
Rapidly growing solitary fibrous tumors of the pleura: a case report and review of the literature
Introduction
A solitary fibrous tumor (SFT) is a rare mesenchymal spindle cell tumor first reported by Wagner in 1870 (1). SFT originates from a CD34(+) dendritic mesenchymal cell, almost diffusely distributed in human connective tissue so it can occur almost anywhere in the body, including mediastinum, pericardium, orbit, peritoneum, intracranial, kidney, prostate, etc. However, pleura is the most common (2-8). Klemperer and Rabin first reported solitary fibrous tumors of the pleura (SFTP) pathological features and was listed as an independent disease in 1931 (9). The etiology of SFT is not clear. There are no known genetic, environmental or susceptibility risk factors, and it is not related to asbestos exposure.SFTP is mostly a painless regional mass with slow growth, and pleural effusion is uncommon (10,11). SFTP is characterized by insidious onset and no obvious clinical symptoms in the early stage. Cough, chest pain, chest tightness and dyspnea occur only after tumor enlargement and compression of surrounding tissues.The best treatment for SFTP is total resection of primary and local recurrent diseases. Long term follow-up is necessary because of the risk of recurrence.We report a case of the rapid growth of SFTP within nine months which with bloody pleural effusion and review of the literature. Compared with other literature reports, this case is characterized by rapid tumor enlargement and bloody pleural effusion. At the same time, we further discussed the clinicopathological features of SFTP and the predictive factors of malignant tumor.
We present the following case in accordance with the CARE reporting checklist (available at http://dx.doi.org/10.21037/atm-20-4974).
Case presentation
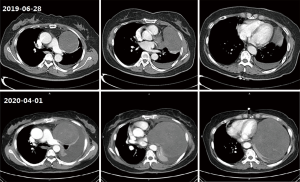
A 60-year-old female patient complained of progressive dyspnea for two months and was admitted to the respiratory department of our hospital. She had a history of diabetes and had been taking metformin and glimepirea, and her blood sugar levels were well controlled. She has no history of smoking or asbestos exposure. Nine months ago, a computed tomography (CT) scanned in the chest revealed a space-occupying lesion in her left chest during (Figure 1), and she gave up further diagnosis and treatment for fear of surgery. In the past two months, she has gradually aggravated breathing difficulties, which are more noticeable after exercise and cannot be relieved after rest, accompanied by a cough and a little sputum. She does not have chills, fever, hemoptysis, chest tightness, or chest pain. Physical examination showed that: the body temperature was 36.4 °C, the superficial lymph nodes in the whole body were not enlarged, the trachea was in the middle, the breath sounds in the left lung were significantly reduced, there was no dry and wet rayed sounds in the two lungs, and both lower limbs were hollow edema.
Her peripheral blood WBC was 12.4×109/L, the percentage of neutrophils was 86.9%, albumin was only 32.4 g/L, and B-type natriuretic peptide (BNP) was measured at 28 pg/mL (normal value 0–100 pg/mL). Tumor markers, including CEA, AFP, NSE, CYFRA211, SCCA, CA-125, CA-50, CA19-9, CA72-4, CA15-3, and CA24-2 were all regular. Closed thoracic drainage was conducted. Finding that pleural effusion was bloody, specific gravity was 1.028, Rivalta test was positive, the WBC was 790/L, the lymphocytes and neutrophils were 60% and 40% respectively; Total protein 39.40 g/L, lactate dehydrogenase (LDH) 181 U/L, adenosine deaminase (ADA) 8 U/L, glucose 7.55 mmol/L. Cytological examination of pleural fluid revealed a small number of mesenchymal cells, but no malignant cells were found.
The CT (2020-04-01) of the patient showed a round slightly low-density tumor about 15.8×13.2 cm in the left thoracic cavity, the margin of the tumor seems to have a capsule, its internal density was uneven, and with the mixed flocculent slightly higher density, no obvious enhanced, considering the pleural origin possible. The trachea was in the middle, and the trachea and bronchus were unobstructed. The bronchus of the left upper and lower lobes was narrow, and the left lung was partially distended. The left thoracic cavity can see the water density shadow. However, there were just a few striations in the upper lobe of the right lung. The pericardium was thickened. Mediastinum was compressed to the right, and lymph nodes can be seen in it. Compared with nine months ago (2019-06-28), the tumor was significantly enlarged, and the pleural effusion was increased (Figure 1).
