Combined liver and extrahepatic bile duct resection for biliary invasion of colorectal metastasis: a case-cohort analysis and systematic review
Introduction
Advancements in liver surgery have gradually extended the indications for resection of colorectal liver metastases (CRLMs) (1). A variety of surgical techniques are currently available to resect CLRMs, including two stage procedures, vascular reconstructions, and portal vein embolization to increase postoperative liver volume. The criteria for resectability of CRLMs have shifted in the last decade to include any patient in whom all disease can radically be removed with the preservation of sufficient functional remnant liver. Recent data justify an aggressive surgical approach of CRLMs with 5-year survival rates of 40–50% (2).
Little is known, however, about the more uncommon manifestation of CRLMs with biliary invasion. The incidence of biliary invasion (intrahepatic or extrahepatic) by CRLMs is reported in 5–40% of patients presenting with these metastases (3). CRLMs with biliary invasion usually present with dilatation of bile ducts due to biliary obstruction, and may cause jaundice if located centrally in the hilar area.
Combined liver resection and extrahepatic bile duct resection with biliary-enteric reconstruction may be useful in treating CRLMs with hilar biliary invasion. The surgical technique has been increasingly used for resection of perihilar cholangiocarcinoma in recent years, which has resulted in improved experience in specialized centers (4). Nonetheless, surgical and oncological outcomes of this technique remain largely unknown when used for CLRM with biliary invasion: previous studies describe mostly single cases or small series of patients.
To assess the feasibility, safety and survival associated with this technique, we present a case-cohort analysis of all patients who underwent major liver resections for CRLMs in our center, and compared patients with and without extrahepatic bile duct resection. To elucidate currently available evidence, we also performed a systematic literature search to provide an overview studies focusing on surgical treatment of patients with CLRM with biliary invasion.
Methods
Case-cohort analysis
This study was conducted in accordance with the declaration of Helsinki, and was approved by the local ethical committee. Patients who underwent a major liver resection for CRLM (i.e., resection of three or more liver segments) at the Academic Medical Centre in Amsterdam between 2003 and 2013 were selected from a prospective database. Retrospective data collected from the medical reports included demographics, presenting symptoms, synchronous or metachronous metastasis, primary tumor origin, surgical reports, and pathology reports. Patients with extrahepatic biliary invasion from a centrally located metastasis underwent a combined liver and extrahepatic bile duct resection and biliodigestive reconstruction. Bile duct resection was similar in all patients, consisting of biliary resection up to the cranial margin of the pancreatic head in combination with complete lymph node dissection of the hepatoduodenal ligament. Biliodigestive reconstruction was performed using single or multiple hepatico-jejunostomies. Neo-adjuvant treatment was not routinely adopted during the study period. None of the patients with biliary obstruction received chemotherapy in the preceding months prior to surgery because of recent jaundice.
Patients with and without biliary invasion were compared with respect to patient characteristics, surgical outcome, and overall survival. Morbidity was defined as a complication requiring intervention (Clavien grade 3 or higher) (5), and overall survival was defined as the time between liver resection and the day of death. Postoperative mortality was defined as any cause of death within 90 days after surgery.
Continuous variables are presented as the median (range), and analyzed using the Mann-Whitney U test. Categorical variables were analyzed using Fisher’s exact test. Kaplan-Meier estimates were used in analysis of overall survival, and differences in overall survival were analyzed using the Log-rank (Mantel-Cox) test. P values below 0.05 were considered significant.
Literature review
We performed a systematic literature search in Cochrane Library, Medline and PubMed using two search strategies: (I) a specific search for articles covering combined liver and extrahepatic biliary resections of CRLMs; and (II) a general search for articles covering resection of CRLMs.
