
Impact of sex difference on clinical outcomes in acute myocardial infarction patients with single-vessel and multi-vessel disease: based on Korea Acute Myocardial Infarction Registry-National Institute of Health
Highlight box
Key findings
• In Korean acute myocardial infarction (AMI) patients, women were older and had poor baseline clinical characteristics.
• Sex-related differences were noted in young AMI patients with AMI with single-vessel disease (SVD).
• There were no sex-related differences in clinical outcomes in patients with AMI with Multi-vessel disease (MVD).
What is known and what is new?
• There have been studies evaluating sex differences among Korean patients with AMI.
• This is the first study to evaluate the impact of sex differences on the clinical outcomes in Korean AMI patients with SVD and MVD.
What is the implication, and what should change now?
• Further specific and detailed prospective studies investigating sex differences in AMI are warranted.
Introduction
Despite remarkable advances in interventional cardiology and pharmacologic therapeutics, coronary artery disease (CAD) remains a leading cause of death in both men and women (1). Several studies have investigated sex-related differences in CAD (2-4). Generally, the incidence of CAD is relatively low in women, and CAD develops nearly 10 years later in women than in men (3,4). In addition, awareness regarding sex differences in the clinical characteristics, management, and mortality of acute myocardial infarction (AMI) has increased over the last several decades. Although there are conflicting results, several previous studies have reported that women with AMI have a poorer baseline risk profile, are less intensively treated, and have worse clinical outcomes (5,6).
Coronary angiographic studies have shown that nearly 50–60% of patients with AMI have multi-vessel disease (MVD). The clinical outcomes of MVD are unfavorable compared to those of single-vessel coronary artery disease (SVD) because patients with MVD tend to have more extensive atherosclerosis and a relatively high ischemic burden (7,8). The clinical impact of sex differences in patients with AMI with MVD has been evaluated in the Western population (9). Although there have been studies evaluating sex differences among Korean patients with AMI, studies evaluating sex differences in SVD and MVD in Korean patients with AMI are lacking (10-12).
Therefore, the main purpose of the present study was to analyze sex differences in the clinical characteristics of AMI patients with SVD and MVD. In addition, we aimed to evaluate the impact of sex differences on the clinical outcomes in patients with AMI with SVD and MVD. We present the following article in accordance with the STROBE reporting checklist (available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-536/rc).
Methods
Study population
A total of 13,903 patients with AMI enrolled in the Korea AMI-National Institute of Health (KAMIR-NIH) between November 2011 and June 2015 were selected. From these, the following patients were excluded: 1,988 patients with failure to achieve successful percutaneous coronary intervention (PCI), 570 patients with left main CAD, and 343 patients with follow-up loss or poor data quality. Finally, 11,002 patients were enrolled in the present study, of which 5,644 and 5,358 were diagnosed with SVD and MVD, respectively. Patients’ data were analyzed to identify sex differences in outcomes according to age (<65 and ≥65 years) (Figure S1).
All patients received a 300 mg loading dose of aspirin and 180 mg of ticagrelor, 600 mg of clopidogrel, or 60 mg prasugrel before diagnostic coronary angiography (CAG). CAG was performed through the radial or femoral artery. PCI was performed using standard techniques. Postoperatively, other medications including β-blockers, renin-angiotensin system inhibitors, and statins were prescribed, and two-dimensional echocardiography was performed to evaluate left ventricular ejection fraction.
Study definition and clinical outcomes
MVD was defined as >50% diameter stenosis in at least two major epicardial coronary arteries on quantitative CAG. The primary end point of this study was the cumulative incidence of major adverse cardiac events (MACEs) during 3 years. MACE was a composite of all-cause death, non-fatal myocardial infarction (MI), repeated PCI, and stroke. Non-fatal MI was defined as recurrent symptoms with new ST-segment elevation on electrocardiography or re-elevation of cardiac markers to at least twice the upper limit of the normal range. Repeated PCI was defined as PCI for a target lesion, target vessel, or non-target vessel (13).
Statistical analysis
The clinical characteristics of the treatment groups were analyzed. Continuous variables are presented as means ± standard deviations and compared using unpaired Student’s t-tests or Mann–Whitney U tests. Discrete variables are expressed as percentages and frequencies and were compared using chi-square or Fisher’s exact tests. Logistic regression analysis with propensity score matching was performed to minimize selection bias in the direct comparison between the groups. The variables included were age, previous chest pain, atypical chest pain, Killip class, ST-segment elevation MI (STEMI), risk factors including hypertension, diabetes mellitus, previous MI, previous PCI, atrial fibrillation, stroke and smoking, left ventricular ejection fraction, infarct-related artery (IRA), preprocedural thrombolysis in myocardial infarction (TIMI) flow grade in IRA, lesion classification, vascular access, three-vessel disease, PCI modalities, and medications. Men and women were matched 1:1 using the nearest neighbor matching method (14), and the clinical characteristics of the matched population were compared. The risk of each clinical endpoint in both matched groups was compared using the Cox proportional hazard regression model with the covariables that showed statistical significance (P<0.1) in the univariate analysis or were considered clinically important in the multivariable model. Hazard ratios (HRs) with 95% confidence intervals (CIs) were calculated.
All analyses were performed using SPSS for Windows, version 25.0 (Armonk, NY). All statistical tests were 2-tailed, and statistical significance was defined as P≤0.05.
Ethical statement
The KAMIR-NIH is a prospective, open, observational, on-line registry, multicenter cohort study that investigates real-world outcomes of Korean patients with AMI. Cases of AMI diagnosed at community and teaching hospitals with facilities for primary PCI and on-site cardiac surgery are registered online on www.kamir.or.kr since November 2005. Trained study coordinators at each participating institution collect data through face-to-face interviews, phone calls, or chart review. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The present study retrospectively evaluated data from the KAMIR-NIH database and the current study was approved by the ethics committee of Chonnam National University Hospital (No. CNUH-2022-341). Written informed consent was obtained from all participants.
Results
Clinical characteristics in SVD patients
Among patients with SVD aged <65 years, women were older than men (56.0±6.8 vs. 52.2±7.6 years, P<0.001). Women frequently complained of previous chest pain and presented with atypical angina symptoms. The proportion of patients classified as Killip class III–IV was higher and that of patients with STEMI was lower in women than in men. Moreover, risk factors such as hypertension, diabetes, and history of stroke were more frequently observed in women. The left anterior descending artery was the most common IRA, and the frequency of pre-TIMI flow grades of 0 or 1 was lower in women. Moreover, women were frequently treated with plain balloon angioplasty and thrombus aspiration (Table 1).