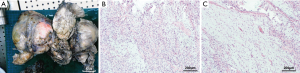
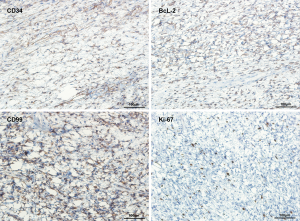
The patient was transferred to cardiothoracic surgery. After adequate preparation, left thoracotomy exploratory surgery was conducted on April 7, 2020. The mass in the chest was broad and hard, which was widely attached to the surrounding tissues, especially the mediastinum pleura and the left upper lung. Then, the mass and surrounding tissue were carefully separated along with the extracapsular mass. Eventually, the mass was completely removed. HE staining showed the presence of both sparse and dense regions (Figure 2). Immunohistochemistry showed that CD34 (+), bcl-2 (+), CD99 (+), Ki-67 (10%+) and S-100 (−), SMA (−), CR (−), Calretinin (−) (Figures 3,4). The final pathological diagnosis was an SFT originating from the mediastinum pleura. The patient recovered well and was dismissed 23 days later. No radiotherapy or chemotherapy was given. At present, there is not any recurrence or pleural effusion on CT, and there is no significant fluctuation of blood glucose.

All procedures performed in studies involving human participants were in accordance with the Helsinki Declaration (as revised in 2013), and this study was approved by the ethics committee of the Affiliated Hospital of Jiangnan University. Written informed consent was obtained from the patient for publication of this study and any accompanying images.
Discussion
SFT was classified as a fibroblast/myofibroblast tumor by the WHO tumor classification criteria for soft and bone tissues in 2013 (12). As an interstitial-derived tumor, the etiology of the SFT is unclear, there are no known genetic, environmental, or predisposing risk factors, and it is not linked to asbestos exposure, perhaps because SFT is not mesothelioma. However, it is currently believed that SFT is a translocation related tumor, which is consistent with the fusion of the NAB2-STAT6 gene caused by repeated intrachromosomal rearrangement of 12q chromosome, and this translocation may be the main factor of its pathogenesis (13). Most of SFT originated from pleura, especially the visceral pleura, a few of which occurred outside pleura and rarely in the lung (14). The incidence of SFTP was less than 5% of all pleural tumors (15). SFT occurred in connective tissue, which unrelated to age, so there is no age difference in the onset of SFT. Some scholars summarized 378 cases of SFTP reported in Chinese and English. They found that 195 cases were male, 183 cases were female, and the age span of onset was 6–81 years. The disease usually occurred in middle age and with no significant gender difference (16).
Generally, SFTP has a concealed onset, slow growth, no obvious clinical symptoms in the early stage, which brings difficulties to diagnosis. Our patient also experienced symptoms only after the tumor rapidly increased and oppressed the surrounding tissues. Common symptoms include cough, chest pain, chest distress and dyspnea. These symptoms are usually secondary to compression of adjacent structures and local invasion of the tumor after enlargement. Exceedingly rare causes of dyspnea have been reported as a result of SFTP invasion of the left atrium through the pulmonary vein and recovery through surgical treatment (17).Tumor compression of lung parenchyma and the laryngeal nerve can cause hemoptysis and hoarseness, respectively (18). Compression of the mediastinum can lead to displacement, compression of the superior vena cava can lead to cyanosis, venous dilation, edema and hypotension of the upper body (face, neck, chest and upper limbs), as well as other symptoms of the superior vena cava syndrome (SVCS) (19). In contrast, compression of the liver can lead to weight loss, loss of appetite, fatigue, and even right upper abdominal mass and lower limb edema (20).
SFTP combined with pleural effusion is uncommon, the incidence is less than 20%, and pleural effusion may play a significant role in the differentiation of benign and malignant tumors (21). A limited number of literature reports suggest that bloody pleural effusion may be involved in rapid tumor enlargement and increased surface tension leading to superficial vascular rupture (18). However, our patient’s may be linked to increased exudation and infection caused by tumor compression on the lung and large blood vessels. Iatrogenic chylothorax has also been reported due to the more massive lymphatic vessel injury following endoscopic ultrasound and fine-needle aspiration (22). As our patient, all cytological results of pleural effusion were negative, which may be related to the complete capsule of the tumor, and the cells are difficult to shed (23).
