
Effect of moxibustion preconditioning on autophagy-related proteins in rats with myocardial ischemia reperfusion injury
Introduction
Myocardial infarction (MI) is one of the leading causes of global mortality and morbidity (1). It is caused by an obstruction of the coronary artery, and the primary treatment method is to restore blood flow to the heart through surgical procedures, such as percutaneous coronary angioplasty (PCI) and coronary artery bypass grafting (2). Although these interventions have improved post-MI survival rates and clinical outcomes, the process of restoring blood and oxygen induces various pathological changes within the myocardium, in a phenomenon known as myocardial ischemia reperfusion injury (MIRI) (3). Research shows that MIRI negatively affects myocardial functions, metabolic processes, and myocardium ultrastructure, increasing the likelihood of adverse ventricular remodeling and chronic heart failure (4). Despite ongoing efforts, there is yet no effective therapy for preventing and treating MIRI. Therefore, there has been a growing public interest in finding safe and effective complementary treatments and increasing scientific inquiry in investigating the effects of these treatments for MIRI.
The pathogenesis of MIRI is associated with increased oxidative stress and inflammation, mitochondrial damage, calcium overload, apoptosis, and autophagy (5-9). Among these possible factors, there has been a growing interest in investigating the role of autophagy in MIRI. Autophagy is an intracellular catabolic mechanism involving sequestration and degradation of damaged proteins and other dysfunctional cellular components (9). Under basal conditions, it serves as a housekeeping mechanism for maintaining cellular homeostasis. During times of energy stress or nutrient starvation, it promotes cellular survival by promoting ATP production and reducing energy crisis. However, when autophagy is excessive and sustained, it can detrimentally activate apoptosis and aggravate cellular injury (10). MIRI research has shown that autophagy activation is prolonged, maladaptive, and damaging to the heart (11). Therefore, the modulation of autophagy represents a novel therapeutic approach to attenuating MIRI and warrants further study (12).
Research has shown that there is a close relationship between Akt, Bcl-2, Beclin 1 and autophagy. Increasing evidence shows that Akt activation plays a critical role in cardioprotection after ischemia/reperfusion (I/R) (13) because Akt signaling and Akt lysosomal co-localization with Phafin2 complex is vital for autophagy induction (14,15). Moreover, the process of autophagy involves a complex network of intersecting proteins, namely Beclin 1 and Bcl-2. Beclin 1 is one of the key players in the regulation of autophagy. It plays a central role in the formation of a membrane structure called autophagosome, which is responsible for engulfing damaged cellular components tagged for degradation (16). Beclin 1, in turn, is regulated by its interactions with Bcl-2, an anti-apoptotic protein that inhibits autophagy by binding to the BH3 domain of Beclin 1. In brief, research demonstrates that they all play essential roles in the regulation of autophagy.
Moxibustion is an integral part of traditional Chinese medicine (TCM) with a wide range of therapeutic benefits. It is a form of heat therapy and involves the burning of mugwort herb (Artemisia vulgaris) directly or indirectly over specific acupuncture points (17). Previous studies have also shown that moxibustion preconditioning has a protective effect on the myocardium (18). In both research and clinical settings, Neiguan (PC6) is a commonly selected acupuncture point for the treatment of heart-related conditions, including hypertension, cardiac hypertrophy, and MIRI (19,20). We previously showed that moxibustion at PC6 could effectively alleviate myocardial ischemia in MIRI rats (21). However, the mechanisms of how moxibustion preconditioning on PC6 modulates MIRI and affects autophagy are still mostly unknown. Therefore, this study aimed to examine the effects of moxibustion preconditioning on MIRI and autophagy and to explore whether the cardioprotective effects of moxibustion preconditioning are mediated by regulation of autophagy-related proteins Beclin 1, Bcl-2 and Akt.
Methods
Experimental animals
A total of 60 male Sprague Dawley rats (2 months old, 250±20 g) were purchased from Shanghai Sippr-BK Laboratory Animal Co. Ltd. For the week preceding the experiment, the rats were acclimatized to a 12-hour light/dark cycle in a controlled environment with a temperature of approximately 25 °C and a relative humidity of 50%. They had free access to standard mouse chow and tap water. This study was approved by the Animal Ethics Committee of Nanjing University of Chinese Medicine Laboratory Animal Center and was conducted in accordance to the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals.
