
Hybrid operating room for the intraoperative CT-guided localization of pulmonary nodules
Introduction
Screening for early lung cancer with low-dose computed tomography (CT) (1,2) has increased the detection of both small pulmonary nodules (<1 cm in size) and ground glass opacities (GGOs). Unfortunately, most of these lesions are thoracoscopically invisible or impalpable with video-assisted thoracic surgery (VATS), ultimately requiring an accurate preoperative CT (POCT)-guided localization. However, this traditional two-stage approach is not devoid of potential complications, including wire dislodgement, pneumothorax, and/or hemothorax (3).
With the advent of hybrid operating rooms (HORs), simultaneous single-stage identification and removal of such lesions has become possible. In this scenario, intraoperative CT (IOCT)-guided lesion localization can be followed by VATS performed within a HOR—thus abating the need for patient transfer. Gill et al. (4) described this approach for the first time in 2015, and several independent investigators subsequently confirmed its feasibility. Here, we review the technical developments and the state-of-the-art in the field of IOCT-guided localization and resection of small pulmonary nodules performed within a HOR.
Methods
We searched PubMed, the most commonly used bibliographic database for biomedical topics, with the following keywords: “hybrid operating room”, “pulmonary nodules”, “video-assisted thoracoscopic surgery”, “image-guided”, and “localization”. Only studies written in English were considered in this review.
HOR setting
The HOR generally consists of two components, i.e., an imaging system and a surgical table. As far as lung localization procedures are concerned, the most commonly used imaging system is the C-arm cone-beam CT (CBCT) scanner (ARTIS zeego®; Siemens Healthcare GmbH, Erlangen, Germany). Multiple detector CT (MDCT) (Definition FLASH CT; Siemens, Washington, DC, USA) (5) and mobile O-arm CBCT (Medtronic Japan Co., Ltd., Tokyo, Japan) (6) have been also proposed for the detection of pulmonary lesions in HORs.
Ujiie et al. (5) described an MDCT system implemented within a “guided therapeutic operating room”. Specifically, lesions were localized using MDCT with the patient being under local anesthesia. After pulmonary lesion localization, the study participants were transferred to a CBCT table for induction of general anesthesia. In spite of localization and surgery being performed in the same operating room, the risk of wire dislodgement and pneumothorax during patient transfer was not negligible.
Ohtaka et al. (6) introduced an O-arm CT scan system integrated with an “intrathoracic stamping” method for localization of pulmonary lesions. However, potentially harmful radiation exposure to the patient remains a primary drawback inherent to this approach. Accordingly, the O-arm center may require numerous time-consuming manual setting adjustments (owing to the lack of a predetermined scanning field).
In this context, C-arm CBCT is superior to the two aforementioned systems in several respects (7). First, it has an open gantry design that maximizes flexibility during lesion targeting, ultimately facilitating localization of the puncture site. Second, C-arm CBCT is capable of performing circumferential scanning around the surgical table, thereby reducing the risk of potential complications occurring during patient transportation (e.g., wire dislodgement, pneumothorax, and/or hemothorax). Finally, the iGuide navigation software integrated with the system offers a user-friendly, intuitive localization procedure.
Procedural workflow
The workflow for CT-guided pulmonary lesion localization has been described in several published studies (4,7-11). First, patients should be intubated (either with a single- or a double-lumen tube) and placed under general anesthesia. Positioning should be performed according to lesion localization, with supine, lateral decubitus, modified semiprone or semisupine positions being all suitable (7). Importantly, the anesthesia workstation pipelines should be gathered and aligned within the table edge to avoid any potential entanglement with the C-arm during CBCT rotation (Figure 1A). After an initial scan with a 6-sec acquisition protocol (6s Dyna-CT Body), the localization path should be planned via a syngo Needle Guidance of a syngo X-Workplace (Siemens Healthcare GmbH) on the CT image (Figure 1B)—ultimately delineating the needle path from the skin entry site to the target lesion. A laser beam that marks both the needle entry point and the proper angle for needle path should subsequently be projected from the C-arm onto the patient’s skin. The marker needle (18-, 19- or 22-gauge) is therefore placed under laser guidance (Figure 1C). An additional 6-sec Dyna-CT scan is performed to confirm an appropriate needle positioning, with the lesion being subsequently localized using hookwire or dye marking. The entire procedure (Dyna-CT scanning, needle puncture, and lesion localization) should be performed under end-inspiratory breath hold to ensure that the lung is kept fully inflated.