For the first search we used the following medical subject headings/Mesh terms and key words: colonic neoplasms, rectal neoplasms, colorectal neoplasms, liver metastastases, metastatic liver disease, hepatectomy, liver resection, metastasectomy, hepatobiliary, bile ducts, extrahepatic, biliary tree, hilar and anastomosis, Roux-en-Y with exclusion of hepatocellular, HCC, Klatskin, Klatskin’s tumor and cholangiocarcinoma. Title and abstract of all articles were scanned for their relevance on the subject. The second search was performed to cover the reports of combined liver and biliary resections within (systematic) review articles for the last 5 years. We used the following search in PubMed: colorectal neoplasms [Mesh] AND hepatectomy [Mesh] AND (“2008/05/23”[PDat]: “2013/05/21”[PDat]). Similar searches were performed in Cochrane Library and Medline.
Two observers (Wouter W. te Riele and Tristan H. van Dongen) inspected the title and abstract of citations to identify relevant papers for which we obtained the full texts. All prospective or retrospective case reports or cohort studies were included in the analysis if they presented an assessment of surgical resection of CRLM with biliary invasion. Selection of articles was limited to articles describing human subjects in English language. Data were extracted from all included manuscripts, including patient characteristics, surgical technique, pathology results, and survival.
Results
Case-cohort analysis
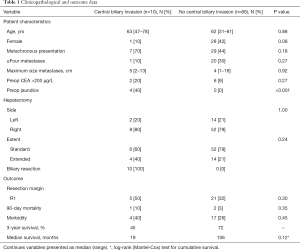
During the study period, 753 liver resections were performed in our single institution, of which 271 were major liver resections (3 or more Couinaud segments). A total of 76 patients underwent major liver resection for histologically proven CRLM, and were included in this study. Ten patients (13%) had biliary invasion from a centrally located hepatic metastasis and underwent a concomitant biliary resection with biliodigestive reconstruction. Clinicopathological, surgical and outcome data from patients with and without biliary invasion are presented in Table 1.
Full table
Four of ten patients with biliary invasion presented with jaundice and underwent preoperative biliary drainage, two percutaneously and two endoscopically. The median preoperative bilirubin level was 16 µmol/L (range, 5–87 µmol/L). After combined liver and extrahepatic bile duct resection, an R0 resection was achieved in five of ten patients (50%). A positive resection margin was accepted in three patients after intra-operative frozen section analysis, because further extending the resection was not considered feasible. In two other patients, a positive resection margin was only found in the definitive resection specimen. All patients had segmental bile duct obstruction from the CLRM, and seven of ten patients were found to have microscopic biliary invasion in intrahepatic or extrahepatic bile ducts. Biliary fibrosis was found in three of ten patients because of longer existing biliary obstruction. Nine out of ten patients had a solitary metastasis, and one patient had multiple (synchronous) metastases.
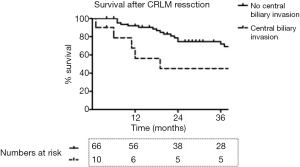
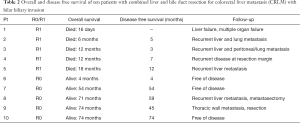
One of ten patients died postoperatively after combined liver resection and extrahepatic bile duct resection. Postoperative morbidity and mortality tended to be higher among patients with biliary invasion compared to patients without biliary invasion, but this was not found to be statistically significant (Table 1). Overall median survival was 19 months among patients who underwent concomitant bile duct resection, versus 106 months among patients treated without biliary resection (P=0.12; Figure 1). Patterns of recurrence among patients with biliary invasion after surgical resection are specified in Table 2.