Animal grouping and experimental design
After one week of acclimatization, the rats were randomly divided into four groups which are outlined below: (I) in the sham operation group (Sham, n=15), the rats were held down by the technician, with gentle but without moxibustion for 5 minutes a day for 4 consecutive days. On the fifth day, they underwent a sham operation, consisting of left thoracotomy but without the ligation of the left anterior descending (LAD) coronary artery; (II) in the MIRI model group (MIR, n=15), the rats were also held down for 5 minutes a day for 4 consecutive days without moxibustion. However, on the fifth day, MIRI was induced by ligating the LAD for 30 minutes, followed by 4 hours of reperfusion; (III) in the moxibustion pretreatment plus MIRI group (Moxi, n=15), the rats were held down gently and received moxibustion at bilateral PC6 with 7 small moxibustion cones a day for 4 consecutive days, and on the fifth day, MIRI was induced; (IV) in the 3-MA plus MIRI group (3-MA, n=15), the rats were held down for 5 minutes a day for 4 consecutive days without moxibustion, and on the 5th day, MIRI was induced. However, 10 minutes before the ligation of LAD, the rats were administered a tail vein injection of an autophagy inhibitor 3-methyladenine (3-MA, dissolved in saline, 15 mg/kg) (AAB, USA) (22).
MIRI model establishment and moxibustion intervention
The MIRI model was established as previously described (21). The rats were anesthetized with an injection of pentobarbitone sodium (50 mg/kg) into their abdominal cavities. They were then placed supine on a temperature-controlled experimental board set at 37±3 °C and intubated with a small animal ventilator (R407, RWD, China) set at a respiratory rate of 60–70 breaths per minute. After disinfecting the surgical area, the left chest was opened to expose the heart at the 3rd intercostal space. The pericardium was separated, the heart was exteriorized, and the LAD was quickly ligated with 6.0 prolene suture, approximately 2 mm in width and depth in order to induce MI (23). The appearance of a paler color below the ligation area and ST-segment elevation on ECG (PowerLab System, AD Instruments, USA) were used to confirm a successful occlusion of the LAD. After 30 minutes of ischemia, the suture was removed to allow reperfusion for 4 hours.
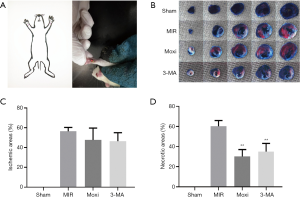
Only the rats in the Moxi group received moxibustion pretreatment before MIRI induction. Moxibustion was performed as previously described (21) by gently restraining the rats and applying a total of 7 small moxibustion cones (5 mg/cone) on PC6 for 40–45 seconds per cone and the cone was moved when it burned to a third of its size (Figure 1A). A 3-year 1:40 golden moxa, obtained from Nanyang Wolong Han medicine moxa factory (Hanan, China), was used for the experiment.
Collection of tissue and serum samples
After 4 hours of reperfusion, the rats were anesthetized with pentobarbitone sodium (80 mg/kg). Six rats from each group were used to assess the myocardial infarction area using Evans blue and TTC staining (Sigma, USA). For the remaining 9 rats in each group, blood samples and left ventricular tissues were collected for further analysis. Blood was withdrawn from the abdominal aorta and centrifuged for 10 minutes at 3,500×g to obtain the serum, which was then stored at −80 °C. Three of the nine heart tissues were placed in 4% paraformaldehyde for morphological evaluation and immunohistochemistry (IHC), and the other 6 were stored in −80 °C freezer for western blot and real-time PCR analysis.
Assessment of myocardial infarction area
Evans Blue and TTC staining were used to visualize the area of myocardial infarction (24). After anesthesia, Evans blue (2%, dissolved in PBS) was injected into the apex cordis, at the caudal end of the rats’ hearts. The hearts were then quickly harvested and frozen at −80 °C. The frozen myocardial tissue below the ligation site was sliced into 5 sections of approximately 1–2 mm each and placed in 2% TTC (dissolved in PBS) for 10 minutes at 37 °C in a dark incubator. The stained heart slices were fixed in 4% paraformaldehyde for 24 hours and then photographed (Nikon-TV, Japan) and analyzed using image analysis software Image-ProPlus. There were 3 main zones in the stained myocardial tissue: the infarct tissue appeared white, the at-risk tissue for infarction appeared red, and the normal tissue with no infarction appeared blue. In this study, the severity of myocardial infarction was assessed by estimating the percentage of total ischemic area and the percentage of necrotic areas using the following formulae: percentage of total ischemic areas = (infarct plus at-risk areas)/total myocardial areas; percentage of necrotic areas = infract areas/(infract plus at-risk areas).