Table 1
| Characteristics | Total | Matched | |||||
|---|---|---|---|---|---|---|---|
| Female (n=340) | Male (n=2,897) | P value | Female (n=339) | Male (n=339) | P value | ||
| Age, years | 56.0±6.8 | 52.2±7.6 | <0.001 | 55.9±6.8 | 54.8±6.9 | 0.074 | |
| Symptom | |||||||
| Previous chest pain | 100 (29.4) | 707 (24.4) | 0.043 | 100 (29.5) | 104 (30.7) | 0.738 | |
| Atypical angina | 40 (11.8) | 204 (7.1) | 0.002 | 39 (11.5) | 47 (13.9) | 0.356 | |
| Killip class III, IV | 35 (10.3) | 187 (6.5) | 0.008 | 34 (10.0) | 31 (9.1) | 0.696 | |
| STEMI | 172 (50.6) | 1,700 (58.7) | 0.004 | 172 (50.7) | 188 (55.5) | 0.218 | |
| Risk factors | |||||||
| Hypertension | 154 (45.3) | 976 (33.7) | <0.001 | 153 (45.1) | 146 (43.1) | 0.588 | |
| Diabetes mellitus | 79 (23.2) | 505 (17.4) | 0.008 | 78 (23.0) | 74 (21.8) | 0.713 | |
| Previous MI | 15 (4.4) | 139 (4.8) | 0.752 | 15 (4.4) | 15 (4.4) | 1.000 | |
| Previous PCI | 26 (7.6) | 190 (6.6) | 0.447 | 26 (7.7) | 27 (8.0) | 0.886 | |
| Atrial fibrillation | 8 (2.4) | 87 (3.0) | 0.502 | 8 (2.4) | 9 (2.7) | 0.806 | |
| Stroke | 16 (4.7) | 76 (2.6) | 0.029 | 16 (4.7) | 16 (4.7) | 1.000 | |
| Smoking | 46 (13.5) | 1,893 (65.3) | <0.001 | 46 (13.6) | 50 (14.8) | 0.659 | |
| LVEF, % | 54.2±10.1 | 53.7±9.3 | 0.398 | 54.2±10.1 | 53.2±9.6 | 0.285 | |
| Infarct related artery | |||||||
| Left anterior descending | 202 (59.4) | 1,554 (53.6) | 0.043 | 201 (59.3) | 190 (56.0) | 0.393 | |
| Right coronary | 85 (25.0) | 862 (29.8) | 0.068 | 85 (25.1) | 95 (28.0) | 0.487 | |
| Left circumflex | 53 (15.6) | 481 (16.6) | 0.633 | 53 (15.6) | 54 (15.9) | 0.924 | |
| Pre-TIMI flow grade 0 or 1 | 192 (56.5) | 1,833 (63.3) | 0.014 | 191 (56.3) | 201 (59.3) | 0.418 | |
| B2/C lesion | 280 (82.4) | 2,472 (85.3) | 0.146 | 279 (82.3) | 285 (84.1) | 0.538 | |
| Trans-radial approach | 136 (40.0) | 1,167 (40.3) | 0.920 | 136 (40.1) | 133 (39.2) | 0.814 | |
| IRA PCI modality | |||||||
| Balloon angioplasty | 33 (9.7) | 190 (6.6) | 0.030 | 33 (9.7) | 27 (8.0) | 0.417 | |
| BMS | 4 (1.2) | 79 (2.7) | 0.087 | 4 (1.2) | 3 (0.9) | 0.704 | |
| 1st generation DES | 13 (3.8) | 94 (3.2) | 0.572 | 13 (3.8) | 11 (3.2) | 0.678 | |
| 2nd generation DES | 290 (85.3) | 2,534 (87.5) | 0.255 | 289 (85.3) | 298 (87.9) | 0.311 | |
| Thrombus aspiration | 78 (22.9) | 931 (32.1) | 0.001 | 78 (23.0) | 90 (26.5) | 0.278 | |
| Medications | |||||||
| Aspirin | 338 (99.4) | 2,869 (99.0) | 0.491 | 337 (99.4) | 337 (99.4) | 1.000 | |
| Clopidogrel | 226 (66.5) | 1,761 (60.8) | 0.042 | 225 (66.4) | 215 (63.4) | 0.421 | |
| Prasugrel | 44 (12.9) | 490 (16.9) | 0.062 | 44 (13.0) | 56 (16.5) | 0.194 | |
| Ticagrelor | 64 (18.8) | 600 (20.7) | 0.415 | 64 (18.9) | 61 (18.0) | 0.766 | |
| β-blocker | 297 (87.4) | 2,544 (87.8) | 0.806 | 296 (87.3) | 296 (87.6) | 1.000 | |
| RAS inhibitors | 280 (82.4) | 2,417 (83.4) | 0.614 | 279 (82.3) | 282 (83.2) | 0.760 | |
| Statin | 318 (93.5) | 2,751 (95.0) | 0.260 | 317 (93.5) | 319 (94.1) | 0.750 | |
Values are presented as the n (%) or mean ± SD. SVD, single-vessel disease; STEMI, ST segment elevation myocardial infarction; MI, myocardial infarction; PCI, percutaneous coronary intervention; LVEF, left ventricular ejection fraction; IRA, infarct related artery; TIMI, thrombolysis in myocardial infarction; BMS, bare metal stent; DES, drug eluting stent; RAS, renin angiotensin system.
Among patients with SVD aged ≥65 years, women were older than men (75.7±6.1 vs. 72.9±5.8 years, P<0.001). The proportion of patients with STEMI was lower in women than in men. Among cardiovascular risk factors, hypertension was more frequently observed in women than in men. The left anterior descending artery was the most common IRA, and patients were frequently treated with plain balloon angioplasty alone (Table 2). After propensity score matching, the clinical characteristics were comparable between women and men and between both age groups.