Full table
Literature review
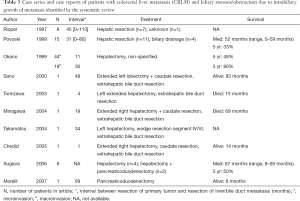
A total of ten studies, comprising four case series and six case reports, were identified by our systematic literature search (Table 3) (3,6-13). These articles included a total of 97 patients with CRLMs and biliary invasion. The largest series reported by Okano identified 62 (42%) patients with biliary invasion in a series of 149 patients after hepatectomy for CRLM (3). They differentiated between microscopic bile duct invasion (30%) and macroscopic bile duct invasion (12%). Two thirds of the patients with macroscopic bile duct invasion had a well-differentiated adenocarcinoma with less vascular involvement. Five-year survival was significantly better for patients with macroscopic bile duct invasion compared to patients with microscopic bile duct invasion or no bile duct invasion, 80% vs. 48% vs. 57%, respectively. They concluded from their multivariate analysis that macroscopic bile duct invasion was an independent prognostic variable for favorable outcome after hepatic resection. Povoski et al. described a series of 15 patients with intrabiliary colorectal metastases, including 11 patients who underwent hepatectomy (6). Five-year survival was 33% for patients who underwent a resection versus 0% for patients who underwent no resection (n=4). Riopel et al. described a series of eight patients with CRLM that demonstrated prominent spread throughout the biliary tree (7). Seven of these patients underwent hepatic resection, but no data on follow-up were provided. These authors observed a pattern of CRLMs that mimics primary biliary neoplasia, as the tumor grows within the biliary tract along intact basement membranes. Sugiura et al. found macroscopic intrabiliary tumor growth in 6 out of 103 patients (6%) who underwent hepatectomy for CLRM and concluded that macroscopic biliary invasion is most often accompanied with microscopic biliary invasion (12). Four patients were treated with a hepatectomy and two with a combined hepatectomy and pancreatoduodenectomy. Median survival was 87 months and 5-year survival was 50%.
Full table
An additional five case reports were identified, in which combined hepatectomy and extrahepatic bile duct resection for CLRM with biliary invasion were reported (8-11,14). Two patients were alive after 83 and 14 months, and two patients died after 69 and 15 months, respectively. Notably, one case report was identified describing a patient with a pancreatic metastasis of a colorectal carcinoma with infiltration of the intrapancreatic tract of the common bile duct (13). This patient underwent a pancreatoduodenectomy and was alive after 6 months.
Discussion
Extrahepatic biliary invasion is not a well-recognized growth pattern of CLRMs, and its implications for treatment have been rarely reported throughout the years. This study aimed to analyze the outcomes of patients with CRLMs and extrahepatic biliary invasion treated at a surgical referral center. We identified ten patients who underwent combined liver and extrahepatic bile duct resection for CLRM with biliary invasions. In addition to our case-cohort analysis, we performed a systematic review of available case series, including ten studies and 97 patients.
Presentation
In our study, four patients with CRLMs with biliary invasion presented with jaundice. The usual cause of jaundice in patients with CRLMs is diffuse parenchymal disease and extensive tumor involvement of the liver. Extrahepatic biliary obstruction is rarely considered when assessing patients with CRLMs. Nonetheless, CLRMs can grow from intrahepatic bile ducts towards the liver hilum, eventually causing extrahepatic biliary obstruction due to mucosal metastasis, sludge formation by floating tumor debris or compression by enlarged lymph nodes (3).
Diagnosis
One of the problems using diagnostic imaging tests like ultrasound, CT, MRI or endoscopic retrograde cholangiography, is the difficulty to discriminate between intrahepatic cholangiocarcinoma and metastatic adenocarcinoma. It has been suggested that rapid growth of the tumor and rapid increase in CEA and/or CA 19.9 levels are more suspicious for bile duct metastases in patients with a history of CRLM, rather than for cholangiocarcinoma (7). Nevertheless, intraoperative recognition of bile duct invasion by a CRLM seems to be more common than preoperative clinical or radiographic recognition.
Treatment
The basic principle of surgical treatment of CRLMs is to obtain negative surgical margins by radical excision of all metastases. This principle should be maintained in this rare type of CRLMs with biliary invasion. The observation of Sugiura et al. that biliary invasion of CLRM mostly comprises both macroscopic and microscopic tumor involvement of bile ducts is supported by the findings from our study. Therefore, a recommendation can be made to use a combined hepatectomy and extrahepatic bile duct resection for patients with CRLMs and biliary invasion, in order to achieve good local control and curative resection (6,12). By analogy with hilar cholangiocarcinoma, the concept of hilar resection in conjunction with liver resection is based on a three-dimensional perception of the tumor located centrally in the liver. Tumor extension can occur from the bile duct confluence to the right and left biliary tree along the main hepatic bile duct and segmental bile ducts. The tumor can also invade anteriorly into the duct of segment 4 and posteriorly into the duct of segment 1. Ductal extension should be evaluated during operation and resection of segment 4 or 1 should be considered (4). Contrary to resection of ‘normal’ parenchymal CRLMs, anatomic resection according to the segmental biliary division seems to be preferred in metastases with biliary invasion to avoid recurrence in the remnant liver.