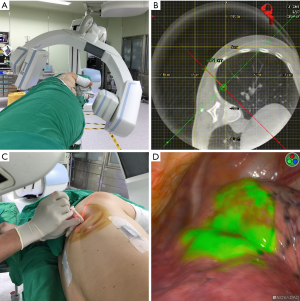
Localization procedure and operators
The CBCT and iGuide system may be operated either by surgeons in cooperation with radiologists (4,5,11,12) or by surgical teams alone (6,7). When the procedure is performed in cooperation with radiologists, the localization time has been shown to vary between 10.5 and 162 min (median: 30−40 min). In a study conducted on ten patients in which the O-arm CT was operated by surgeons, Ohtaka et al. (6) have reported a median localization time of 67 min (range, 58−110 min). Hsieh et al. (7) reported their experience on 30 consecutive patients whose pulmonary nodules were localized by a surgical team. The case series was divided into two sequential groups based on the timing of operation. Specifically, the early group consisted of the first 15 patients, whereas the late group comprised the last 15 cases. The localization time of the late group was significantly shorter than that of the early group (median: 49 vs. 22 min, respectively). In addition, a detailed analysis of the factors influencing the localization time (e.g., reciprocal localization of the C-arm and the surgical table, patient positioning, and tumor anatomical location) was performed. In a subsequent study conducted by the same research group in 100 consecutive cases, the mean localization time was 20.58 min (10). These results suggest that—compared to radiologists—surgeons require a significant learning curve to be proficient in lesion localization. However, increased procedural experience allows surgeons to achieve very favorable treatment outcomes.
Techniques to localize pulmonary nodules within a HOR
Several techniques for preoperative localization of pulmonary nodules in HORs have been proposed. However, no firm recommendation can be made on the optimal methodology (Table 1). Hookwire localization remains the standard method for the preoperative localization of non-palpable pulmonary nodules (3), albeit being limited by not negligible risks (e.g., pneumothorax, pulmonary hemorrhage, and wire dislodgement, most frequently related to lung collapse occurring during one-lung ventilation). Although other metallic markers (e.g., fiducials and coils) allow multiple lesions to be localized, they require intraoperative fluoroscopic guidance—ultimately increasing radiation exposure to both the patient and the operator (13-15).

Full table
Besides metallic materials, dye injections have been proposed for localization purposes. This approach is technically easier to perform and carries a lower risk of pneumothorax and pulmonary hemorrhage. However, some of the dyes currently in use (e.g., barium and lipiodol) are not water soluble and may increase the risk of embolism (16). In addition, they require the use of intraoperative fluoroscopy to confirm a correct localization. In this context, a water blue dye solution has been proposed a safe, cost-effective, and less sensitizing marker. However, its use is limited by the risk of parenchymal diffusion and dye spillage—which may hamper unequivocal lesion localization (17,18). Bellomi et al. (19) have investigated the potential utility of radiotracer technetium (99m) macroaggregates for localizing nonpalpable lung nodules. However, extravasation of the radiotracer into the pleural cavity represents a significant shortcoming. In addition, this approach has not been previously utilized in a HOR setting.
In recent years, indocyanine green (ICG)—a fluorescent dye characterized by specific absorption and emission wavelengths in the low-energy near-infrared (NIR) spectrum—has been utilized for a variety of clinical applications, including monitoring of cardiac output, measurements of liver blood flow, and ophthalmic angiography (20,21). Wen et al. (18) have previously reported their experience with 26 patients harboring pulmonary nodules who underwent NIR marking in a HOR (Figure 1D). Compared to other injection markers, ICG offers significant advantages, including an intuitive real-time delineation of margins (resulting in a clearer operating field) and a reduced radiation exposure. The main shortcoming inherent in ICG use is the requirement of a specific NIR detection system.
Safety and efficacy of intraoperative versus preoperative localization
Although the IOCT approach has numerous potential advantages, few studies have provided a direct head-to-head comparison of IOCT versus traditional POCT-guided localization. Chen et al. (22) retrospectively compared cases who underwent IOCT (n=25) and POCT (n=283), with the former technique being performed with C-arm CBCT using the blue dye localization technique. Compared with the POCT group, the global operating time was shorter in the IOCT group (192.6 vs. 244.1 min, P=0.003), despite a longer localization time (33.1 vs. 22.3 min, P<0.001). Although two major complications occurred in the IOCT group (i.e., large pneumothorax and diaphragm injury), patients who underwent POCT showed a relatively higher rate of minor complications (e.g., small pneumothorax, intrapulmonary focal hemorrhage). All of these cases regressed under conservative treatment alone. In a prospective study, we have previously compared 34 cases who underwent IOCT with 30 patients whose lesions were localized with POCT (23). The IOCT approach was based on C-arm CBCT using the hookwire/dye localization technique. The results indicated that localization time was similar in the two groups, although the IOCT group was characterized by (I) a shorter “time at risk” (defined as the time interval from completion of localization to skin incision), (II) a longer time under general anesthesia, and (III) a longer total operating room utilization time. With regard to procedural complications, pneumothorax and pulmonary hemorrhage occurred more frequently in the POCT group, although no specific therapeutic interventions were required (Table 2).