Table 2
| Characteristics | Total | Matched | |||||
|---|---|---|---|---|---|---|---|
| Female (n=978) | Male (n=1,429) | P value | Female (n=844) | Male (n=844) | P value | ||
| Age, year | 75.7±6.1 | 72.9±5.8 | <0.001 | 74.8±5.9 | 74.3±6.0 | 0.095 | |
| Symptom | |||||||
| Previous chest pain | 257 (26.3) | 344 (24.1) | 0.220 | 218 (25.8) | 201 (23.8) | 0.338 | |
| Atypical angina | 147 (15.0) | 211 (14.8) | 0.858 | 128 (15.2) | 126 (14.9) | 0.892 | |
| Killip class III, IV | 141 (14.4) | 183 (12.8) | 0.255 | 122 (14.5) | 114 (13.5) | 0.574 | |
| STEMI | 480 (49.1) | 796 (55.7) | 0.001 | 429 (50.8) | 444 (52.6) | 0.465 | |
| Risk factors | |||||||
| Hypertension | 654 (66.9) | 758 (53.0) | <0.001 | 554 (65.6) | 525 (62.2) | 0.149 | |
| Diabetes mellitus | 301 (30.8) | 389 (27.2) | 0.058 | 260 (30.8) | 243 (28.8) | 0.366 | |
| Previous MI | 71 (7.3) | 124 (8.7) | 0.211 | 60 (7.1) | 69 (8.2) | 0.410 | |
| Previous PCI | 110 (11.2) | 194 (13.6) | 0.091 | 100 (11.8) | 110 (13.0) | 0.461 | |
| Atrial fibrillation | 58 (5.9) | 115 (8.0) | 0.048 | 55 (6.5) | 63 (7.5) | 0.445 | |
| Stroke | 75 (7.7) | 118 (8.3) | 0.601 | 70 (8.3) | 78 (9.2) | 0.491 | |
| Smoking | 51 (5.2) | 448 (31.4) | <0.001 | 51 (6.0) | 61 (7.2) | 0.328 | |
| LVEF, % | 51.3±10.7 | 51.9±10.9 | 0.241 | 51.4±10.4 | 51.5±10.8 | 0.511 | |
| Infarct related artery | |||||||
| Left anterior descending | 550 (56.2) | 722 (50.5) | 0.006 | 472 (55.9) | 458 (54.3) | 0.493 | |
| Right coronary | 266 (27.2) | 503 (35.2) | <0.001 | 242 (28.7) | 265 (31.4) | 0.222 | |
| Left circumflex | 162 (16.6) | 204 (14.3) | 0.125 | 130 (15.4) | 121 (14.3) | 0.538 | |
| Pre-TIMI flow grade 0 or 1 | 578 (59.1) | 817 (57.2) | 0.347 | 487 (57.7) | 492 (58.3) | 0.805 | |
| B2/C lesion | 834 (85.3) | 1,231 (86.1) | 0.549 | 710 (84.1) | 725 (85.9) | 0.306 | |
| Trans-radial approach | 324 (35.1) | 532 (37.2) | 0.039 | 287 (34.0) | 293 (34.7) | 0.758 | |
| IRA PCI modality | |||||||
| Balloon angioplasty | 99 (10.1) | 98 (6.9) | 0.004 | 74 (8.8) | 69 (8.2) | 0.662 | |
| BMS | 52 (5.3) | 73 (5.1) | 0.821 | 45 (5.3) | 39 (4.6) | 0.502 | |
| 1st generation DES | 16 (1.6) | 39 (2.7) | 0.078 | 16 (1.9) | 22 (2.6) | 0.325 | |
| 2nd generation DES | 811 (82.9) | 1,219 (85.3) | 0.115 | 709 (84.0) | 714 (84.6) | 0.738 | |
| Thrombus aspiration | 217 (22.2) | 367 (25.7) | 0.051 | 194 (23.0) | 201 (23.8) | 0.687 | |
| Discharge medications | |||||||
| Aspirin | 951 (97.2) | 1,396 (97.7) | 0.485 | 823 (97.5) | 819 (97.0) | 0.550 | |
| Clopidogrel | 757 (77.4) | 1,004 (70.3) | <0.001 | 641 (75.9) | 625 (74.1) | 0.368 | |
| Prasugrel | 33 (3.4) | 116 (8.1) | <0.001 | 33 (3.9) | 36 (4.3) | 0.712 | |
| Ticagrelor | 157 (16.1) | 270 (18.9) | 0.073 | 145 (17.2) | 157 (18.6) | 0.446 | |
| β-blocker | 827 (84.6) | 1,108 (77.5) | <0.001 | 706 (83.6) | 697 (82.5) | 0.586 | |
| RAS inhibitors | 767 (78.4) | 1,097 (76.8) | 0.339 | 662 (78.4) | 649 (76.9) | 0.447 | |
| Statin | 884 (90.4) | 1,305 (91.3) | 0.433 | 766 (90.8) | 760 (90.0) | 0.620 | |
Values are presented as the n (%) or mean ± SD. SVD, single-vessel disease; STEMI, ST segment elevation myocardial infarction; MI, myocardial infarction; PCI, percutaneous coronary intervention; LVEF, left ventricular ejection fraction; IRA, infarct related artery; TIMI, thrombolysis in myocardial infarction; BMS, bare metal stent; DES, drug eluting stent; RAS, renin angiotensin system.
Clinical characteristics in MVD patients
Among patients with MVD aged <65 years, women were older than men (57.9±5.7 vs. 53.8±6.9 years, P<0.001). The proportion of patients classified as Killip III–IV was higher, and that of patients with STEMI was lower in women than in men. Among the cardiovascular risk factors, hypertension and diabetes were more frequently observed in women. Comparison of IRA treatment modalities showed that thrombus aspiration was less frequently performed in women (Table 3).