Enzyme-linked immunosorbent assay (ELISA)
The serum levels of cTnT and lactate dehydrogenase (LDH) were determined using cTnT ELISA kit and LDH ELISA kit respectively (Yifeixue Biotech, China), according to the manufacturer’s protocol.
Hematoxylin & eosin (H&E) staining
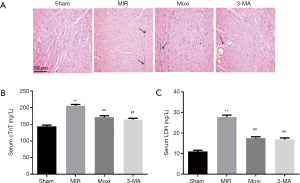
H&E staining was used for morphological evaluation. The ventricular tissues fixed in 4% paraformaldehyde were dehydrated, paraffin embedded, and sliced into 4 µm thick sections. The sections were then stained with hematoxylin and eosin. After H&E staining, the sections were dehydrated and observed under an optical microscope. For each tissue section, 6 random fields were photographed, analyzed, and averaged by an investigator blinded to the group assignment.
Quantitative real-time polymerase chain reaction (RT-qPCR)
RT-qPCR was used to measure the mRNA expressions of Beclin 1, Bcl-2, and Akt. After extracting total RNA in myocardial tissues with TRIzol (Abcam, USA), cDNA was synthesized by reverse transcription and then amplified by a real-time fluorescence quantitative PCR system (IQ5TM, Bio-Rad, USA). Amplification was carried out by denaturing at 94 °C for 30 seconds, annealing at 55 °C for 45 seconds, and extending at 72 °C for 30 seconds for 40 cycles. The primer sequences are listed in Table 1. GAPDH was used as the reference gene for all PCR experiments. Each sample was analyzed 3 times and the relative expressions of genes were determined using the 2−ΔΔCT method.

Full table
IHC
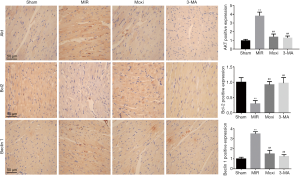
IHC was performed to assess the expressions of Beclin 1, Bcl-2, and Akt. Ventricular tissue sections placed on slides were deparaffinized in xylene for 20 minutes and rehydrated through a graded concentration series of ethanol. The sections were then placed in citrate butter (10 mM, pH 6.0) and heated to 60 °C for 5–10 minutes for antigen retrieval. Following this, sections were incubated in 3% hydrogen peroxide in methanol for 10–15 minutes to block endogenous peroxidase activity. After washing in distilled water, the sections were blocked with 5% BSA blocking buffer for 20 minutes at room temperature and incubated with primary antibodies for Beclin 1, Bcl-2, and Akt (diluted 1:200; catalogue number respectively: 29,281, 40,415, 40,576; SAB, USA) for 1–2 hours at 37 °C and overnight at 4 °C. After washing in PBS, they were incubated with secondary antibodies (diluted 1:10,000) for 30 minutes at 37 °C. The sections were then incubated with SABC for 20 minutes, stained with DAB solution, and observed under a light microscope. The absorbance of each image was analyzed using image analytic system. The positive expressions of total-Akt and Beclin 1 were identified in the cell nucleus and cytoplasm, while that of Bcl-2 was identified in the cell cytoplasm. A semi-quantitative evaluation of total-Akt, Bcl-2, Beclin 1 was performed using a method described in the literature (25).
Western blot analysis
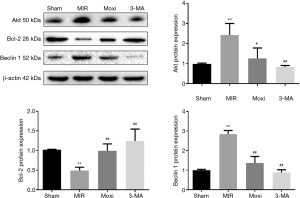
In addition to IHC, the levels of autophagy-related proteins Beclin 1, Bcl-2, and Akt were evaluated with western blot. Frozen myocardial tissues were homogenized in radioimmunoprecipitation assay (RIPA) buffer at a ratio of 100 mg: 1 mL and centrifuged at 12,000 ×g for 10 minutes at 4 °C. BCA protein assay (Pierce, USA) was performed using the supernatants to determine protein concentration. The proteins were then loaded and separated on SDS-polyacrylamide gel electrophoresis (SDS-PAGE), followed by a transfer to PVDF membrane. Following this, the membranes were blocked with 5% nonfat milk for 2 hours and then incubated with primary antibodies for Beclin 1, Bcl-2, and Akt (diluted 1:1,000; catalogue number respectively: 29,281, 40,415, 40,576; SAB, USA) overnight at 4 °C. After washing 3 times for 10 minutes each, the membranes were incubated with secondary antibodies (diluted 1:10,000) for 2 hours. Anti-β-actin (diluted 1:1,000) was used as the loading control protein. The bands were imaged and analyzed using the Bio-Imaging system.