The function of the pleura and endocrine system is not fully independent. Occasionally, SFTP can induce paraneoplastic syndromes, such as hypertrophic osteoarthropathy (24) and hypoinsulinemic hypoglycemia caused by ectopic secretion of insulin-like growth factor II which knew as Doege Potter syndrome (DPS) (25).Hypertrophic osteoarthropathy, known as Pierre-Marie-Bamberger syndrome, is characterized by clubbing of the fingers or toes caused by calcification of the bone surface and soft tissue. Also, the increase of human beta chorionic gonadotropin-releasing factor leads to gynecomastia (26), and cerebellar degeneration is also occasionally seen (27). Distant metastasis of SFTP is very rare, but it has been reported in the pancreas, lung, and thyroid (28,29).Similarly, malignant transformation occurs occasionally (30). Our patient has no paraneoplastic syndrome.
SFTP mostly intrudes into the thoracic cavity and forms pedicle, while a few may invade lung tissue. CT is an essential method for the clinical diagnosis of SFTP. Benign SFTP usually presents solitary masses on CT, which are usually round or spindle-shaped, with an uneven size, clear boundaries, homogeneous density of soft tissue, with the capsule, with or without lobulation. Mediastinum and lung parenchyma are often displaced under pressure. Malignant SFTP usually has unclear boundaries, adhesion with or invasion of surrounding tissues, uneven density, calcification, and pleural effusion is often the case (11). Additional signs include obtuse, acute, or tapering angles between lesions and mediastinum due to tumor size, “geographic pattern” due to the presence of large vessels, necrosis, and calcification, the presence of cleavage planes and the absence of lymphadenopathy or pleural edema (31). Magnetic resonance (MR) imaging is a supplement to other imaging methods for evaluating the patient’s condition (32), while (18)F-FDG PET/CT has limited diagnostic value for malignant SFTP in suspected patients (33). Bronchoscopy was used for large tumors and compression of the lung, which has a significant impact on the bronchial tree (14). And it has been reported that endobronchial ultrasound (EBUS) was used to evaluate tumor angiogenesis before the operation, and tissue samples were obtained under thoracoscopy for diagnosis (34).
Descriptions of lung function are scarce. Mendez-Sanchez et al. reported three patients with SFTP, all of them presented mild to moderate restrictive ventilation dysfunction at baseline, and all showed significant improvement after surgical treatment. Indicators such as FVC and FEV1 all recovered to close to the normal level to 30% of the normal predicted value. The authors believe that if the tumor occupies a specific volume in the chest, respiratory mechanics and lung compliance will be damaged. The reduction of lung volume and the increase of airway resistance need to be compensated by the increase of inspiratory muscle strength and the change of breathing mode (rapid shallow breathing), but this may be at the expense of the increase of dyspnea. At the same time, the transmural pressure gradient decreased, which affected the ventilation/perfusion ratios and, therefore, gas exchange;this is a different reason for dyspnea. Also, the authors believe that lung function and CT scans are excellent objective indicators for follow-up (35).
The histopathological feature of SFT is the coexistence of sparse and dense regions, which are separated by fibrous stroma, and with hemangiopericytoma branching vessels (36). The cell’s dense area is composed of mild fusiform cells, which are arranged in short interlaced bundles, forming a herringbone or mat-like arrangement. The sparse area may be highly collagenated, or it may present an uncommon myxoid change. Malignant SFT is composed of large cell components, with infiltrative growth, moderate to prominent cell atypia, and mitotic images are standard (37,38). The most valuable positive markers of SFT immunohistochemistry were CD34, CD99, Bcl-2, and vimentin, cytokeratin (CK) was always negative. Recent studies have shown that nuclear STAT6 immunoreactivity is a highly sensitive and specific marker of SFT, and almost all SFT contain NP2-STAT6 fusion gene (39). The positive expression rate of STAT6 was even 100% in benign SFT but decreased in malignant SFT (40). Also, the Ki67 proliferation index of 10% or more was helpful in the diagnosis of malignant SFT (21). Gene analysis based on next generation sequencing may be helpful to reveal the mutation characteristics of SFTP (41).