Table 3
| Characteristics | Total | Matched | |||||
|---|---|---|---|---|---|---|---|
| Female (n=247) | Male (n=2,221) | P value | Female (n=243) | Male (n=243) | P value | ||
| Age, year | 57.9±5.7 | 53.8±6.9 | <0.001 | 57.8±5.7 | 56.5±7.2 | 0.061 | |
| Symptom | |||||||
| Previous chest pain | 68 (27.5) | 570 (25.7) | 0.525 | 66 (27.2) | 67 (27.6) | 0.919 | |
| Atypical angina | 28 (11.3) | 210 (9.5) | 0.342 | 28 (11.5) | 29 (11.9) | 0.888 | |
| Killip class III, IV | 34 (13.8) | 214 (9.6) | 0.041 | 32 (13.2) | 34 (14.0) | 0.791 | |
| STEMI | 103 (41.7) | 1,199 (54.0) | <0.001 | 103 (42.4) | 109 (44.9) | 0.583 | |
| Risk factors | |||||||
| Hypertension | 138 (55.9) | 936 (42.1) | <0.001 | 134 (55.1) | 119 (49.0) | 0.173 | |
| Diabetes mellitus | 113 (45.7) | 597 (26.9) | <0.001 | 110 (45.3) | 102 (42.0) | 0.464 | |
| Previous MI | 15 (6.1) | 158 (7.1) | 0.543 | 15 (6.2) | 16 (6.6) | 0.853 | |
| Previous PCI | 25 (10.1) | 200 (9.0) | 0.563 | 24 (9.9) | 23 (9.5) | 0.878 | |
| Atrial fibrillation | 3 (1.2) | 57 (2.6) | 0.191 | 3 (1.2) | 3 (1.2) | 1.000 | |
| Stroke | 16 (6.5) | 86 (3.9) | 0.051 | 16 (6.6) | 14 (5.8) | 0.706 | |
| Smoking | 32 (13.0) | 1,403 (63.2) | <0.001 | 32 (13.2) | 42 (17.3) | 0.207 | |
| LVEF, % | 51.4±11.9 | 52.5±10.4 | 0.113 | 51.7±11.5 | 51.9±10.3 | 0.854 | |
| Infarct related artery | |||||||
| Left anterior descending | 119 (48.2) | 927 (41.7) | 0.052 | 116 (47.7) | 107 (44.0) | 0.413 | |
| Right coronary | 90 (36.4) | 840 (37.8) | 0.670 | 89 (36.6) | 89 (36.6) | 1.000 | |
| Left circumflex | 38 (15.4) | 454 (20.4) | 0.059 | 38 (15.6) | 47 (19.3) | 0.283 | |
| Pre-TIMI flow grade 0 or 1 | 141 (57.1) | 1,345 (60.6) | 0.290 | 138 (56.8) | 142 (58.4) | 0.713 | |
| B2/C lesion | 220 (89.1) | 1,945 (87.6) | 0.497 | 216 (88.9) | 209 (86.0) | 0.338 | |
| Trans-radial approach | 91 (36.8) | 908 (40.9) | 0.220 | 90 (37.0) | 95 (39.1) | 0.640 | |
| Three-vessel disease | 78 (31.6) | 755 (34.0) | 0.446 | 77 (31.7) | 81 (33.3) | 0.698 | |
| Culprit only PCI | 110 (44.5) | 1,102 (49.6) | 0.130 | 110 (45.3) | 120 (49.4) | 0.364 | |
| IRA PCI modality | |||||||
| Balloon angioplasty | 18 (7.3) | 129 (5.8) | 0.351 | 16 (6.6) | 13 (5.3) | 0.566 | |
| BMS | 1 (0.4) | 23 (1.0) | 0.338 | 1 (0.4) | 3 (1.2) | 0.315 | |
| 1st generation DES | 10 (4.0) | 111 (5.0) | 0.512 | 10 (4.1) | 8 (3.3) | 0.631 | |
| 2nd generation DES | 218 (88.3) | 1,958 (88.2) | 0.963 | 216 (88.9) | 219 (90.1) | 0.657 | |
| Thrombus aspiration | 44 (17.8) | 573 (25.8) | 0.006 | 44 (18.1) | 56 (23.0) | 0.178 | |
| Medications | |||||||
| Aspirin | 242 (98.0) | 2,188 (98.5) | 0.514 | 238 (97.9) | 241 (99.2) | 0.253 | |
| Clopidogrel | 177 (71.7) | 1,337 (60.2) | <0.001 | 173 (71.2) | 161 (66.3) | 0.240 | |
| Prasugrel | 17 (6.9) | 335 (15.1) | <0.001 | 17 (7.0) | 29 (11.9) | 0.063 | |
| Ticagrelor | 48 (19.4) | 513 (23.1) | 0.192 | 48 (19.8) | 49 (20.2) | 0.910 | |
| β-blocker | 211 (85.4) | 1,963 (88.4) | 0.173 | 209 (86.0) | 204 (84.0) | 0.526 | |
| RAS inhibitors | 198 (80.2) | 1,799 (81.0) | 0.751 | 195 (80.2) | 196 (80.7) | 0.909 | |
| Statin | 229 (92.7) | 2,112 (95.1) | 0.108 | 226 (93.0) | 228 (93.8) | 0.715 | |
Values are presented as the n (%) or mean ± SD. MVD, multi-vessel disease; STEMI, ST segment elevation myocardial infarction; MI, myocardial infarction; PCI, percutaneous coronary intervention; LVEF, left ventricular ejection fraction; IRA, infarct related artery; TIMI, thrombolysis in myocardial infarction; BMS, bare metal stent; DES, drug eluting stent; RAS, renin angiotensin system.
Among patients with MVD aged ≥65 years, women were older than men (76.7±6.3 vs. 73.3±5.9 years, P<0.001). Women frequently reported previous chest pain and complained of atypical angina symptoms. The proportion of patients classified as Killip III–IV was higher and that of patients with STEMI was lower in women than that in men. Risk factors such as hypertension and diabetes were more frequent in women than in men. Three-vessel disease was more frequently observed in women than in men (Table 4). After propensity score matching, the clinical characteristics were comparable between women and men and between both age groups.
Table 4
| Characteristics | Total | Matched | |||||
|---|---|---|---|---|---|---|---|
| Female (n=1,200) | Male (n=1,690) | P value | Female (n=741) | Male (n=741) | P value | ||
| Age, year | 76.7±6.3 | 73.3±5.9 | <0.001 | 75.7±5.8 | 74.8±6.1 | 0.062 | |
| Symptom | |||||||
| Previous chest pain | 342 (28.5) | 421 (24.9) | 0.031 | 293 (27.6) | 281 (26.5) | 0.558 | |
| Atypical angina | 244 (20.3) | 281 (16.6) | 0.011 | 194 (18.3) | 188 (17.7) | 0.735 | |
| Killip class III, IV | 236 (19.7) | 288 (17.0) | 0.071 | 201 (19.0) | 190 (17.9) | 0.538 | |
| STEMI | 521 (43.4) | 785 (46.4) | 0.106 | 465 (43.9) | 466 (44.0) | 0.965 | |
| Risk factors | |||||||
| Hypertension | 899 (74.9) | 997 (59.0) | <0.001 | 765 (72.2) | 724 (68.3) | 0.052 | |
| Diabetes mellitus | 472 (39.3) | 581 (34.3) | 0.006 | 410 (38.7) | 401 (37.8) | 0.688 | |
| Previous MI | 72 (6.0) | 158 (9.3) | 0.001 | 67 (6.3) | 77 (7.3) | 0.388 | |
| Previous PCI | 119 (9.9) | 233 (13.8) | 0.002 | 118 (11.1) | 128 (12.1) | 0.498 | |
| Atrial fibrillation | 81 (6.8) | 122 (7.2) | 0.627 | 72 (6.8) | 74 (7.0) | 0.864 | |
| Stroke | 131 (10.9) | 155 (9.2) | 0.122 | 115 (10.8) | 120 (11.3) | 0.729 | |
| Smoking | 77 (6.4) | 514 (30.4) | <0.001 | 77 (7.3) | 92 (8.7) | 0.229 | |