Although the concept of combined hemihepatectomy and extrahepatic bile duct resection was applied in all ten patients in this series, we could not avoid an R1 resection in five patients (50%). In three of eight patients (38%) in whom frozen sections analysis was used an R1 resection could not be avoided. The major concern leading us to accept these non-radical resections was anticipated insufficient liver remnant if a radical resection had been pursued. In two patients with R1 resections, frozen sections analysis was not performed. We therefore feel that routine examination of frozen sections should be recommended, in order to improve the chance of a radical resection.
The current study was not able to analyze the effect of an R1 resection compared to the effect of chemotherapy only, which would be interesting to see in future studies. Nevertheless, patients with an R1 resection in this study had remarkably short disease-free and overall survival, so an R1 resection does not seem to justify the burden of major liver surgery and associated mortality. It seems preferable to select patients with CRLMs and extrahepatic biliary invasion for surgical treatment only if radiological staging predicts a high chance of a radical (R0) resection, although this can only be ascertained at surgical exploration.
Pathology findings
The pattern of invasion of CLRMs into the bile duct has been described by Riopel and Takamatsu (7,14). Firstly, the tumor cells infiltrate along an intact basement membrane and replace the non-neoplastic bile duct epithelium. Secondly, the transition between the tumor cells and the native biliary epithelium was abrupt and hyperplasia/dysplasia was not seen. Thirdly, the morphologic features of the liver and intrabiliary ductal tumor were reminiscent of colorectal cancer. In addition to the intraluminal and intraepithelial extension as described by Sugiura et al., Minagawa described metastatic colorectal tumor as showing papillary growth in the bile duct (10). According to Okano et al., in most cases macroscopic bile duct invasion tends to be a better differentiated adenocarcinoma with less vascular involvement compared to microscopic bile duct invasion (3).
Prognosis
The systematic literature search on resection of CLRMs with biliary invasion revealed a large variability in 5-year survival rates, ranging between 33–80%. The large variation may in part be explained by differences in study populations, with some studies including any patient with intra- or extrahepatic intrabiliary growth in the analysis. The postoperative morbidity (40%) and 90-day mortality (10%) in our study was acceptable, and seems comparable to outcomes after major liver resection of hilar cholangiocarcinoma (15). The median overall survival was only 19 months and remarkably lower compared to a median survival of 106 months among patients treated without biliary resection. This difference, which could be attributed to the high percentage (50%) of R1 resections among patients with biliary invasion, was however, not found to be statistically significant, due to the small size of the study group.
Macroscopic bile duct invasion of CRLMs, as opposed to microscopic invasion, appears to be an independent prognostic variable for favorable outcome after hepatic resection (3). In our study however, most resections were found to have both macroscopic and microscopic biliary invasion in intrahepatic or extrahepatic bile ducts, which is in accordance with the findings of Sugiura et al. It therefore remains questionable if macroscopic bile duct invasion can be used as a prognostic predictor (12).
This retrospective study is limited by the small number of patients. However, CRLM with biliary invasion is relatively rare. There are two reasons why the number of large resections for CRLM in this series is relatively low: (I) many patients with CRLM are resected using parenchyma sparing techniques, i.e., multiple local or (sub)segmental excisions rather than major resections; and (II) as we are a national referral center for intrahepatic and hilar cholangiocarcinoma, most major liver resections have been performed for these diagnoses. The latter also explains our interest in the more uncommon biliary obstruction caused by CRLM. The present study summarizes all available literature data in the systematic review, and comprises the first case-cohort analysis that compares outcomes after resection of CRLM with and without biliary invasion.
