
Minimal change disease induced by tiopronin: a rare case report and a review of the literature
Introduction
Tiopronin (TP), formally 2-mercaptopropionylglycine, has been demonstrated to be a useful therapy in cystinuria (in-label indication) and rheumatoid arthritis (RA) (off-label indication) in Western countries since the 1980s (1,2). Additionally, TP has been extensively used in numerous liver diseases in China, including fatty liver disease, viral hepatitis, and drug-induced liver injury (3-5). Previous research has verified that the reduced sulfhydryl of TP plays a vital role in hepatic protection and tissue detoxification (5). The thiol provided by TP acts like a scavenger of toxin and reactive oxygen species (4), which results in hepatoxicity. TP can also potentially decrease ATPase function to enhance the mitochondrial energy, consequently guaranteeing hepatic cell function. The prevalence of adverse effects of TP is lower than other sulfhydryl agents (SA) like D-penicillamine (6). However, intolerance, including cutaneous and mucosae side effects (stomatitis, pruritus, erythema, and pemphigus), renal injury (proteinuria), and hematological disorders, have been reported (7,8). Reports regarding TP-induced nephrotic syndrome (NS) are rare, especially minimal change disease (MCD) due to TP (9,10). In current study, we firstly describe a Chinese patient who developed MCD after the administration of TP for six months.
Case presentation
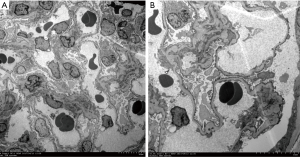
In June 2016, a 36-year-old female was diagnosed as pulmonary tuberculosis (TB), with the use of isoniazid (INH) 300 mg qd po, rifampicin (RIF) 450 mg qd po, ethambutol (EMB) 750 mg qd po, and pyramide (PZA) 500 mg tid po. After two months of treatment, the patient was radiographically improved. On 2nd September 2016, laboratory tests revealed an increasing glutamic pyruvic transaminase (ALT) of 101 IU/L. TP at the dosage of 200 mg three times daily was initiated for hepatic protection. However, ALT increased to 1032 IU/L on 9th September 2016, suggesting that the patient had generalized drug-induced liver injury. INH, RIF and PZA were suspected as the causative agents. Therefore, anti-TB treatment was discontinued. On 14th September 2016, the patient recovered from liver injury (ALT 62 IU/L). After that, anti-TB therapies including pasiniazid 300 mg tid po, rifapentine 450 mg orally twice weekly, EMB 750 mg qd po, and levofloxacin 400 mg qd po were administrated again. Six months after the initiation of TP (total dosage: 108 g), the patient developed the following symptoms: foamy urine, frequent nocturia, abdominal pain, and edema. She was subsequently hospitalized. Laboratory tests revealed that the hematology parameters were normal. However, urinalysis showed proteinuria (4+) and urine occult blood (2+). Daily urinary excretion of total protein was 8,024 mg (167 mg/kg; nephrotic range >50 mg/kg); serum biochemistry showed urea 5.5 mmol/L, creatinine 54 µmol/L, ALT 68 U/L, glutamic oxaloacetic transaminase (AST) 66 U/L [more than upper normal value (UNV)], bilirubin 11.2 µmol/L, total protein 37.6 g/L [less than lower normal value (LNV)], albumin 18.5 g/L (less than LNV), cholesterol 10.74 mmol/L (more than UNV), triglycerides 1.47 mmol/L, Na+ 131 mmol/L (less than LNV), K+ 3.9 mmol/L, Cl− 103 mmol/L. Immunologic tests revealed negative results of anti-glomerular basement membranous antibody, anti-phospholipase A2 receptor, anti-neutrophil cytoplasmic antibodies, rheumatoid factors, and antinuclear factors. Serological tests revealed that HBs antigen (Ag), HCV antibody (Ab), and HIV Ag/Ab were negative. According to a renal biopsy, twenty glomeruli were detected under the light scope. Most glomeruli were normal in spite of one sclerotic glomerulus observed. Sporadic glomeruli observed slight mesangial expansion and matrix segmental hyperplasia. Meanwhile, the tubules showed slight interstitium injury and minimal atrophy. There was minimal interstitial inflammation and fibrosis. Additionally, the endomembrane of the arterioles was slightly widened. Immunofluorescence analysis of the specimen revealed the minimal deposition of IgM diffusely distributed in the mesangium despite the deposition of IgG, IgA, C3, Clq, κ, λ was negative. Electron microscopy images showed that the podocyte foot processes were diffusely fused. The mesangial matrix and glomerular basement membrane were normal. There was no electron-dense deposition detected under the electron microscopy (Figure 1). MCD was diagnosed accordingly. As the possibility of disease-associated MCD could be excluded based on the above information, we focused on the possibility of drug-related adverse effects. World Health Organization-Uppsala Monitoring Centre (WHO-UMC) system was used to assess the causality of each drug (11). Results showed that TP was classified as “probable”, while other 4 concomitant medications were categorized as “possible”. Likewise, the score was 7 (categorized as “probable”) for TP, while 3 (categorized as “possible”) for other agents according to the Naranjo Adverse Drug Reaction Probability Scale (Table 1) (12). Therefore, TP was suspected as the mainly probable cause of MCD. Considering that pulmonary TB was recovered, all the previous treatments were discontinued. Symptomatic treatments including furosemide 20 mg qd po, losartan 100 mg qd po, and rosuvastatin 10 mg qd po were initiated simultaneously. Two weeks later, MCD was resolved clinically, with the apparent reduction of daily total urinary protein (77.5 mg). Five weeks after the withdrawal of TP, the serum proteins and cholesterol were normalized as well. During the first year of follow-up, her urinalysis did not show proteinuria again.

