and faster operative times without compromising long-term radiographic fusion (19). In the current study we analyze a retrospective cohort analysis of 314 patients undergoing posterior cervicothoracic fusion in order to evaluate the clinical and radiographic benefits of skipping C7 instrumentation (termed “C7 bridge” by the authors). We present the following article in accordance with the STROBE reporting checklist (available at https://jss.amegroups.com/article/view/10.21037/jss-21-85/rc).
Methods
Patient population
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Institutional Review Board of The University of Illinois at Chicago (Approval number: UIC #2014-0137) and individual consent for this retrospective analysis was waived. 314 patients were identified who underwent PSF across the CTJ between November 2010 and September 2019 at our institution. Retrospective chart review was performed on all of these cases. Inclusion criteria consisted of: age over 18 years old, posterior approach for spinal fusion procedure, and multi-level instrumentation across the CTJ. The control group for our study consisted of 19 out of these 314 patients who underwent instrumentation at the C7 vertebra. Instrumentation at C7 was performed for a variety of reasons including technical inability to instrument C6 or T1 vertebral levels, prior anterior fusion at C7, fracture of C7, and cage placement.
Each patient’s clinical documentation, operative reports, anesthesia reports, nursing records, and postoperative follow-up documentation, and radiographic imaging were thoroughly reviewed, and data was recorded regarding age, clinical diagnosis, surgery performed, staging of surgery (standalone or as part of a 1, 2, or 3 stage procedure), vertebral levels instrumented, presence of instrumentation at C7 vertebra, intraoperative complications, operative time, estimated blood loss (EBL), bone-morphogenetic protein (BMP) utilization, time to postoperative follow-up, significant long-term complication, and documentation of long-term radiographic fusion. Long-term complications were identified on post-operative follow-up and included hardware failure, wound infections, worsening pain (relative to preoperative symptoms), or muscle weakness.
Surgical procedures
Posterior cervicothoracic fusion was performed either as a standalone approach (stage 0) or as part of a multistage surgical plan (stages 1–3). Surgeries were performed by the same team of surgeons at our institution with operative technique as detailed below. Two-stage surgical plans involved anterior cervical discectomy and fusion (ACDF) in addition to posterior fusion, and three-stage surgical plans involved removal of previous failed hardware in addition to the two-stage procedures. Intraoperative neuro-monitoring was utilized throughout the procedure with pre-and-post positioning signals recorded for baseline. Motor-evoked potentials were recorded in four downstream muscle groups bilaterally. A midline longitudinal posterior incision was made and extended along all spinal levels to be instrumented. Posterior spinal elements were carefully dissected to expose anatomical landmarks (cervical lateral masses and thoracic pedicles). Cervical instrumentation was placed in lateral masses according to An’s method while thoracic instrumentation was placed in thoracic pedicles following cannulation according to standard Lenke’s technique. Intra-operative AP and lateral radiographs as well as a O-arm intraoperative CT scan were used to confirm proper positioning of instrumentation. In C7 bridge procedures, screws were placed bilaterally at C6 and T1, with rods secured across the junction. Laminectomy was performed using a high-speed drill and rongeurs for cases in which decompression was indicated. In certain cases, multistage fusion was accomplished via PSF done in conjunction with anterior instrumentation. Contouring of rods was performed prior to securing in screw tulips in order to achieve optimal degrees of cervical lordotic/thoracic kyphotic correction. Thorough decortication and bone graft placement (+/− BMP) were performed to promote successful bony arthrodesis. In C7 bridge cases, fusion construct was extended at least 2 vertebral levels above and below C7 in order to ensure construct stability.