| LVEF, % | 50.6±11.4 | 49.8±11.1 | 0.085 | 50.6±11.3 | 50.1±11.1 | 0.455 | |
| Infarct related artery | |||||||
| Left anterior descending | 498 (41.5) | 697 (41.2) | 0.890 | 438 (41.3) | 438 (41.3) | 1.000 | |
| Right coronary | 456 (38.0) | 658 (38.9) | 0.611 | 401 (37.8) | 412 (38.9) | 0.623 | |
| Left circumflex | 246 (20.5) | 335 (19.8) | 0.654 | 221 (20.8) | 210 (19.8) | 0.553 | |
| Pre-TIMI flow grade 0 or 1 | 648 (54.0) | 912 (54.0) | 0.985 | 572 (54.0) | 572 (54.0) | 1.000 | |
| B2/C lesion | 1,050 (87.5) | 1,497 (88.6) | 0.376 | 927 (87.5) | 935 (88.2) | 0.595 | |
| Trans-radial approach | 421 (35.1) | 645 (38.2) | 0.091 | 384 (36.2) | 412 (38.9) | 0.209 | |
| Three-vessel disease | 500 (41.7) | 638 (37.8) | 0.034 | 432 (40.8) | 411 (38.8) | 0.351 | |
| Culprit only PCI | 640 (53.3) | 926 (54.8) | 0.438 | 564 (53.2) | 555 (52.4) | 0.695 | |
| IRA PCI modality | |||||||
| Balloon angioplasty | 84 (7.0) | 112 (6.6) | 0.695 | 73 (6.9) | 80 (7.5) | 0.557 | |
| BMS | 50 (4.2) | 52 (3.1) | 0.118 | 39 (3.7) | 34 (3.2) | 0.551 | |
| 1st generation DES | 35 (2.9) | 50 (3.0) | 0.948 | 34 (3.2) | 28 (2.6) | 0.438 | |
| 2nd generation DES | 1,031 (85.9) | 1,476 (87.3) | 0.267 | 914 (86.2) | 918 (86.6) | 0.800 | |
| Thrombus aspiration | 232 (19.3) | 348 (20.6) | 0.405 | 210 (19.8) | 215 (20.3) | 0.786 | |
| Discharge medications | |||||||
| Aspirin | 1,162 (96.8) | 1,640 (97.0) | 0.748 | 1,025 (96.7) | 1,024 (96.6) | 0.904 | |
| Clopidogrel | 934 (77.8) | 1,245 (73.7) | 0.010 | 813 (76.7) | 804 (75.8) | 0.646 | |
| Prasugrel | 29 (2.4) | 84 (5.0) | <0.001 | 29 (2.7) | 35 (3.3) | 0.446 | |
| Ticagrelor | 196 (16.3) | 308 (18.2) | 0.187 | 180 (17.0) | 181 (17.1) | 0.954 | |
| β-blocker | 964 (80.3) | 1,364 (80.7) | 0.801 | 851 (80.3) | 846 (79.8) | 0.786 | |
| RAS inhibitors | 943 (78.6) | 1,323 (78.3) | 0.847 | 837 (79.0) | 823 (77.6) | 0.461 | |
| Statin | 1,070 (89.2) | 1,521 (90.0) | 0.469 | 954 (90.0) | 950 (89.6) | 0.774 | |
Values are presented as the n (%) or mean ± SD. MVD, multi-vessel disease; STEMI, ST segment elevation myocardial infarction; MI, myocardial infarction; PCI, percutaneous coronary intervention; LVEF, left ventricular ejection fraction; IRA, infarct related artery; TIMI, thrombolysis in myocardial infarction; BMS, bare metal stent; DES, drug eluting stent; RAS, renin angiotensin system.
Clinical outcomes
Among patients with SVD aged <65 years, the cumulative incidence and 3-year risk of MACE in the crude population was higher in women than in men (15.0% vs. 8.4%; HR, 1.46; 95% CI, 1.07–1.99; P=0.017). Moreover, the incidence of stroke was higher in women than in men (2.1% vs. 0.6%; HR, 3.09; 95% CI, 1.23–7.76; P=0.016). The incidence and 3-year risk of MACE (15.0% vs. 9.4%; HR, 1.86; 95% CI, 1.10–3.13; P=0.020) and stroke (2.1% vs. 0.6%; HR, 6.85; 95% CI, 1.16–12.3; P=0.034) in the matched population were also significantly higher in women than in men.
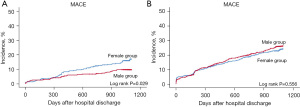
The 3-year risk of MACE in patients with SVD aged ≥65 years was similar for men and women (21.6% vs. 20.9%; HR, 0.90; 95% CI, 0.75–1.08; P=0.258). However, the incidence of stroke events was significantly higher in women than in men (3.2% vs. 1.4%; HR, 2.49; 95% CI, 1.38–4.53; P=0.003). In the matched population, the 3-year risk and incidence of MACE and other individual clinical events were comparable between men and women (Table 5) (Figure 1).
Table 5
| Clinical outcomes | Total | Matched | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Female | Male | Adjusted HR (95% CI) | P value | Female | Male | Adjusted HR (95% CI) | P value | ||
| <65 years old | |||||||||
| n | 340 | 2,897 | 339 | 339 | |||||
| MACE† | 51 (15.0) | 242 (8.4) | 1.46 (1.07–1.99) | 0.017 | 51 (15.0) | 32 (9.4) | 1.86 (1.10–3.13) | 0.020 | |
| All-cause death | 10 (2.9) | 71 (2.5) | 0.71 (0.35–1.45) | 0.348 | 10 (2.9) | 9 (2.7) | 2.10 (0.66–6.64) | 0.209 | |
| Cardiac death | 6 (1.8) | 49 (1.7) | 0.57 (0.22–1.47) | 0.243 | 6 (1.8) | 5 (1.5) | 4.71 (0.81–27.5) | 0.085 | |
| Non-fatal MI | 10 (2.9) | 42 (1.4) | 1.79 (0.88–3.63) | 0.107 | 10 (2.9) | 4 (1.2) | 1.78 (0.44–7.18) | 0.420 | |
| Any repeated PCI | 29 (8.5) | 152 (5.2) | 1.48 (0.99–2.23) | 0.058 | 29 (8.6) | 19 (5.6) | 1.56 (0.79–3.09) | 0.201 | |
| Stroke | 7 (2.1) | 17 (0.6) | 3.09 (1.23–7.76) | 0.016 | 7 (2.1) | 2 (0.6) | 6.85 (1.16–12.3) | 0.034 | |
| ≥65 years old | |||||||||
| n | 978 | 1,429 | 844 | 844 | |||||
| MACE† | 211 (21.6) | 298 (20.9) | 0.90 (0.75–1.08) | 0.258 | 173 (20.5) | 181 (21.4) | 0.93 (0.76–1.15) | 0.524 | |
| All-cause death | 147 (15.0) | 202 (14.1) | 0.98 (0.71–1.13) | 0.309 | 117 (13.9) | 123 (14.6) | 0.98 (0.76–1.26) | 0.858 | |
| Cardiac death | 113 (11.6) | 139 (9.7) | 0.99 (0.77–1.29) | 0.955 | 84 (10.0) | 86 (10.2) | 1.01 (0.74–1.37) | 0.973 | |
| Non-fatal MI | 23 (2.4) | 35 (2.4) | 0.86 (0.47–1.43) | 0.821 | 19 (2.3) | 21 (2.5) | 0.86 (0.46–1.61) | 0.643 | |
| Any repeated PCI | 40 (4.1) | 85 (5.9) | 0.68 (0.46–1.01) | 0.052 | 36 (4.3) | 48 (5.7) | 0.73 (0.47–1.13) | 0.155 | |
| Stroke | 31 (3.2) | 20 (1.4) | 2.49 (1.38–4.53) | 0.003 | 27 (3.2) | 14 (1.7) | 1.87 (0.97–3.57) | 0.061 | |
Values are presented as the n (%). †, composite of all-cause death, non-fatal MI, any repeated PCI, stroke. SVD, single-vessel disease; HR, hazard ratio; CI, confidence interval; MACE, major adverse cardiac event; MI, myocardial infarction; PCI, percutaneous coronary intervention.