Full table
Discussion
In this case, we reported a 36-year-old female who received TP as the hepatic protection therapy. MCD occurred 6 months after TP treatment (600 mg/day, 12.5 mg/kg/day). Withdrawal of TP had completely resolved the MCD within five weeks. The patient had no relevant medical history. Therefore, the disease-associated MCD was excluded. Subsequently, the searches were performed in the PubMed database using the syntax of (“tiopronin” OR “thiopronin” OR “isoniazid” OR “pasiniazid” OR “rifampicin” OR “rifapentine” OR “ethambutol” OR “levofloxacin” OR “pyramide”) AND (“proteinuria” OR “nephrotic syndrome” OR “minimal change disease”). RIF were well known to cause acute kidney injury (13), there were 34 relevant articles demonstrating that RIF could cause NS with acute kidney injury during the initial treatment for TB (14,15). There was only one case of INH reporting an MCD developed after five days of INH monotherapy (16). The patient has discontinued the RIF and INH for six months. Thus, it was unlikely that MCD in the present case was due to these two medicines. Tanaka et al. report a case of MPO-ANCA-related nephritis due to EMB with the concurrent therapy of RIF (17). Considering EMB-induced nephrotoxicity was rare, EMB was usually not suspected as the causative medicine of renal injury developed during concomitant therapy. No relevant article was retrieved for the pasiniazid, rifapentine, levofloxacin, and PZA. It was documented that high doses or long-term administration of TP occasionally caused adverse renal effects, from proteinuria to NS (18). Fifteen articles were associated with TP-induced NS (6,8). In addition, both of WHO-UMC system assessments and the Naranjo scores supported the probability of TP-induced MCD. Therefore, in the present case, TP was considered as the most likely causative agent of MCD.
TP, a glycine differential with a free sulfhydryl, has the same mechanism as D-penicillamine (8). SA, including TP and D-penicillamine, can interrupt the disulfide bond of cystine and bind the thiol to form a more soluble drug-cystine complex (19). Additionally, SA might restore the efficacy of sialyltransferase at the B lymphocyte level to decrease the functional affinity of the rheumatoid factors (20). Thus, SA has been demonstrated to be a useful therapy in cystinuria and RA (1,2). However, TP is preferred for its lower incidence of adverse effects (6,21).
The most severe adverse effect of TP is NS (6). A literature review has revealed that only fifteen articles have been published reporting 31 clinical cases about TP-induced NS thus far (6-10,18,21-29). Among these articles, TP was used as treatment agent for cystinuria (6 studies) (6,21,22,26,27,29), RA (4 studies) (7,9,18,25), and hepatoprotection (1 study) (8). NS occurred six weeks to 28 months after TP initiation. Also, NS was resolved within 10 to 103 days after TP discontinuation with or without steroid treatment. However, only MCD induced by TP was reported (Table 2) (9,10). Salvarani et al. describe a 60-year-old woman, who was diagnosed as definite RA and developed MCD after five months of TP treatment with arising human leukocyte antigen (HLA)-DR3 (9). Moreover, Lecoules et al. report a 73-year-old patient who developed MCD six weeks after TP therapy (10). In both cases, MCD was severe on initiation and recovered rapidly after drug withdrawal.