Statistical analysis
All data are presented as mean ± standard deviation and were analyzed with SPSS 21.0. The normality of data distribution was tested with the Shapiro-Wilk test. If the data were normally distributed, groups were compared by one-way analysis of variance (ANOVA), followed by the Fisher’s LSD method. If the data were not normally distributed, the Dunnett’s T3 method was used for group comparisons. A value of P≤0.05 was considered statistically significant.
Results
Moxibustion preconditioning decreased necrotic areas caused by MIRI
The protective effect of moxibustion preconditioning against MIRI was assessed by determining myocardial infarction sizes (Figure 1B,C,D). The percentage of the necrotic area was significantly smaller for the Moxi and 3-MA groups compared to the MIR group (P<0.01). However, there was no significant difference in the percentage of ischemia area among the MIR, 3-MA, and Moxi groups.
Moxibustion preconditioning attenuated MIRI-induced morphological alterations and myocardial enzyme levels
H&E staining was performed to observe the morphological changes in myocardial tissue below the ligation site (Figure 2A). MIRI resulted in myocardial cellular loss, myofibrillar degeneration, and inflammatory cell infiltration. These MIRI-induced morphological changes were attenuated by the administration of moxibustion preconditioning and 3-MA. ELISA was performed to test the levels of cTnT and LDH in serum (Figure 2B,C). The levels of cTnT and LDH were significantly higher for the MIR group compared to the Sham group. Both moxibustion preconditioning and 3-MA attenuated these MIRI-induced changes in cTnT and LDH levels (P<0.01).
Moxibustion preconditioning modulated myocardial autophagy-related proteins
The effect of moxibustion preconditioning on MIRI-induced myocardial autophagy was evaluated by assessing the expressions of autophagy-related proteins Beclin 1, Bcl-2, and Akt. Gene expressions of Beclin 1, Bcl-2 and Akt were evaluated using RT-qPCR (Figure 3). The induction of MIRI caused a significant up-regulation of Beclin 1 and Akt mRNA expressions and a down-regulation of Bcl-2 (P<0.01). Compared to the MIR group, the mRNA expressions of Beclin 1 and Akt were significantly down-regulated by the application of moxibustion preconditioning and 3-MA (P<0.01), while that of Bcl-2 was up-regulated by moxibustion preconditioning (P=0.05).

According to the IHC data (Figure 4), the rats in the MIR group compared to the Sham group had increased expressions of Beclin 1 and Akt and lower expression of Bcl-2 (P<0.01). An injection of 3-MA, an autophagy inhibitor, normalized these changes in the MIR group by reducing Beclin 1 and Akt, and increasing Bcl-2 levels (P<0.01). Moxibustion preconditioning exerted similar normalizing effects on MIRI-induced changes, significantly reducing Beclin 1 and Akt and increasing Bcl-2 (P<0.01).
These IHC findings were consistent with the western blot data (Figure 5). Compared to the Sham group, the rats in the MIR group showed higher expressions of Beclin 1 and Akt (P<0.05) and a lower expression of Bcl-2 (P<0.01). These changes were normalized in both the 3-MA and Moxi groups; compared to the MIR group, the rats in the 3-MA and Moxi groups had significantly lower levels of Beclin 1 and Akt, and a higher level of Bcl-2 (P<0.01).
Discussion
MIRI is a significant and paradoxical problem arising from the reperfusion of ischemic myocardium. While the timely restoration of blood is critical to reducing myocardial ischemic injury, the process of reperfusion can itself lead to further myocardial damage and death. Moxibustion, an essential intervention in TCM, involves thermal stimulation of specific acupuncture points and is effective in treating various cardiovascular diseases (19). In this study, we found that moxibustion preconditioning at PC6 reduced myocardial necrotic area and myocardial enzyme levels. It also normalized myocardial cellular degeneration and myofibrillar disorganization induced by MIRI, indicating that moxibustion preconditioning has a cardioprotective effect against MIRI, which consistent with the previous (21).