Because the clinical manifestations of SFTP are nonspecific, it is usually diagnosed in the advanced stage of development. Fortunately, 80% of the time are benign (42). Adhesions or unclear boundaries with surrounding tissues, pleural effusion or calcification, maximum tumor diameter higher than 10 cm, invasive growth, uneven density, metastasis or recurrence, paraneoplastic syndrome, moderate to significant cell heterogeneity, high Ki67 proliferation index, and low STAT6 expression suggest that SFTP may be malignant. A CT-guided transthoracic biopsy is helpful for its diagnosis and differential diagnosis, but it requires extensive sampling. Classification stages based on histologic and morphologic indicators of SFTP have proposed by de Perrot et al. (43).
SFTP needs to be differentiated from a variety of pleural soft tissue tumors. Such as pleural mesothelioma, desmoid fibromatosis, inflammatory myofibroblastoma, epithelioid hemangioendothelioma, pseudotumor of pleura, synovial sarcoma, and primary lymphoma of pleura (44). In most cases, under the correct clinical background, using the appropriate immunohistochemistry method to consider the morphological characteristics can make a reliable diagnosis (45).
The best treatment for SFTP is the total resection of primary and local recurrent diseases. Patients with SFTP who received surgery had better overall survival than those who did not (46). After resection, the compressed lung tissue can be fully expanded, and the related paraneoplastic syndrome can also be successfully treated (47). The standard surgical methods include lung tumor resection, wedge resection, segmental resection and lobectomy (48). Video-assisted thoracoscopic surgery is mostly used for resection of small tumors, but it was also used for resection of large fibromas around 10 cm (49). Da Vinci Surgical System has been reported to be used for the complete surgical resection of an anterior mediastinal tumor in an obese patient (50). For patients with a large tumor or extensive adhesions, thoracotomy is often used, and in case of incomplete resection or recurrence, the secondary operation may be needed (51,52). Originated from the pleural wall, extrapleural resection can be carried out without removing the chest wall (53), and patients with pericardial cavity involvement may need cardiopulmonary bypass support (54). Preoperative percutaneous embolization has been reported to reduce the risk of intraoperative bleeding significantly; however, SFTP embolization may lead to a rare potential life-threatening complication (55).
Surgical resectability is the most important prognostic factor. For unresectable patients, the effect of chemotherapy and radiotherapy is controversial, and more research is needed to confirm. Radiotherapy can be used for preoperative tumor reduction or adjuvant treatment in patients with a malignant tumor or surgical margin defect (56). Conventional chemotherapy may effectively control or stabilize locally advanced and metastatic SFTP (57). Hyperthermic intrathoracic chemotherapy has been used in primary and secondary pleural malignancies such as SFTP to improve local control, prolong survival, and improve quality of life (58). Also, safe, efficient, and fewer side effects of local microwave-thermal ablation has been used for the treatment of postoperative recurrence of SFTP (59).
Surgical resection is the gold standard of SFTP treatment. For malignant SFTP, radiotherapy, and conventional chemotherapy drugs only show limited efficacy (13), while the research on targeted therapy is either still in progress (60). High expression of multiple growth factors is common in SFT, which also provides an objective basis for antiangiogenesis therapy of SFT (61). Sunitinib and pazopanib have shown specific clinical and preclinical efficacy (62,63). The combination of temozolomide and bevacizumab may be a feasible choice for patients with the inoperable disease (64), and it has been proved to be an effective and tolerable treatment in the local advanced, recurrent and metastatic malignant SFT (65). Also, chemotherapy combined with molecular targeted therapy has produced promising results, and the consequences of further phases are awaited (56). In recent years, increased attention has been paid to the research of immune checkpoint blockade therapy. It has been reported that patients with malignant SFTP have received concurrent chemoradiotherapy and Te-mozolomide/bevacizumab treatment, it was found ineffective, but programmed cell death protein 1 (PD-1) has played a specific role (66). It has been found that low tissue miR-125b level can predict the malignant degree of SFTP, suggesting that miR-125b plays a potential role in the growth and malignant transformation of SFTP, and miRNA-125b may be a biomarker or potential therapeutic target of SFTP (67).