Full table
The mechanisms underlying MCD stimulated by TP are still obscure. Firstly, it has been discovered that TP-induced NS is dose-dependent, and the threshold dose is 50 mg/kg/day (23). In contrast, Salvarani et al. suggested there is no relationship between mean daily dose and toxicity (9). Taking the present patient into account, the TP dosage administrated was 12.5 mg/kg/day, which is below the threshold dosage but still capable of leading to MCD. Secondly, the highest prevalence of NS occurs during the first half of the year of treatment, and it seems to be self-limiting upon cessation of the related drug. Similarly, in the present patient, MCD had developed after six months of TP treatment and rapidly terminated after TP withdrawal without steroid or immunosuppressive agents. Thirdly, the sensitization process which occurred in TP-induced MCD was identical to that which occurs after exposure to other allergens (30). Moreover, Stratta et al. has identified the immune complex after incubation of kidney specimens with TP. Thus, it is speculated that TP acts like an allergen in renal injury (31). Fourthly, Salvarani et al. showed the positive result of HLA-DR3 in a patient with TP-induced MCD (9). Likewise, Ferraccioli et al. reported a strong association between nephritis due to TP and HLA class I antigens B35-Cw4 (24). Unfortunately, no HLA series was analyzed in the present patient.
Conclusions
We present a patient with TP-induced MCD. It is probable that TP meddles with the podocytes function, although the underlying mechanism has not yet been fully elucidated. The occurrence of reversible glomerular lesions reported by us should be considered as a potential adverse effect when administrating the prolonged treatment of TP. Urine should be monitored at a minimum once a week, to detect early proteinuria in patients receiving TP.
Acknowledgments
Funding: This work was supported by the Program for Key but Weak Discipline of Shanghai Municipal Commission of Health and Family Planning (2016ZB0304), Research Funds of Shanghai Health and Family Planning commission (20184Y0022), Research Funds of Clinical Pharmacy Innovation Research Institute of Shanghai Jiao Tong University School of Medicine (CXYJY2019ZD001).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Sany J, Combe B, Delecoeuillerie G, et al. A comparative controlled trial of 2 administration modalities of tiopronin in rheumatoid arthritis. Rev Rhum Ed Fr 1993;60:36s-44s. [PubMed]
- Knoll T, Zollner A, Wendt-Nordahl G, et al. Cystinuria in childhood and adolescence: recommendations for diagnosis, treatment, and follow-up. Pediatr Nephrol 2005;20:19-24. [Crossref] [PubMed]
- Li XP, Wen F, Yang W, et al. The role of tiopronin for the prevention of chemotherapy-related liver toxicity in advanced colorectal cancer patients treated with mFOLFOX7: a prospective analysis. Tumori 2014;100:446-51. [Crossref] [PubMed]
- Tang MC, Cheng L, Qiu L, et al. Efficacy of Tiopronin in treatment of severe non-alcoholic fatty liver disease. Eur Rev Med Pharmacol Sci 2014;18:160-4. [PubMed]
- Li J, Qiu X, Guo W, et al. Prospective analysis of tiopronin in prevention of sorafenib and antiviral therapy inducing liver toxicity in advanced hepatitis B virus-related hepatocellular carcinoma. Med Oncol 2015;32:238. [Crossref] [PubMed]
- Tasic V, Lozanovski VJ, Ristoska-Bojkovska N, et al. Nephrotic syndrome occurring during tiopronin treatment for cystinuria. Eur J Pediatr 2011;170:247-9. [Crossref] [PubMed]
- Sany J, Combe B, Verdie-Petibon D, et al. Long-term tolerability of tiopronin (Acadione) in the treatment of rheumatoid arthritis. Apropos of 140 personal cases. Rev Rhum Mal Osteoartic 1990;57:105-11. [PubMed]
- Zheng Z, Xue Y, Jia J, et al. Tiopronin-induced membranous nephropathy: a case report. Ren Fail 2014;36:1455-60. [Crossref] [PubMed]
- Salvarani C, Macchioni P, Rossi F, et al. Nephrotic syndrome induced by tiopronin: association with the HLA-DR3 antigen. Arthritis Rheum 1985;28:595-6. [Crossref] [PubMed]