There is a growing body of research demonstrating the complex role of autophagy in both physiological and pathological conditions. While autophagy plays an essential role in maintaining cellular homeostasis, research demonstrates that when it is excessive and sustained, such as in the case of MIRI, it can be maladaptive and damaging to the heart (26,27). Previous experimental studies have shown that myocardial injury could be mitigated by using autophagy inhibitors such as 3-MA (28). So, for our study, we used autophagy inhibitor 3-MA as a positive control drug to compare and confirm the cardioprotective effect of moxibustion preconditioning. We found that the administration of 3-MA decreased necrotic area and myocardial enzyme levels while improving MIRI-induced morphological changes. Moxibustion preconditioning exerted similar cardioprotective effects on MIRI as the 3-MA intervention. Based on these findings, we hypothesized that moxibustion preconditioning protects the myocardium during MIRI by inhibiting autophagy in a similar way to 3-MA. 3-MA is a widely used autophagy inhibitor, and it suppresses autophagy by blocking the enzymatic action of class III phosphatidylinositol 3-kinase (PI3K), which plays a central role in the initiation and progression of autophagy (28,29). Once activated during MIRI, PI3K can phosphorylate and upregulate other key regulators of autophagy, including Akt (30,31). By inhibiting PI3K, we speculated that 3-MA would also downregulate Akt. Thus, autophagy-related proteins, Akt, Bcl-2, and Beclin 1 were examined.
Accordingly, in our study, the upregulation of Akt in the MIRI group was normalized by 3-MA intervention. Similarly, moxibustion preconditioning also resulted in a decreased level of Akt expression. There was a significant difference in total Akt between the groups, so we did not further investigate the expression of phospho-Akt. However, the total Akt cannot reflect the level of phospho-Akt, so we could not explore the role of Akt thoroughly; phospho-Akt should be detected in future studies. Previous studies have also demonstrated that Akt activation and Akt-mediated phosphorylation of Beclin 1 inhibit autophagy (32). While, in our study, Akt was associated with increased Beclin 1 and decreased Bcl-2 expressions, suggesting an upregulation of autophagy. Compared to the sham group, MIRI induction increased both Akt and Beclin 1 expressions, which were significantly attenuated by 3-MA and moxibustion preconditioning. These conflicting data might be explained by the complexity of PI3K/Akt signaling (29,33). Research shows that Akt is involved in both inhibition and activation of autophagy, depending on its interactions with either class I or class III PI3K, respectively (14,15,31). Class I PI3K/Akt signaling has been well-documented and is widely known to suppress autophagy and encourage cellular survival, growth, and proliferation (31). On the other hand, class III PI3K/Akt interaction promotes autophagy, with recent studies demonstrating the importance of Akt signaling and Akt lysosomal localization for autophagy induction (14,15). Furthermore, as reported in the literature (28), 3-MA works by suppressing the function of class III PI3K, and then downregulates proteins downstream of PI3K, including Akt. In our study, we only observed the expression of Akt, but not PI3K. Therefore, further studies are needed to clarify the relationships between PI3K/Akt signaling, moxibustion, and autophagy.
Beclin 1 plays a central role in the initial step of autophagy by promoting the formation and maturation of autophagosome, a double membrane vesicle that engulfs dysfunctional cellular components and fuses with lysosomes for degradation. Bcl-2 interacts with Beclin 1 and inhibits its autophagic activities. Cellular stress disrupts this Bcl-2/Beclin 1 interaction by phosphorylating ser70, ser87 and thr69 of Bcl-2 and displacing Beclin 1 from Bcl-2 (34). Research also shows that downregulation of Bcl-2 protein subsequently leads to Beclin 1 upregulation and autophagy induction (35). When there is cellular stress, however, Beclin 1 is displaced from Bcl-2 and autophagy is triggered (27). In our study, the induction of MIRI decreased the protein and mRNA expressions of Bcl-2 and increased that of Beclin 1 in the ventricular tissues, suggesting that MIRI acted as a stressor to promote autophagy. However, the administration of 3-MA and moxibustion preconditioning normalized these changes by increasing Bcl-2 expressions and decreasing Beclin 1, thereby reducing the level of autophagy.