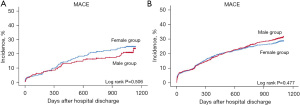
In patients with MVD aged <65 years, the cumulative 3-year incidence of MACE in the crude population was 21.1% and 17.6% for women and men, respectively (HR, 1.09; 95% CI, 0.80–1.47; P=0.596). The incidence of individual clinical events was also similar between the groups. Multivariable analysis after propensity score matching showed that the 3-year risk of MACE was similar in both groups (21.0% vs. 18.5%; HR, 1.12; 95% CI, 0.75–1.69; P=0.575).
Regarding clinical outcomes of patients with MVD aged ≥65 years, the cumulative 3-year incidence of MACE in the crude population was 28.1% and 28.2% in women and men, respectively (HR, 0.95; 95% CI, 0.82–1.10; P=0.452), and the incidence of individual clinical events was also comparable. Multivariable analysis after propensity score matching showed that the 3-year incidence and risk of MACE were similar in both groups (26.3% vs. 29.5%; HR, 0.93; 95% CI, 0.79–1.10; P=0.404) (Table 6, Figure 2).
Table 6
| Clinical outcomes | Total | Matched | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Female | Male | Adjusted HR (95% CI) | P value | Female | Male | Adjusted HR (95% CI) | P value | ||
| <65 years old | |||||||||
| n | 247 | 2,221 | 243 | 243 | |||||
| MACE† | 52 (21.1) | 391 (17.6) | 1.09 (0.80–1.47) | 0.596 | 51 (21.0) | 45 (18.5) | 1.12 (0.75–1.69) | 0.575 | |
| All-cause death | 18 (7.3) | 81 (3.6) | 1.17 (0.67–2.04) | 0.578 | 17 (7.0) | 10 (4.1) | 1.37 (0.58–3.22) | 0.472 | |
| Cardiac death | 13 (5.3) | 68 (3.1) | 1.01 (0.53–1.92) | 0.971 | 12 (4.9) | 9 (3.7) | 1.04 (0.34–2.57) | 0.900 | |
| Non-fatal MI | 7 (2.8) | 59 (2.7) | 0.93 (0.41–2.07) | 0.850 | 7 (2.9) | 7 (2.9) | 0.96 (0.32–2.94) | 0.948 | |
| Any repeated PCI | 34 (13.8) | 278 (12.5) | 1.15 (0.79–1.66) | 0.457 | 34 (14.0) | 32 (13.2) | 1.08 (0.67–1.77) | 0.747 | |
| Stroke | 2 (0.8) | 29 (1.3) | 0.35 (0.08–1.52) | 0.162 | 2 (0.8) | 6 (2.5) | 0.19 (0.04–1.10) | 0.064 | |
| ≥65 years old | |||||||||
| n | 1,200 | 1,690 | 1,060 | 1,060 | |||||
| MACE† | 338 (28.1) | 477 (28.2) | 0.95 (0.82–1.10) | 0.452 | 279 (26.3) | 313 (29.5) | 0.93 (0.79–1.10) | 0.404 | |
| All-cause death | 205 (17.1) | 273 (16.2) | 0.92 (0.76–1.11) | 0.387 | 162 (15.3) | 185 (17.5) | 0.94 (0.76–1.16) | 0.543 | |
| Cardiac death | 154 (12.8) | 191 (11.3) | 0.96 (0.76–1.20) | 0.701 | 118 (11.1) | 128 (12.1) | 1.01 (0.78–1.29) | 0.964 | |
| Non-fatal MI | 38 (4.2) | 54 (3.2) | 0.87 (0.56–1.34) | 0.523 | 32 (3.0) | 35 (3.3) | 0.90 (0.55–1.46) | 0.662 | |
| Any repeated PCI | 112 (9.3) | 184 (10.9) | 0.97 (0.76–1.24) | 0.807 | 104 (9.8) | 119 (11.2) | 0.91 (0.69–1.18) | 0.468 | |
| Stroke | 33 (2.8) | 41 (2.4) | 1.12 (0.69–1.82) | 0.643 | 26 (2.5) | 21 (2.0) | 1.25 (0.70–2.23) | 0.452 | |
Values are presented as n (%). †, composite of all-cause death, non-fatal MI, any repeated PCI, stroke. MVD, multi-vessel disease; HR, hazard ratio; CI, confidence interval; MACE, major adverse cardiac event; MI, myocardial infarction; PCI, percutaneous coronary intervention.
Independent predictors for MACE
Table S1 lists the independent predictors for MACE in patients with AMI with SVD and MVD. Patients with AMI aged ≥65 years had more statistically significant clinical predictors for MACE compared patients with AMI aged <65 years. For instance, medical treatment with statins, β-blockers, and renin-angiotensin system inhibitors and use of second-generation drug-eluting stents in the IRA and in patients with Killip class III and IV were independent predictors for MACE in patients with SVD or MVD aged ≥65 years. Female sex was an independent clinical predictor for 3-year MACE in patients with SVD aged <65 years (HR, 1.86; 95% CI, 1.10–3.13; P=0.020).
Discussion
The principal findings from this study based on the KAMIR-NIH registry are as follows: (I) baseline clinical characteristics were different between men and women. Compared with men, women were older, commonly presented with Killip class III or IV, and showed a lower incidence of STEMI. Generally, women had more cardiovascular risk factors, including hypertension and diabetes, but their past history of cigarette smoking was significantly lower than that of men. (II) Among young (<65 years) patients with SVD, women had a higher incidence and 3-year risk of MACE and stroke. (III) However, there were no sex-related differences in clinical outcomes in patients with AMI with MVD or SVD aged ≥65 years.