- Lecoules S, Duvic C, Herody M, et al. Tiopronin-induced nephrotic syndrome with minimal glomerular lesions. Presse Med 1999;28:273-5. [PubMed]
- Behera SK, Das S, Xavier AS, et al. Comparison of different methods for causality assessment of adverse drug reactions. Int J Clin Pharm 2018;40:903-10. [Crossref] [PubMed]
- Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 1981;30:239-45. [Crossref] [PubMed]
- Chiba S, Tsuchiya K, Sakashita H, et al. Rifampicin-induced acute kidney injury during the initial treatment for pulmonary tuberculosis: a case report and literature review. Intern Med 2013;52:2457-60. [Crossref] [PubMed]
- Park DH, Lee SA, Jeong HJ, et al. Rifampicin-induced minimal change disease is improved after cessation of rifampicin without steroid therapy. Yonsei Med J 2015;56:582-5. [Crossref] [PubMed]
- Kim JS, Kim KJ, Choi EY. Minimal change disease related to rifampicin presenting with acute renal failure during treatment for latent tuberculosis infection: A case report. Medicine (Baltimore) 2018;97:e10556. [Crossref] [PubMed]
- Mori S, Matsushita Y, Arizono K. Minimal-change nephrotic syndrome associated with isoniazid in anti-tuberculosis chemoprophylaxis for a patient with rheumatoid arthritis. Intern Med 2011;50:253-7. [Crossref] [PubMed]
- Tanaka H, Oshiro Y, Kawanaka N, et al. A case of MPO-ANCA-related nephritis caused by an anti-tuberculosis drug. Nihon Jinzo Gakkai Shi 2013;55:172-6. [PubMed]
- Ambanelli U, Manganelli P, Ferraccioli GF. Clinical efficacy and adverse effects of tiopronin in rheumatoid arthritis. Report of a follow-up in 50 patients. Z Rheumatol 1982;41:235-9. [PubMed]
- Dolin DJ, Asplin JR, Flagel L, et al. Effect of cystine-binding thiol drugs on urinary cystine capacity in patients with cystinuria. J Endourol 2005;19:429-32. [Crossref] [PubMed]
- Albouy R, Bendaoud B, Casburn-Budd R, et al. Decrease of the functional affinity of rheumatoid factors under the effect of tiopronin. Rev Rhum Mal Osteoartic 1991;58:43s-9s. [PubMed]
- Asanuma H, Nakai H, Takeda M, et al. Clinical study on cystinuria in children--the stone management and the prevention of calculi recurrence. Nihon Hinyokika Gakkai Zasshi 1998;89:758-65. [Crossref] [PubMed]
- Reveillaud RJ, Blanc G, Daudon M. Nephrotic syndrome and skin disorders appearing during alpha-mercapto-propionyl-glycine treatment of 2 cases of cystinic lithiasis. J Urol Nephrol (Paris) 1978;84:663-7. [PubMed]
- Rizzoni G, Pavanello L, Dussini N, et al. Nephrotic syndrome during treatment with alpha-mercaptopropionylglycine. J Urol 1979;122:381-2. [Crossref] [PubMed]
- Ferraccioli GF, Peri F, Nervetti A, et al. Tiopronin-nephropathy: clinical, pathological, immunological and immunogenetic characteristics. Clin Exp Rheumatol 1986;4:9-15. [PubMed]
- Mordini M, Guidoni G, Maestrini M, et al. Basic treatment of rheumatoid arthritis with tiopronin. A study of 25 cases. Minerva Med 1989;80:1019-23. [PubMed]
- Lindell A, Denneberg T, Enestrom S, et al. Membranous glomerulonephritis induced by 2-mercaptopropionylglycine (2-MPG). Clin Nephrol 1990;34:108-15. [PubMed]
- Shibasaki T, Murai S, Kodama K, et al. A case of nephrotic syndrome due to alpha-mercaptopropionyl glycine in a patient with familial cystinuria. Nihon Jinzo Gakkai Shi 1990;32:933-7. [PubMed]
- Koeger AC, Palazzo E, De Person JF, et al. Subacute development of nephrotic syndrome caused by tiopronin therapy. A propos of 4 cases. Rev Rhum Ed Fr 1993;60:78. [PubMed]
- Alvarez Navascues R, Vidau Arguelles P, Rodriguez Suarez C, et al. Nephrotic syndrome and anasarca status, secondary to treatment with tiopronin in a case of cystinuria. Arch Esp Urol 2001;54:438-40. [PubMed]
- Lupo A, Faraggiana T, Loschiavo C, et al. Nephrotic Syndrome During 2-Mercapto-Propionyl-Glycine (Thiola) Therapy. Nephron 1981;28:96-9. [Crossref]
- Stratta P, Coratelli P, Camussi G, et al. Sindrome nefrosica durante trattamento con 2-mercaptopropionilglicina. Minerva Nefrol 1978;25:87-92.