Radiographic follow-up
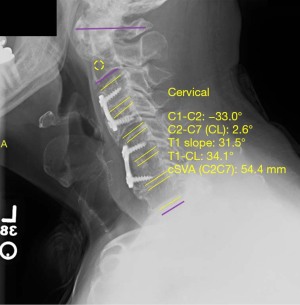
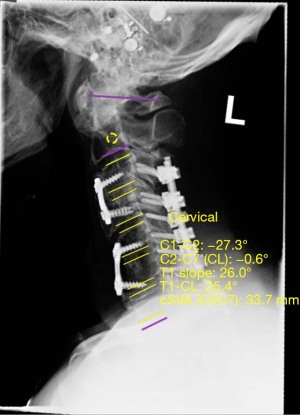
Analysis of postoperative lateral X-ray imaging was performed to assess postoperative fusion stability. Values of sagittal vertical axis (SVA), T1 slope, and cervical cobb angle (both pre-and-post-operatively) were obtained using a validated software, Surgimap® by Nemaris, Inc. (Figures 1,2, respectively) (21). Indicators of successful fusion included lack of hardware malfunction, lack of instrument loosening or displacement, spinal stability, lack of vertebral fractures, and bony fusion seen on postoperative radiographs. In the control group, 9 (47.4%) patients had satisfactory preoperative and postoperative imaging. In the C7 bridge group, 166 (56.2%) had satisfactory preoperative imaging and 177 (60.0%) had satisfactory postoperative imaging.
Statistical analysis
Fisher’s exact tests were used to analyze all categorical data and unpaired t-tests or 2-way analyses of variance were used to analyze all parametric data using SPSS software (version 26.0, IBM, Armonk, New York, USA). Data are presented as the mean +/− standard deviation, and a probability value of less than 0.05 was considered statistically significant.
Results
Patient demographics
A total of 314 patients met all inclusion criteria with 19 patients in the control group and 295 patients in the C7 bridge group. The control group consisted of 11 male (57.9%) and 8 female (42.1%) patients. The C7 bridge group consisted of a similar gender distribution with 170 male (57.6%) and 125 female (42.4%) patients. In terms of age, the average age of the control group was 63.0±10.6 years, which did not significantly differ from the average age of the C7 bridge group which was 58.7±14.4 years (P=0.704). The most common primary diagnoses for which patients in the C7 bridge group received PSF were cervical myelopathy (56.6%), pseudoarthrosis/hardware failure (11.5%), vertebral fracture (10.5%), and kyphotic deformity (6.4%). Similarly, in the control group, the most common primary diagnoses were cervical myelopathy (68.4%), pseudoarthrosis/hardware failure (10.5%), vertebral fracture (10.5%), and cord compression (5.3%) (Table 1).
Table 1
| Baseline characteristics | Control (N=19) | C7 bridge (N=295) | P value |
|---|---|---|---|
| Sex: male | 11 (57.9) | 170 (57.6) | 0.963 |
| Mean age, years | 63.0±10.6 | 58.7±14.4 | 0.704 |
| Primary diagnosis | |||
| Cervical myelopathy | 13 (68.4) | 167 (56.6) | 0.730 |
| Pseudoarthrosis/hardware failure | 2 (10.5) | 34 (11.5) | |
| Vertebral fracture | 2 (10.5) | 31 (10.5) | |
| Kyphotic deformity | 0 (0) | 19 (6.4) | |
| Osteomyelitis | 0 (0) | 11 (3.7) | |
| Cord compression | 1 (5.3) | 9 (3.1) | |
| Cancer mass/metastasis | 0 (0) | 8 (2.7) | |
| Ankylosing spondylitis | 0 (0) | 5 (1.7) | |
| Central cord syndrome | 0 (0) | 4 (1.4) | |
| Other* | 1 (5.3) | 7 (2.4) |
Numbers in parentheses are percentages. *, other diagnoses include traumatic spondylolisthesis, epidural lesion, extradural lesion, cervical AVM, and vertebral lytic lesion. AVM, arteriovenous malformation.