In addition to autophagy itself, the interaction between autophagy and apoptosis mediated by Bcl-2/Beclin 1 also plays a vital role in MIRI (36), so we took Bcl-2 and Beclin 1 as the focus of our study. Studies have shown that Bcl-2 can regulate apoptosis along with autophagy; specifically, Bcl-2 not only binds to Beclin 1 to suppress autophagy, but it also interacts with Bax/Bak to inhibit apoptosis. According to other recent research, acupuncture pretreatment at PC6 has been shown to exert a cardioprotective effect by modulating gene expressions and regulating various signaling pathways, including apoptosis (37,38). During cellular stress, Bcl-2 is displaced from both Beclin 1 and Bax/Bak, activating autophagy and apoptosis, respectively. Thus, upregulation of Bcl-2 is associated with modulation of both autophagy and apoptosis. In our study, moxibustion preconditioning upregulated Bcl-2 expression, indicating that it can promote cellular survival in MIRI through attenuation of both autophagy and apoptosis. In order to verify the hypothesis that moxibustion can inhibit myocardial apoptosis, further study is needed.
Autophagy is a lysosome-dependent cellular catabolic process. Besides Beclin 1, LC3, p62, and TFEB also play an essential role in autophagy (39). The transformation from LC3 I to LC3 II is the key step in autophagosome lipid membrane fusion which increases the volume of autophagosomes. P62 protein itself is one of the selective substrates of autophagy, which means when autophagy is activated, p62 will be degraded. TFEB is a member of the MiTF/TFE family, and the activation of TFEB increases the synthesis of lysosomal-associated membrane protein (LAMP) and accelerates the autophagy process. According to previous studies, there was an upregulation of LC3II/I and downregulation of p62 in MIRI, while moxibustion could reverse these changes (40). However, to completely demonstrate the involvement of autophagy, other autophagic markers apart from Beclin 1 should also be detected. Finally, in a previous study, moxibustion promoted autophagy against a lethal bacterial infection (41). Nevertheless, we observed the inhibition of autophagy by moxibustion. Combined, these data suggest that moxibustion might exert a dual effect of either activating or suppressing autophagy, depending on the autophagic activity level. Anyhow, more evidence is required to clarify the underlying mechanism and possible bi-directional modulatory effect of moxibustion preconditioning on autophagy.
Conclusions
Autophagy during MIRI is characterized as excessive, sustained, and maladaptive. Attenuating autophagy may present a novel therapeutic approach to reducing myocardial injury. This study showed that moxibustion preconditioning at PC6 could mitigate myocardial injury in MIRI. The cardio-protective effect of moxibustion preconditioning may be mediated by modulation of autophagy.
Acknowledgments
Funding: This work was supported by the Natural Science Foundation of China (No.81674063 and No.81704169), Jiangsu Provincial Leading Talents in Chinese Medicine (No.SLJ0226), the Natural Science Foundation of Jiangsu Province (No.BK20171067) and the Special Subject of TCM Standardization of the State Administration of Traditional Chinese Medicine [ZYYS-2016 (0002)].
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Animal Ethics Committee of Nanjing University of Chinese Medicine Laboratory Animal Center and was conducted in accordance to the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals.