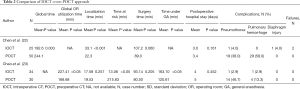
Full table
Besides the success rates and the occurrence of procedural complications, the radiation dose delivered to the patient is a factor that needs careful consideration. A previous study has shown that the dose delivered by CBCT and MDCT was similar when the head was imaged; however, as far as abdominal studies were concerned, the use of CBCT resulted in a higher radiation dose delivered to the patient (24). Unfortunately, data in the field of thoracic surgery are inconsistent. Using O-arm CT (which lacks a predetermined scanning field), Ohtaka et al. (6) reported an increased patient exposure. Notably, both Hsieh et al. (9) and Zhao et al. (25) showed that the radiation dose associated with the use of C-arm CBCT was similar to that delivered during conventional CT-guided biopsies (223.2 vs. 281 mGy, respectively). In contrast, Chen et al. (22) demonstrated that the radiation exposure related to preoperative localization in a HOR was higher than that of a conventional CT room. Image quality and operator experience are among the contributing factors that may explain such discrepancies.
Because all of the aforementioned studies focused on the radiation dose emitted by the CT scanner, we specifically investigated the dose received by the patient (23). To this aim, we placed four thermoluminescent dosimeters (UD-802A; Panasonic, Osaka, Japan) at the lesion level around the patient’s chest wall. The mean dose measured by the dosimeters was used for the purpose of analysis. The results showed a trend toward higher doses in the MDCT group compared with patients operated in a HOR (median values: 6.88 versus 3.65 mSv, respectively), albeit not significantly so.
Open issues and limitations of IOCT localization
The HOR technology may offer significant advantages in the field of thoracic surgery, including: (I) a significant shortening in the time from diagnosis (through IOCT localization) to curative treatment of pulmonary nodules or GGOs and (II) a reduced need for patient mobilization. However, the HOR environment is not without limitations as far as the VATS surgery is concerned. As previously discussed by our group (23), the surgical table in current HORs is not specifically for thoracic operations and lacks a hinge joint for bending. This technical limitation may decrease the available intercostal space—ultimately hampering the feasibility of VATS and increasing the risk of intercostal nerve injury. Another shortcoming lies in the simultaneous localization of multiple lung nodules, which is presently unfeasible. With the current C-arm CBCT technology (ARTIS zeego®), only one nodule at a time can be localized. If needle puncture is complicated by significant pneumothorax, the localization procedure should be aborted. Notably, a robot-supported C-arm angiography system (ARTIS pheno®) has recently allowed the simultaneously localization of multiple nodules. However, more studies are needed before firm conclusions on this technical development can be made. Our research group is also conducting a clinical trial (https://clinicaltrials.gov/ct2/show/NCT03395964) that will provide a head-to-head comparison of IOCT vs. POCT.
Conclusions
Although some issues still need to be solved, the current literature clearly indicates that the HOR setting—which offers imaging capabilities within the operating room—offers great potential as a safe and effective tool to localize small pulmonary nodules or GGOs. In the upcoming years, further technical advances in the field of HORs will be introduced—including the integration of electromagnetic navigation bronchoscopy (26) and robotic surgery. In summary, the HOR is expected to provide a paradigm shift for prompt diagnosis and removal of early lung cancer, ultimately decreasing the burden and mortality due to this malignancy.
Acknowledgements
Funding: This study was financially supported by a grant (No. CMRPG3F1813) from the Chang Gung Memorial Hospital, Taiwan.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [Crossref] [PubMed]
- Gould MK, Donington J, Lynch WR, et al. Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e93S-120S.