Operative results
The clinical outcomes of skipping C7 instrumentation were determined by comparing surgical data between control and C7 bridge groups (Table 2). The majority of patients in both the C7 bridge group and the control group underwent PSF across the CTJ as a standalone procedure or a stage 1 of a multi-staged procedure; however, a significantly greater proportion of patients in the control group underwent standalone or stage 1 procedures (n=17, 89.5%) compared to patients in the C7 bridge group (n=193, 65.4%) (P<0.001). Accordingly, a significantly greater proportion of patients in the C7 bridge group underwent PSF as stage 2 or stage 3 of a multi-staged procedure (n=102, 34.6%) compared to patients in the control group (n=2, 10.5%) (P<0.001). Other stages of multi-staged procedures commonly involved ACDF as well as removal of failed hardware. Patients in the C7 bridge group had significantly decreased overall EBL compared to patients in the control group (488±576 vs. 822±1,137 mL respectively, P=0.007). Additionally, when comparing staged approaches, patients in the C7 bridge group that received standalone or stage 1 procedures had significantly decreased EBL compared to patients in the control group (498±628 vs. 845±1,186 mL, respectively, P=0.022). There was no significant difference between groups in patients who received PSF as stage 2 or stage 3 of a multi-staged operation. In regard to operative time, there was no significant difference in total operative time between the C7 bridge group and the control group. However, in terms of the number of vertebral levels fused, patients in the C7 bridge group had significantly more vertebral levels fused per operation (7.5±1.7 levels, range 3–17) compared to patients in the control group (7.3±2.5 levels, range 3–11) (P=0.019) as expected for biomechanical buttress effect (22). BMP was utilized significantly more frequently in the C7 bridge group than the control group (30.2% vs. 21.1% respectively, P=0.042). BMP usage was off label for selected cases, especially for patients with pseudo-arthrosis.
Table 2
| Surgical characteristics | Control (N=19) | C7 bridge (N=295) | P value |
|---|---|---|---|
| Stage of surgical plan* | <0.001# | ||
| Standalone/stage 1 | 17 (89.5) | 193 (65.4) | |
| Stage 2/3 | 2 (10.5) | 102 (34.6) | |
| Blood loss (mL) | 822±1,137 | 488±576 | 0.007# |
| Standalone/stage 1 | 845±1,186 | 498±628 | 0.022# |
| Stage 2/3 | 625±813 | 470±465 | 0.295 |
| Operative time (min) | 184±86 | 174±95 | 0.844 |
| Standalone/stage 1 | 181±90 | 158±81 | 0.410 |
| Stage 2/3 | 201±95 | 207±115 | 0.547 |
| Levels fused (range) | 7.3±2.5 (3.0–11.0) | 7.5±1.7 (3.0–17.0) | 0.019# |
| Intraoperative complications** | 1 (5.3) | 9 (3.1) | 0.299 |
| BMP usage | 4 (21.1) | 89 (30.2) | 0.042# |
| Length of follow-up (months) | 12.9±16.2 | 13.9±15.4 | 0.307 |
| Long-term complications† | 2 (10.5) | 19 (6.4) | 0.265 |
| Long-term radiographic fusion‡ | 10 (90.9) | 105 (82.0) | 0.097 |
Numbers in parentheses are percentages. *, posterior spinal fusion was performed as a standalone procedure or as part of a multistage surgical plan; **, intraoperative complications include durotomy, vertebral artery injury, loss of motor signals, extruded anterior implant, and fractured C7 body; †, long-term complications include pseudoarthrosis/hardware failure, adjacent segment disease, proximal junctional kyphosis, and distal junctional kyphosis; ‡, N=11 in control group; N=128 in C7 skip group with long-term radiographic data available; #, P<0.05. BMP, bone morphogenic protein
Intraoperative and long-term complications
The safety of skipping C7 instrumentation was determined by comparing intraoperative and long-term complications between the C7 bridge and control groups (Table 2). There was no significant difference in the rate of intraoperative complications between the control group and the C7 bridge group (5.3% vs. 3.1% respectively, P=0.299). One patient (5.3%) in the control group had intraoperative complications associated with morbid obesity (body mass index >44) that prolonged operative time by over 1 hour. A total of 9 patients (3.1%) in the C7 bridge group had intraoperative complications. Three of these patients experienced incidental durotomies that were all repaired intraoperatively, two patients had vertebral artery injuries, two patients had decreased upper extremity motor signals on neuromonitoring, one patient had intraoperative fracture of C7 vertebra prolonging operative time, and one patient had extrusion of an anterior implant which required additional stage 3 operation for revision.