References
- Rayner M, Scarborough P, Townsend N, et al. Cardiovascular disease in Europe — epidemiological update 2015. Eur Heart J 2015;36:2696-705. [Crossref] [PubMed]
- Ye Y, Yang M, Zhang S, et al. Percutaneous coronary intervention versus cardiac bypass surgery for left main coronary artery disease: A trial sequential analysis. Medicine (Baltimore) 2017;96:e8115. [Crossref] [PubMed]
- Hausenloy DJ, Yellon DM. Myocardial ischemia-reperfusion injury: a neglected therapeutic target. J Clin Invest 2013;123:92-100. [Crossref] [PubMed]
- Frank A, Bonney M, Bonney S, et al. Myocardial ischemia reperfusion injury: from basic science to clinical bedside. Semin Cardiothorac Vasc Anesth 2012;16:123-32. [Crossref] [PubMed]
- de Vries DK, Kortekaas KA, Tsikas D, et al. Oxidative damage in clinical ischemia/reperfusion injury: a reappraisal. Antioxid Redox Signal 2013;19:535-45. [Crossref] [PubMed]
- Diaz I, Smani T. New insights into the mechanisms underlying vascular and cardiac effects of urocortin. Curr Vasc Pharmacol 2013;11:457-64. [Crossref] [PubMed]
- Rafiq K, Kolpakov MA, Seqqat R, et al. c-Cbl inhibition improves cardiac function and survival in response to myocardial ischemia. Circulation 2014;129:2031-43. [Crossref] [PubMed]
- Liu J, Wang H, Li J. Inflammation and Inflammatory Cells in Myocardial Infarction and Reperfusion Injury: A Double-Edged Sword. Clin Med Insights Cardiol 2016;10:79-84. [Crossref] [PubMed]
- Thapalia BA, Zhou Z, Lin X. Autophagy, a process within reperfusion injury: an update. Int J Clin Exp Pathol 2014;7:8322-41. [PubMed]
- Ouyang C, You J, Xie Z. The interplay between autophagy and apoptosis in the diabetic heart. J Mol Cell Cardiol 2014;71:71-80. [Crossref] [PubMed]
- Ma S, Wang Y, Chen Y, et al. The role of the autophagy in myocardial ischemia/reperfusion injury. Biochim Biophys Acta 2015;1852:271-6. [Crossref] [PubMed]
- Gao C, Wang R, Li B, et al. TXNIP/Redd1 Signaling and Excessive Autophagy: A Novel Mechanism of Myocardial Ischemia/Reperfusion Injury in Mice. Cardiovasc Res 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Jiang YQ, Chang GL, Wang Y, et al. Geniposide Prevents Hypoxia/Reoxygenation-Induced Apoptosis in H9c2 Cells: Improvement of Mitochondrial Dysfunction and Activation of GLP-1R and the PI3K/AKT Signaling Pathway. Cell Physiol Biochem 2016;39:407-21. [Crossref] [PubMed]
- Jaber N, Dou Z, Chen JS, et al. Class III PI3K Vps34 plays an essential role in autophagy and in heart and liver function. Proc Natl Acad Sci U S A 2012;109:2003-8. [Crossref] [PubMed]
- Matsuda-Lennikov M, Suizu F, Hirata N, et al. Lysosomal interaction of Akt with Phafin2: a critical step in the induction of autophagy. PLoS One 2014;9:e79795. [Crossref] [PubMed]
- Kang R, Zeh HJ, Lotze MT, et al. The Beclin 1 network regulates autophagy and apoptosis. Cell Death Differ 2011;18:571-80. [Crossref] [PubMed]
- Kim MJ, Namgung U, Hong KE. Regenerative effects of moxibustion on skeletal muscle in collagen-induced arthritic mice. J Acupunct Meridian Stud 2012;5:126-35. [Crossref] [PubMed]
- Wang C, Xie WJ, Liu M, et al. Effect of electroacupuncture and moxibustion preconditioning on blood endothelin and creatine kinase contents and myocardial HSP 70 expression in rabbits with myocardial ischemia-reperfusion injury. Zhen Ci Yan Jiu 2014;39:372-6. [PubMed]
- Wang S, Ren L, Jia L, et al. Effect of acupuncture at Neiguan (PC 6) on cardiac function using echocardiography in myocardial ischemia rats induced by isoproterenol. J Tradit Chin Med 2015;35:653-8. [Crossref] [PubMed]
- Li J, Li J, Chen Z, et al. The influence of PC6 on cardiovascular disorders: a review of central neural mechanisms. Acupunct Med 2012;30:47-50. [Crossref] [PubMed]
- Bai H, Lu SF, Chen WY, et al. Effect of Moxibustion Preconditioning with Seed-sized Moxa Cones on Myocardial Infarction Size and Beclin 1 Expression in Myocardial Ischemia-reperfusion Injury Rats. Zhen Ci Yan Jiu 2017;42:471-6. [PubMed]