- Ichinose J, Kohno T, Fujimori S, et al. Efficacy and complications of computed tomography-guided hook wire localization. Ann Thorac Surg 2013;96:1203-8. [Crossref] [PubMed]
- Gill RR, Zheng Y, Barlow JS, et al. Image-guided video assisted thoracoscopic surgery (iVATS) - phase I-II clinical trial. J Surg Oncol 2015;112:18-25. [Crossref] [PubMed]
- Ujiie H, Kato T, Hu HP, et al. A novel minimally invasive near-infrared thoracoscopic localization technique of small pulmonary nodules: A phase I feasibility trial. J Thorac Cardiovasc Surg 2017;154:702-11. [Crossref] [PubMed]
- Ohtaka K, Takahashi Y, Kaga K, et al. Video-assisted thoracoscopic surgery using mobile computed tomography: new method for locating of small lung nodules. J Cardiothorac Surg 2014;9:110. [Crossref] [PubMed]
- Hsieh MJ, Wen CT, Fang HY, et al. Learning curve of image-guided video-assisted thoracoscopic surgery for small pulmonary nodules: A prospective analysis of 30 initial patients. J Thorac Cardiovasc Surg 2018;155:1825-32.e1. [Crossref] [PubMed]
- Fang HY, Chao YK, Hsieh MJ, et al. Image-guided video-assisted thoracoscopic surgery for small ground glass opacities: a case series. J Vis Surg 2017;3:142. [Crossref] [PubMed]
- Hsieh MJ, Fang HY, Lin CC, et al. Single-stage localization and removal of small lung nodules through image-guided video-assisted thoracoscopic surgery. Eur J Cardiothorac Surg 2017. [Epub ahead of print]. [PubMed]
- Chao YK, Wen CT, Fang HY, et al. A single-center experience of 100 image-guided video-assisted thoracoscopic surgery procedures. J Thorac Dis 2018;10:S1624-30. [Crossref] [PubMed]
- Yang SM, Ko WC, Lin MW, et al. Image-guided thoracoscopic surgery with dye localization in a hybrid operating room. J Thorac Dis 2016;8:S681-9. [Crossref] [PubMed]
- Kostrzewa M, Kara K, Rathmann N, et al. Computed Tomography-Assisted Thoracoscopic Surgery: A Novel, Innovative Approach in Patients With Deep Intrapulmonary Lesions of Unknown Malignant Status. Invest Radiol 2017;52:374-80. [Crossref] [PubMed]
- Sancheti MS, Lee R, Ahmed SU, et al. Percutaneous fiducial localization for thoracoscopic wedge resection of small pulmonary nodules. Ann Thorac Surg 2014;97:1914-8; discussion 1919.
- Park CH, Han K, Hur J, et al. Comparative Effectiveness and Safety of Preoperative Lung Localization for Pulmonary Nodules: A Systematic Review and Meta-analysis. Chest 2017;151:316-28. [Crossref] [PubMed]
- Mayo JR, Clifton JC, Powell TI, et al. Lung nodules: CT-guided placement of microcoils to direct video-assisted thoracoscopic surgical resection. Radiology 2009;250:576-85. [Crossref] [PubMed]
- Watanabe K, Nomori H, Ohtsuka T, et al. Usefulness and complications of computed tomography-guided lipiodol marking for fluoroscopy-assisted thoracoscopic resection of small pulmonary nodules: experience with 174 nodules. J Thorac Cardiovasc Surg 2006;132:320-4. [Crossref] [PubMed]
- Lin MW, Tseng YH, Lee YF, et al. Computed tomography-guided patent blue vital dye localization of pulmonary nodules in uniportal thoracoscopy. J Thorac Cardiovasc Surg 2016;152:535-44.e2. [Crossref] [PubMed]
- Wen CT, Liu YY, Fang HY, et al. Image-guided video-assisted thoracoscopic small lung tumor resection using near-infrared marking. Surg Endosc 2018;32:4673-80. [Crossref] [PubMed]
- Bellomi M, Veronesi G, Trifiro G, et al. Computed tomography-guided preoperative radiotracer localization of nonpalpable lung nodules. Ann Thorac Surg 2010;90:1759-64. [Crossref] [PubMed]
- Yannuzzi LA. Indocyanine green angiography: a perspective on use in the clinical setting. Am J Ophthalmol 2011;151:745-51.e1. [Crossref] [PubMed]
- Zelken JA, Tufaro AP. Current Trends and Emerging Future of Indocyanine Green Usage in Surgery and Oncology: An Update. Ann Surg Oncol 2015;22 Suppl 3:S1271-83. [Crossref] [PubMed]
- Chen PH, Hsu HH, Yang SM, et al. Preoperative Dye Localization for Thoracoscopic Lung Surgery: Hybrid versus Computed Tomography Room. Ann Thorac Surg 2018;106:1661-7. [Crossref] [PubMed]
- Chao YK, Pan KT, Wen CT, et al. A comparison of efficacy and safety of preoperative versus intraoperative computed tomography-guided thoracoscopic lung resection. J Thorac Cardiovasc Surg 2018;156:1974-83.e1. [Crossref] [PubMed]
- Kwok YM, Irani FG, Tay KH, et al. Effective dose estimates for cone beam computed tomography in interventional radiology. Eur Radiol 2013;23:3197-204. [Crossref] [PubMed]
- Zhao ZR, Lau RW, Yu PS, et al. Image-guided localization of small lung nodules in video-assisted thoracic surgery. J Thorac Dis 2016;8:S731-7. [Crossref] [PubMed]
- Zhao ZR, Lau RW, Ng CS. Hybrid theatre and alternative localization techniques in conventional and single-port video-assisted thoracoscopic surgery. J Thorac Dis 2016;8:S319-27. [PubMed]