Length of follow up was similar in both groups at 12.9±16.2 months in the control group and 13.9±15.4 months in the C7 bridge group (P=0.307). 4 deaths were recorded in the follow-up period, however none occurred during immediate postoperative period nor were they related to the PSF operation. Within the postoperative follow-up period, there were 2 (10.5%) complications in the control group and 19 (6.4%) in the C7 bridge group (P=0.265). One patient in the control group presented with new onset acute neck pain and muscle spasms that resolved with pain management and required no further intervention; the other patient experienced postoperative wound infection requiring wound exploration and washout. In the C7 bridge group, postoperative complications included 8 patients with wound drainage/infection requiring exploration and washout, 10 patients experienced hardware failure/pseudoarthrosis requiring revision surgery, and one patient with superior laryngeal nerve neuropraxia with gradual improvement over the follow-up period. All other patients underwent uneventful postoperative courses.
Radiographic outcomes
Immediate postoperative radiographs were assessed in both groups to confirm proper hardware placement, spinal stability and alignment. Analysis and comparison of preoperative and postoperative biomechanical parameters revealed that compared to controls, patients in the C7 bridge group had similar preoperative SVA (P=0.793) but significantly greater postoperative SVA (20.2±3.1 vs. 29.3±13.1 mm, P=0.008). However, there was no significant difference in millimeters of overall SVA correction between groups (P=0.867). Preoperative (P=0.499) and postoperative (P=0.069) T1 slope measurements were similar between the groups, as was the degree of T1 slope correction (P=0.127). Lastly, preoperative cervical cobb angle was significantly lower in C7 bridge patients compared to controls (−4.0°±15.4° vs. −1.3°±26.3° respectively, P=0.016); however, there was no difference in postoperative cervical cobb angle measurement (P=0.376) or degree of cervical cobb angle correction between the groups (P=0.519) (Table 3). Follow-up radiographs throughout the postoperative course revealed similar rates of successful fusion in both groups with 90.9% of patients in the control group and 82.0% of patients in the C7 bridge group achieving long-term radiographic fusion (P=0.097) (Table 2).
Table 3
| Radiographic measurements | Control (N=9) | C7 bridge (N=177) | P value |
|---|---|---|---|
| SVA (mm) | |||
| Preoperative | 32.9±15.9 | 28.9±18.9 | 0.793 |
| Postoperative | 20.2±3.1 | 29.3±13.1 | 0.008# |
| Correction | −16.1±16.0 | −0.3±16.2 | 0.867 |
| T1 slope (degrees) | |||
| Preoperative | 30.3±7.2 | 28.4±10.7 | 0.499 |
| Postoperative | 30.8±4.4 | 32.2±10.0 | 0.069 |
| Correction | 3.2±5.5 | 3.4±9.9 | 0.127 |
| Cobb angle (degrees) | |||
| Preoperative | −1.3±26.3 | −4.0±15.4 | 0.016# |
| Postoperative | 0.2±20.8 | −11.4±13.1 | 0.376 |
| Correction | −7.0±12.2 | −5.7±14.2 | 0.519 |
#, P<0.05. SVA, sagittal vertical axis.
Discussion
Our study aimed to determine the clinical and radiographic benefits of skipping C7 instrumentation in multi-level PSF crossing the CTJ. We hypothesized that skipping C7 could improve clinical outcomes without sacrificing postoperative radiographic correction or fusion. Our analysis revealed that skipping C7 instrumentation results in significantly decreased operative blood loss with no loss of radiographic correction and a similar rate of long-term radiographic fusion. Thus, skipping C7 instrumentation provides a safer technique of achieving PSF across with CTJ compared to instrumenting C7.