- Zhang LX, Zhao HJ, Sun DL, et al. Niclosamide attenuates inflammatory cytokines via the autophagy pathway leading to improved outcomes in renal ischemia/reperfusion injury. Mol Med Rep 2017;16:1810-6. [Crossref] [PubMed]
- Li X, Xie Z, Lin M, et al. Renalase protects the cardiomyocytes of Sprague-Dawley rats against ischemia and reperfusion injury by reducing myocardial cell necrosis and apoptosis. Kidney Blood Press Res 2015;40:215-22. [Crossref] [PubMed]
- Xiong J, Wang Q, Xue FS, et al. Comparison of cardioprotective and anti-inflammatory effects of ischemia pre- and postconditioning in rats with myocardial ischemia-reperfusion injury. Inflamm Res 2011;60:547-54. [Crossref] [PubMed]
- Wang W, Wen Q, Xu L, et al. Activation of Akt/mTOR pathway is associated with poor prognosis of nasopharyngeal carcinoma. PLoS One 2014;9:e106098. [Crossref] [PubMed]
- Choi AM, Ryter SW, Levine B. Autophagy in human health and disease. N Engl J Med 2013;368:1845-6. [Crossref] [PubMed]
- Przyklenk K, Dong Y, Undyala VV, et al. Autophagy as a therapeutic target for ischaemia/reperfusion injury? Concepts, controversies, and challenges. Cardiovasc Res 2012;94:197-205. [Crossref] [PubMed]
- Liu H, Lei H, Shi Y, et al. Autophagy inhibitor 3-methyladenine alleviates overload-exercise-induced cardiac injury in rats. Acta Pharmacol Sin 2017;38:990-7. [Crossref] [PubMed]
- Noguchi M, Hirata N, Suizu F. The links between AKT and two intracellular proteolytic cascades: ubiquitination and autophagy. Biochim Biophys Acta 2014;1846:342-52. [PubMed]
- Hofler A, Nichols T, Grant S, et al. Study of the PDK1/AKT signaling pathway using selective PDK1 inhibitors, HCS, and enhanced biochemical assays. Anal Biochem 2011;414:179-86. [Crossref] [PubMed]
- Chen Q, Xu T, Li D, et al. JNK/PI3K/Akt signaling pathway is involved in myocardial ischemia/reperfusion injury in diabetic rats: effects of salvianolic acid A intervention. Am J Transl Res 2016;8:2534-48. [PubMed]
- Wang RC, Wei Y, An Z, et al. Akt-mediated regulation of autophagy and tumorigenesis through Beclin 1 phosphorylation. Science 2012;338:956-9. [Crossref] [PubMed]
- Yu X, Long YC, Shen HM. Differential regulatory functions of three classes of phosphatidylinositol and phosphoinositide 3-kinases in autophagy. Autophagy 2015;11:1711-28. [Crossref] [PubMed]
- Maejima Y, Kyoi S, Zhai P, et al. Mst1 inhibits autophagy by promoting the interaction between Beclin 1 and Bcl-2. Nat Med 2013;19:1478-88. [Crossref] [PubMed]
- Fukui M, Yamabe N, Choi HJ, et al. Mechanism of Ascorbate-Induced Cell Death in Human Pancreatic Cancer Cells: Role of Bcl-2, Beclin 1 and Autophagy. Planta Med 2015;81:838-46. [Crossref] [PubMed]
- Zhong ZH, Lu SF, Chen WY, et al. Time-dependent Protective Effect of Electroacupuncture on Ischemic Myocardium and Changes of Myocardial Autophagy and Apoptosis Related Protein Expression in Rats. Zhen Ci Yan Jiu 2018;43:550-5. [PubMed]
- Fu SP, He SY, Xu B, et al. Acupuncture promotes angiogenesis after myocardial ischemia through H3K9 acetylation regulation at VEGF gene. PLoS One 2014;9:e94604. [Crossref] [PubMed]
- Li X, Huang Q, Wang M, et al. Compound K Inhibits Autophagy-Mediated Apoptosis Through Activation of the PI3K-Akt Signaling Pathway Thus Protecting Against Ischemia/Reperfusion Injury. Cell Physiol Biochem 2018;47:2589-601. [Crossref] [PubMed]
- Mizushima N, Yoshimori T, Levine B. Methods in mammalian autophagy research. Cell 2010;140:313-26. [Crossref] [PubMed]
- Liu NN, Jia XZ, Wang J, et al. Moxibustion improves cardiac function by up-regulating autophagy-related proteins of cardiomyocytes in rats with chronic heart failure. Zhen Ci Yan Jiu 2019;44:25-30. [PubMed]
- Li X, Guo G, Shen F, et al. Moxibustion Activates Macrophage Autophagy and Protects Experimental Mice against Bacterial Infection. Evid Based Complement Alternat Med 2014;2014:450623. [Crossref] [PubMed]