Many studies have compared the clinical characteristics and outcomes of patients with AMI based on sex differences over the last several decades (5-12). Although there is a paucity of data, numerous studies have reported sex differences in clinical, angiographic, and procedural factors, and clinical outcomes among patients with AMI (15-17). Women tend to be under-treated with both revascularization strategies and medical treatment compared with men (18,19). In a global case–control study, Anand et al. (20) demonstrated that women experience their first AMI event on average nine years later than men, and older age led to a higher proportion of women suffering from comorbidities such as hypertension. Among the 27,098 study participants, the median age at the first AMI event was 65 years in women and 56 years in men and women were significantly more likely to have hypertension than men (28.3% vs. 19.7%). The delay in the occurrence of AMI could be explained by the protective effect of estrogen until menopause (21,22). Matthews et al. (22) evaluated the high-density lipoprotein cholesterol and low-density lipoprotein cholesterol levels in premenopausal women and women with menopause 2.5 years earlier. In women who had a natural menopause and did not receive hormone-replacement therapy, serum levels of high-density lipoprotein cholesterol were lower, and levels of low-density lipoprotein cholesterol were higher than those in premenopausal controls (−0.09 vs. 0.00 mmol/L, P=0.01; 0.31 vs. 0.14 mmol/L, P=0.04, respectively). Thus, loss of estrogen could lead to unfavorable lipid metabolism, which may contribute to an increased risk of AMI in later life in women (22). The findings in the present study were similar to those of previous studies (15-20). Moreover, women were older and had a more frequent history of hypertension compared with men in the SVD and MVD groups and in both age groups.
In this study, women with SVD had a significantly higher incidence and risk of MACE in the <65 years group; however, clinical characteristics were similar in women with SVD aged ≥65 years. Vaccarino et al. (23) first reported that in younger patients with AMI, early mortality was higher in women than in men. Based on a nationwide prospective multicenter registry in the United States, the overall mortality during hospitalization was 16.7% among women and 11.5% among men. The mortality for women was more than twice that for men in patients aged <50 years; the difference in mortality decreased with increasing age and was not significant after the age of 74 years. Khera et al. (24) reported that among patients with STEMI aged <60 years in the US, the risk of risk-adjusted in-hospital mortality and longer hospital stay was greater in women than in men. In addition, a multicenter prospective cohort study by Vaccarino et al. (25) demonstrated that the overall 2-year mortality was higher in women (28.9%) than in men (19.6%), and after adjusting demographic confounders, women aged <60 years had a higher mortality than men of a similar age.
Although many questions remain unanswered, the potential reasons for poor clinical outcomes in young women with AMI could be explained by several pathophysiological and psychosocial factors (26-28). According to the Variation in Recovery: Role of Gender on Outcomes of Young Acute Myocardial Infarction Patients (VIRGO) study (27), women were more likely to exceed symptom to medical contact time and door-to-needle time than men. Furthermore, some experts have assumed that younger women have a lower burden of CAD than men, which gives a lower chance of myocardial ischemic preconditioning, leading to a vulnerable condition in AMI (29). Concurrently, the VIRGO study (28) showed that women exhibited higher levels of depression and stress, poorer physical and mental health status, and lower quality of life, leading to poor clinical outcomes. For instance, young women in the VIRGO study were significantly more likely to be divorced or separated than men. In addition, they were significantly more likely to be unemployed and have lower household incomes. Socioeconomic strain can lead to psychological risks such as depression, and a previous study have demonstrated that depression could increase a woman’s risk of cardiovascular disease and atherosclerosis due to low-grade inflammation (30).
In the current study, the 3-year incidence of MACE was comparable between men and women with MVD. These findings are consistent with those of several previous studies (31-33). A meta-analysis by Berger et al. (31) revealed that 30-day mortality among women and men was largely explained by baseline angiographic severity, and MVD could be regarded as a severe angiographic disease compared with SVD. The 30-day mortality was significantly higher in women with STEMI; however, no significant interaction was detected between STEMI and angiographic severity in the adjusted model. The Providing Regional Observations to Study Predictors of Events in the Coronary Tree (PROSPECT) trial (34) showed that women had fewer and more focal non-culprit lesions and necrotic cores and significantly less plaque rupture compared with men. However, despite the fact that women had less extensive coronary atherosclerosis, cardiovascular events including cardiac death and MI during 3 years of follow-up were similar in both groups. The PROSPECT sub-study (34) concluded that women had other high-risk plaque characteristics, including less calcium concentration and a smaller minimal lumen area, compared with men.
Limitations
This study had some limitations. First, this was a retrospective analysis and not a randomized clinical trial. The study was based on registry data; consequently, there could have been a selection bias. Although propensity score matching was performed, and most potential confounders were adjusted prior to analysis, other variables that could influence the clinical outcomes might have not been included. Second, the current study population lacked laboratory data, including cardiac biomarker levels or renal function test findings, due to a significant amount of missing data. Such laboratory data could have a significant impact on the results of the current study. Third, we did not evaluate socioeconomic factors or medical compliance data, which might show great disparities between the groups. Fourth, the interpretation of the current study results has potential for errors due to multiple testing based on sex and age differences. Although there have been previous studies on sex differences in different age groups, further analysis for multiple testing correction was not performed in this study. Also, external validation was not done. Therefore, to clarify the generalizability of the current data result, external validation is needed based on other large scale clinical patient data.
Conclusions
Women were older and had a higher prevalence of comorbidities than men in Korean AMI patients. Further, women with SVD aged <65 years had a significantly higher risk of MACE after adjusting for other clinical confounders. However, the risk of MACE was comparable in older patients with SVD and in patients with MVD. Further specific and detailed prospective studies investigating sex differences in AMI are warranted.