The C7 vertebra is anatomically unique, and thus imposes significant difficulties regarding instrumentation placement as well as construct integrity. Pedicle height, width, and angulation on the vertebral body change significantly through the CTJ with the C7 vertebral body at the epicenter of this transition. Significant consideration must be given to these anatomical changes in order to properly place pedicle screws in the C7 vertebral body (16). Additionally, representing the junction between the mobile cervical spine and the rigid thoracic spine, the C7 segment is under increased biomechanical stress and prone to complications such as implant failure and adjacent-segment disease when it is the last segment instrumented in multi-level PCF (23-25). Thus, it has long been proposed that extension of posterior fusion constructs across the CTJ to end distally at T1 or T2 can potentially reduce stress placed on the C7 vertebra and subsequently reduce rates of hardware failure or distal junctional degeneration. Osterhoff et al. found that patients with multisegmental PCF terminating distally at T1/T2 had a decreased rate of clinically symptomatic pathology at the adjacent level below the instrumentation compared to those whose instrumentation ended at C7 (25). Nagashima et al. found that concomitant screw placement adjacent to the C7 vertebra, in either the C6 or the T1 vertebral body, imparts a buttress effect on the C7 pedicle screw dissipating the force from leverage and reducing stress concentration thereby reducing rates of implant failure (19,22). Thus, in theory, given the anatomical difficulties in instrumenting C7 combined with the improved outcomes of extending multi-level PCF across the CTJ to T1 or T2, skipping C7 instrumentation in posterior cervicothoracic fusion could improve safety without sacrificing stability.
Our study of 314 total patients, 295 of which were not instrumented at C7, found that skipping C7 resulted in significantly decreased operative blood loss compared to patients who received C7 instrumentation. We initially hypothesized that this may be due to decreased operative time considering one fewer vertebral levels requires instrumentation and time spent fashioning the needed coronal bent for a single rod construct or adding a second rod to join a second rod with side connectors. However, operative time did not significantly differ between groups. Interestingly, patients in the C7 bridge group did have a significantly greater average number of vertebral levels fused per operation compared to the control group ranging anywhere from 3-17 vertebral levels fused. Furthermore, the functional operative time per level fused would be lower in the C7 skip group than the control. Thus, perhaps skipping C7 does permit faster operation and subsequently decreased blood loss, while longer fusions overall accounted for lack of significant difference in operative time between groups. There was no significant difference in long term complication rates between those who were instrumented at C7 and those who were not, including any recorded incidence of hardware failure or pseudoarthrosis requiring revision surgery. None of the control group patients experienced hardware failure in comparison to 10 patients in the C7 bridge group (3.4%). While this may seem to indicate that skipping C7 results in increased rates of hardware failure, this is more likely the result of our limited cohort of patients receiving instrumentation at C7. According to Okamoto et al. the overall rate of hardware failure in PCF is 4.2%, thus our findings would indicate that skipping C7 results in potentially reduced incidence of hardware failure compared to rates reported in the literature (26).
The most significant concern with skipping instrumentation at any level during PSF is reduced construct stability and loss of correction in spinal alignment. Our study found that based on comparison of radiographic parameters such as SVA, T1 slope, and cervical cobb angle between preoperative and postoperative radiographs, skipping C7 instrumentation did not significantly affect correction of any of these parameters compared to those with instrumentation at C7. While there is no defined optimal correction of cervical spine curvature, it is generally recommended to return spinal alignment to neutral position (27). Thus, our study shows that constructs in which C7 instrumentation is skipped are still able to maintain sagittal balance. Additionally, analysis of follow-up imaging showed that there was no significant difference between rates of long-term radiographic fusion either (with average mean follow up of ~1 year in both groups) suggesting that skipping C7 still permits adequate bony fusion and postoperative spine stability. To our knowledge, skipping C7 instrumentation is commonly done, but very little literature is available regarding long-term outcomes and potential failures of these constructs including implant failure, non-union/pseudarthrosis, or loss of radiographic correction. Our previous study found similar results; however, it was limited by its small sample size and this study scaled up nearly 3-fold (19).
The current study also describes an off-label use of BMP to augment local autograft in the facilitation of bony fusion in the cervical spine. BMP was used significantly more frequently in the C7 bridge group. While the FDA issued a formal warning in 2008 regarding the risk for airway complications with the use of BMP in cervical spine fusion, we did not see any such complications over the 10-year course of our study (28). In fact, the C7 bridge group, in whom significantly more patients received BMP, had a lower overall complication rate (6.4%) than the control group (10.5%) though not statistically significant. While we acknowledge the potential for complications in a larger study, BMP has been used safely in our institution over the past decade and course of this study.