Conclusions
Major progression has been made in the possibilities of liver surgery over the last decades. In this study, we have shown that patients with CRLMs with biliary invasion are distinct in a large group of patients with CRLMs. These metastases can present with central hilar biliary obstruction and should be treated with combined liver and extrahepatic bile duct resection according to strategies used in hilar cholangiocarcinoma. However, survival in these patients tends to be worse compared to patients having undergone resection for CLRMs without biliary invasion, possibly due to a high rate of non-radical resections. Therefore, it seems preferable to select only patients for surgical treatment if radiological staging predicts a high chance of a radical (R0) resection. Future research is necessary to obtain more knowledge on the biology of this manifestation in terms of growth pattern and prognosis.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by institutional ethics board (NO. IRB00003512).
References
- de Haas RJ, Wicherts DA, Andreani P, et al. Impact of expanding criteria for resectability of colorectal metastases on short- and long-term outcomes after hepatic resection. Ann Surg 2011;253:1069-79. [Crossref] [PubMed]
- Nathan H, de Jong MC, Pulitano C, et al. Conditional survival after surgical resection of colorectal liver metastasis: an international multi-institutional analysis of 949 patients. J Am Coll Surg 2010;210:755-64, 764-6.
- Okano K, Yamamoto J, Moriya Y, et al. Macroscopic intrabiliary growth of liver metastases from colorectal cancer. Surgery 1999;126:829-34. [Crossref] [PubMed]
- van Gulik TM, Kloek JJ, Ruys AT, et al. Multidisciplinary management of hilar cholangiocarcinoma (Klatskin tumor): extended resection is associated with improved survival. Eur J Surg Oncol 2011;37:65-71. [Crossref] [PubMed]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- Povoski SP, Klimstra DS, Brown KT, et al. Recognition of intrabiliary hepatic metastases from colorectal adenocarcinoma. HPB Surg 2000;11:383-90; discussion 390-1. [Crossref] [PubMed]
- Riopel MA, Klimstra DS, Godellas CV, et al. Intrabiliary growth of metastatic colonic adenocarcinoma: a pattern of intrahepatic spread easily confused with primary neoplasia of the biliary tract. Am J Surg Pathol 1997;21:1030-6. [Crossref] [PubMed]
- Sano T, Kamiya J, Nagino M, et al. Pancreatoduodenectomy after hepato-biliary resection for recurrent metastatic rectal carcinoma. J Hepatobiliary Pancreat Surg 2000;7:516-9. [Crossref] [PubMed]
- Tomizawa N, Ohwada S, Tanahashi Y, et al. Liver metastasis of rectal cancer with intraluminal growth in the extrahepatic bile duct. Hepatogastroenterology 2003;50:1625-7. [PubMed]
- Minagawa M, Makuuchi M, Takayama T, et al. Surgical approach to liver metastasis with hepatic hilar invasion. Hepatogastroenterology 2004;51:1467-9. [PubMed]
- Chedid AD, Chedid MF, Kruel CR, et al. Extended right hepatectomy with total caudate lobe resection and biliary tree resection for a large colorectal liver metastasis involving both the right and left hepatic lobes and the umbilical fissure: a case report. Am Surg 2005;71:447-9. [PubMed]
- Sugiura T, Nagino M, Oda K, et al. Hepatectomy for colorectal liver metastases with macroscopic intrabiliary tumor growth. World J Surg 2006;30:1902-8. [Crossref] [PubMed]
- Morelli L, Faraci R, Piscioli I, et al. Colon carcinoma metastasis to the intrapancreatic tract of the common biliary duct: a first case report. Scand J Gastroenterol 2007;42:777-8. [Crossref] [PubMed]
- Takamatsu S, Teramoto K, Kawamura T, et al. Liver metastasis from rectal cancer with prominent intrabile duct growth. Pathol Int 2004;54:440-5. [Crossref] [PubMed]
- Popescu I, Dumitrascu T. Curative-intent surgery for hilar cholangiocarcinoma: prognostic factors for clinical decision making. Langenbecks Arch Surg 2014;399:693-705. [Crossref] [PubMed]