Acknowledgments
Funding: This research was supported by Research of Korea Centers for Disease Control and Prevention(No. 2016-ER6304-02), National Research Foundation of Korea (Nos. 2019R1A2C3003547, and 2019R1A4A1028534), and Ministry of Health & Welfare, Republic of Korea (No. HI18C1352).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-536/rc
Data Sharing Statement: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-536/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-536/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional ethics committee of Chonnam National University Hospital (No. CNUH-2022-341). Written informed consent was obtained from all participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation 2015;131:e29-322. [Crossref] [PubMed]
- Sytkowski PA, D'Agostino RB, Belanger A, et al. Sex and time trends in cardiovascular disease incidence and mortality: the Framingham Heart Study, 1950-1989. Am J Epidemiol 1996;143:338-50. [Crossref] [PubMed]
- Lawlor DA, Ebrahim S, Davey Smith G. Sex matters: secular and geographical trends in sex differences in coronary heart disease mortality. BMJ 2001;323:541-5. Erratum in: BMJ 2002;325:580. [Crossref] [PubMed]
- Bellasi A, Raggi P, Merz CN, et al. New insights into ischemic heart disease in women. Cleve Clin J Med 2007;74:585-94. [Crossref] [PubMed]
- Steingart RM, Packer M, Hamm P, et al. Sex differences in the management of coronary artery disease. Survival and Ventricular Enlargement Investigators. N Engl J Med 1991;325:226-30. [Crossref] [PubMed]
- Maynard C, Litwin PE, Martin JS, et al. Gender differences in the treatment and outcome of acute myocardial infarction. Results from the Myocardial Infarction Triage and Intervention Registry. Arch Intern Med 1992;152:972-6. [Crossref] [PubMed]
- van der Schaaf RJ, Timmer JR, Ottervanger JP, et al. Long-term impact of multivessel disease on cause-specific mortality after ST elevation myocardial infarction treated with reperfusion therapy. Heart 2006;92:1760-3. [Crossref] [PubMed]
- Sorajja P, Gersh BJ, Cox DA, et al. Impact of multivessel disease on reperfusion success and clinical outcomes in patients undergoing primary percutaneous coronary intervention for acute myocardial infarction. Eur Heart J 2007;28:1709-16. [Crossref] [PubMed]
- Ghauharali-Imami S, Bax M, Haasdijk A, et al. The impact of gender on long-term mortality in patients with multivessel disease after primary percutaneous coronary intervention. Neth Heart J 2015;23:592-9. [Crossref] [PubMed]
- Korea Acute Myocardial Infarction Registry (KAMIR) Investigators. Gender differences of success rate of percutaneous coronary intervention and short term cardiac events in Korea Acute Myocardial Infarction Registry. Int J Cardiol 2008;130:227-34. [Crossref] [PubMed]
- Kim YR, Jeong MH, Ahn Y, et al. Sex differences in long-term clinical outcomes of acute myocardial infarction according to the presence of diabetes mellitus. Korean J Intern Med 2021;36:S99-S113. [Crossref] [PubMed]
- Lee M, Kim DW, Park MW, et al. Gender differences in clinical outcomes of acute myocardial infarction undergoing percutaneous coronary intervention: insights from the KAMIR-NIH Registry. J Geriatr Cardiol 2020;17:680-93. [PubMed]
- Cutlip DE, Windecker S, Mehran R, et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation 2007;115:2344-51. [Crossref] [PubMed]
- Austin PC. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav Res 2011;46:399-424. [Crossref] [PubMed]
- Weaver WD, White HD, Wilcox RG, et al. Comparisons of characteristics and outcomes among women and men with acute myocardial infarction treated with thrombolytic therapy. GUSTO-I investigators. JAMA 1996;275:777-82. [Crossref] [PubMed]
- Bonarjee VV, Rosengren A, Snapinn SM, et al. Sex-based short- and long-term survival in patients following complicated myocardial infarction. Eur Heart J 2006;27:2177-83. [Crossref] [PubMed]
- Millett ERC, Peters SAE, Woodward M. Sex differences in risk factors for myocardial infarction: cohort study of UK Biobank participants. BMJ 2018;363:k4247. [Crossref] [PubMed]
- Lansky AJ, Hochman JS, Ward PA, et al. Percutaneous coronary intervention and adjunctive pharmacotherapy in women: a statement for healthcare professionals from the American Heart Association. Circulation 2005;111:940-53. [Crossref] [PubMed]
- Poon S, Goodman SG, Yan RT, et al. Bridging the gender gap: Insights from a contemporary analysis of sex-related differences in the treatment and outcomes of patients with acute coronary syndromes. Am Heart J 2012;163:66-73. [Crossref] [PubMed]
- Anand SS, Islam S, Rosengren A, et al. Risk factors for myocardial infarction in women and men: insights from the INTERHEART study. Eur Heart J 2008;29:932-40. [Crossref] [PubMed]
- Rosano GM, Vitale C, Marazzi G, et al. Menopause and cardiovascular disease: the evidence. Climacteric 2007;10:19-24. [Crossref] [PubMed]
- Matthews KA, Meilahn E, Kuller LH, et al. Menopause and risk factors for coronary heart disease. N Engl J Med 1989;321:641-6. [Crossref] [PubMed]
- Vaccarino V, Parsons L, Every NR, et al. Sex-based differences in early mortality after myocardial infarction. National Registry of Myocardial Infarction 2 Participants. N Engl J Med 1999;341:217-25. [Crossref] [PubMed]
- Khera S, Kolte D, Gupta T, et al. Temporal Trends and Sex Differences in Revascularization and Outcomes of ST-Segment Elevation Myocardial Infarction in Younger Adults in the United States. J Am Coll Cardiol 2015;66:1961-72. [Crossref] [PubMed]
- Vaccarino V, Krumholz HM, Yarzebski J, et al. Sex differences in 2-year mortality after hospital discharge for myocardial infarction. Ann Intern Med 2001;134:173-81. [Crossref] [PubMed]
- Dreyer RP, Sciria C, Spatz ES, et al. Young Women With Acute Myocardial Infarction: Current Perspectives. Circ Cardiovasc Qual Outcomes 2017;10:e003480. [Crossref] [PubMed]
- D'Onofrio G, Safdar B, Lichtman JH, et al. Sex differences in reperfusion in young patients with ST-segment-elevation myocardial infarction: results from the VIRGO study. Circulation 2015;131:1324-32. [Crossref] [PubMed]
- Bucholz EM, Strait KM, Dreyer RP, et al. Editor's Choice-Sex differences in young patients with acute myocardial infarction: A VIRGO study analysis. Eur Heart J Acute Cardiovasc Care 2017;6:610-22. [Crossref] [PubMed]
- Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation 1986;74:1124-36. [Crossref] [PubMed]
- Pizzi C, Santarella L, Costa MG, et al. Pathophysiological mechanisms linking depression and atherosclerosis: an overview. J Biol Regul Homeost Agents 2012;26:775-82. [PubMed]
- Berger JS, Elliott L, Gallup D, et al. Sex differences in mortality following acute coronary syndromes. JAMA 2009;302:874-82. [Crossref] [PubMed]
- Krumholz HM, Douglas PS, Lauer MS, et al. Selection of patients for coronary angiography and coronary revascularization early after myocardial infarction: is there evidence for a gender bias? Ann Intern Med 1992;116:785-90. [Crossref] [PubMed]
- Oe K, Shimizu M, Ino H, et al. Effects of gender on the number of diseased vessels and clinical outcome in Japanese patients with acute coronary syndrome. Circ J 2002;66:435-40. [Crossref] [PubMed]
- Lansky AJ, Ng VG, Maehara A, et al. Gender and the extent of coronary atherosclerosis, plaque composition, and clinical outcomes in acute coronary syndromes. JACC Cardiovasc Imaging 2012;5:S62-72. [Crossref] [PubMed]