Limitations
Our study is limited by relatively unbalanced comparison considering of the 314 total patients, only 19 received C7 instrumentation. Similarly, adequate radiographic data was not available for all patients in either group, thus the sample sizes were smaller for radiographic analyses. Limitations inherent to retrospective analyses also exist in our study including potential for selection bias based on surgeon practice, loss of patient information, and patients lost to follow-up. All the included procedures and follow-up care occurred at a tertiary academic center, slightly limiting generalizability, and the senior author always led the surgical team performing each procedure, minimizing variation in technique for the C7 skip approach to PSF surgery.
Conclusions
Skipping C7 instrumentation results in clinically safer operation with significantly reduced operative blood loss without sacrificing long-term stability, fusion, or correction. Given the challenges associated with instrumenting C7 and its unique anatomical characteristics, skipping C7 instrumentation provides a valuable and effective technique in PSF across the CTJ without imparting undue risks of instrumenting the C7 vertebra. Further prospective studies with larger, better matched sample sizes and data from strict 3, 6, and 12-month follow-up are recommended in order to perform comprehensive analysis regarding clinical and radiographic outcomes of skipping C7 instrumentation in posterior cervicothoracic fusion.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jss.amegroups.com/article/view/10.21037/jss-21-85/rc
Data Sharing Statement: Available at https://jss.amegroups.com/article/view/10.21037/jss-21-85/dss
Peer Review File: Available at https://jss.amegroups.com/article/view/10.21037/jss-21-85/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jss.amegroups.com/article/view/10.21037/jss-21-85/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Institutional Review Board of the University of Illinois at Chicago (Approval number: UIC #2014-0137) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wang MC, Kreuter W, Wolfla CE, et al. Trends and variations in cervical spine surgery in the United States: Medicare beneficiaries, 1992 to 2005. Spine (Phila Pa 1976) 2009;34:955-61; discussion 962-3. [Crossref] [PubMed]
- Hughes JT, Brownell B. Necropsy observations on the spinal cord in cervical spondylosis. Riv Patol Nerv Ment 1965;86:196-204. [PubMed]
- Pallis C, Jones AM, Spillane JD. Cervical spondylosis; incidence and implications. Brain 1954;77:274-89. [Crossref] [PubMed]
- Irvine DH, Foster JB, Newell DJ, et al. Prevalence of cervical spondylosis in a general practice. Lancet 1965;1:1089-92. [Crossref] [PubMed]
- Patil PG, Turner DA, Pietrobon R. National trends in surgical procedures for degenerative cervical spine disease: 1990-2000. Neurosurgery 2005;57:753-8; discussion 753-8. [Crossref] [PubMed]
- Chiles BW 3rd, Leonard MA, Choudhri HF, et al. Cervical spondylotic myelopathy: patterns of neurological deficit and recovery after anterior cervical decompression. Neurosurgery 1999;44:762-9; discussion 769-70. [Crossref] [PubMed]
- Fouyas IP, Statham PF, Sandercock PA. Cochrane review on the role of surgery in cervical spondylotic radiculomyelopathy. Spine (Phila Pa 1976) 2002;27:736-47. [Crossref] [PubMed]
- Kumar VG, Rea GL, Mervis LJ, et al. Cervical spondylotic myelopathy: functional and radiographic long-term outcome after laminectomy and posterior fusion. Neurosurgery 1999;44:771-7; discussion 777-8. [Crossref] [PubMed]
- Salzmann SN, Derman PB, Lampe LP, et al. Cervical Spinal Fusion: 16-Year Trends in Epidemiology, Indications, and In-Hospital Outcomes by Surgical Approach. World Neurosurg 2018;113:e280-95. [Crossref] [PubMed]
- Youssef JA, Heiner AD, Montgomery JR, et al. Outcomes of posterior cervical fusion and decompression: a systematic review and meta-analysis. Spine J 2019;19:1714-29. [Crossref] [PubMed]
- Hart RA, Tatsumi RL, Hiratzka JR, et al. Perioperative complications of combined anterior and posterior cervical decompression and fusion crossing the cervico-thoracic junction. Spine (Phila Pa 1976) 2008;33:2887-91. [Crossref] [PubMed]
- Bueff HU, Lotz JC, Colliou OK, et al. Instrumentation of the cervicothoracic junction after destabilization. Spine (Phila Pa 1976) 1995;20:1789-92. [Crossref] [PubMed]
- Korovessis P, Katonis P, Aligizakis A, et al. Posterior compact Cotrel-Dubousset instrumentation for occipitocervical, cervical and cervicothoracic fusion. Eur Spine J 2001;10:385-94. [Crossref] [PubMed]
- Hilibrand AS, Yoo JU, Carlson GD, et al. The success of anterior cervical arthrodesis adjacent to a previous fusion. Spine (Phila Pa 1976) 1997;22:1574-9. [Crossref] [PubMed]
- Cannada LK, Scherping SC, Yoo JU, et al. Pseudoarthrosis of the cervical spine: a comparison of radiographic diagnostic measures. Spine (Phila Pa 1976) 2003;28:46-51. [Crossref] [PubMed]
- Wang VY, Chou D. The cervicothoracic junction. Neurosurg Clin N Am 2007;18:365-71. [Crossref] [PubMed]
- Stanescu S, Ebraheim NA, Yeasting R, et al. Morphometric evaluation of the cervico-thoracic junction. Practical considerations for posterior fixation of the spine. Spine (Phila Pa 1976) 1994;19:2082-8. [Crossref] [PubMed]
- Tobin MK, Gragnaniello C, Sun FW, et al. Safety and Efficacy of Skipping C7 Instrumentation in Posterior Cervicothoracic Fusion. World Neurosurg 2019;130:e68-73. [Crossref] [PubMed]
- Rhee JM, Kraiwattanapong C, Hutton WC. A comparison of pedicle and lateral mass screw construct stiffnesses at the cervicothoracic junction: a biomechanical study. Spine (Phila Pa 1976) 2005;30:E636-40. [Crossref] [PubMed]
- Ilgenfritz RM, Gandhi AA, Fredericks DC, et al. Considerations for the use of C7 crossing laminar screws in subaxial and cervicothoracic instrumentation. Spine (Phila Pa 1976) 2013;38:E199-204. [Crossref] [PubMed]
- Lafage R, Ferrero E, Henry JK, et al. Validation of a new computer-assisted tool to measure spino-pelvic parameters. Spine J 2015;15:2493-502. [Crossref] [PubMed]
- Nagashima K, Koda M, Abe T, et al. Implant failure of pedicle screws in long-segment posterior cervical fusion is likely to occur at C7 and is avoidable by concomitant C6 or T1 buttress pedicle screws. J Clin Neurosci 2019;63:106-9. [Crossref] [PubMed]
- Bhatia NN. Long-Term Outcomes and Complications Following Anterior and Posterior Cervical Spine Surgery. Seminars in Spine Surgery 2009;21:177-84. [Crossref]
- Kretzer RM, Hu N, Umekoji H, et al. The effect of spinal instrumentation on kinematics at the cervicothoracic junction: emphasis on soft-tissue response in an in vitro human cadaveric model. J Neurosurg Spine 2010;13:435-42. [Crossref] [PubMed]
- Osterhoff G, Ryang YM, von Oelhafen J, et al. Posterior Multilevel Instrumentation of the Lower Cervical Spine: Is Bridging the Cervicothoracic Junction Necessary? World Neurosurg 2017;103:419-23. [Crossref] [PubMed]
- Okamoto T, Neo M, Fujibayashi S, et al. Mechanical implant failure in posterior cervical spine fusion. Eur Spine J 2012;21:328-34. [Crossref] [PubMed]
- Scheer JK, Tang JA, Smith JS, et al. Cervical spine alignment, sagittal deformity, and clinical implications: a review. J Neurosurg Spine 2013;19:141-59. [Crossref] [PubMed]
- Poeran J, Opperer M, Rasul R, et al. Change in Off-Label Use of Bone Morphogenetic Protein in Spine Surgery and Associations with Adverse Outcome. Global Spine J 2016;6:650-9. [Crossref] [PubMed]