SFT was mostly benign, and 10–20% were malignant or potentially malignant. Surgical removal of SFTP usually produces satisfactory results, with a 5-year disease-free survival rate of about 80%. However, the recurrence rate of malignant SFTP was 63%, even after complete resection (66). Age, size, mitosis, and/or necrosis have been proposed as risk stratification factors for predicting prognosis (36). Recurrence mostly occurs within two years after surgery, and long-term follow-up after resection is necessary (68).
Conclusions
SFT is a rare spindle cell tumor-derived from mesenchymal cells. It does not have any known genetic, environmental, or predisposing risk factors. As a translocation related tumor, SFT may be related to the fusion of the NAB2-STAT6 gene caused by repeated rearrangement of the 12q chromosome. It is most frequent in the pleura. The clinical manifestations of SFTP were persistent painless mass and slow growth. With the increase of tumor, there will be corresponding compression symptoms such as cough, chest pain, chest distress and dyspnea. Pleural effusion is uncommon, and the cytological of pleural effusion is negative. Occasionally, SFTP can induce paraneoplastic syndrome, distant metastasis, and malignant transformation.
Lung function may have mild to moderate restrictive ventilation dysfunction. CT is a crucial method for the clinical diagnosis of SFTP. The histopathological characteristics of SFTP are the coexistence of sparse and dense regions. The most valuable positive markers for immunohistochemistry are CD34, CD99, Bcl-2, and vimentin. Recent studies show that the immunoreactivity of STAT6 is a highly sensitive and specific marker of SFT. The diagnosis of SFTP must be differentiated from various kinds of pleural soft tissue tumors.
Adhesion or unclear boundary with surrounding tissue, pleural effusion or calcification, maximum tumor diameter greater than 10 cm, invasive growth, uneven density, metastasis or recurrence, paraneoplastic syndrome, moderate to severe cell heterogeneity, high Ki67 proliferation index, and low STAT6 expression suggest that SFTP may be a malignant tumor. Gene analysis based on next generation sequencing may serve to reveal the mutation characteristics of SFTP. Fortunately, 80% of SFTP is benign.
Complete tumor resection is the gold standard of SFTP. For a patient with a large tumor or extensive adhesions, thoracotomy is often used. If not completely removed or recurred, a second operation may be necessary. Also, local microwave ablation and other methods have been applied in the clinic. For malignant SFTP, the curative effect of radiotherapy and chemotherapy is limited, and chemotherapy combined with molecular targeted therapy has produced promising results. Resectability is the most important prognostic factor. Age, size, mitosis, and necrosis have been proposed as risk stratification factors for predicting prognosis. The prognosis of SFTP is good and needs long-term follow-up.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-4974
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-4974).The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the Helsinki Declaration (as revised in 2013), and this study was approved by the ethics committee of the Affiliated Hospital of Jiangnan University. Written informed consent was obtained from the patient for publication of this study and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Castellani D, Sebastiani G, Maurelli S, et al. Solitary fibrous tumor/hemangiopericytoma of the penis: Report of the first case. Urologia 2015;82:127-9. [Crossref] [PubMed]
- Ema T, Funai K, Kawase A, et al. A solitary fibrous tumor of the pleura for which the tumor doubling time could be calculated by computed tomography: a case report. J Thorac Dis 2018;10:E592-5. [Crossref] [PubMed]
- Czimbalmos C, Csecs I, Polos M, et al. Uncommon presentation of a rare tumour - incidental finding in an asymptomatic patient: case report and comprehensive review of the literature on intrapericardial solitary fibrous tumours. BMC Cancer 2017;17:612. [Crossref] [PubMed]
- Blessing NW, Bermudez-Magner JA, Fernandez MP, et al. Solitary Fibrous Tumor of the Orbit: A Case Series With Clinicopathologic Correlation and Evaluation of STAT6 as a Diagnostic Marker. Ophthalmic Plast Reconstr Surg 2020;36:164-71. [Crossref] [PubMed]
- Schöffski P, Timmermans I, Hompes D, et al. Clinical Presentation, Natural History, and Therapeutic Approach in Patients with Solitary Fibrous Tumor: A Retrospective Analysis. Sarcoma 2020;2020:1385978. [Crossref] [PubMed]
- Glauser G, Sharma N, Kritikos M, et al. Cervical, Intradural Extramedullary Solitary Fibrous Tumor of the Spinal Cord: A Case Report and Review of the Literature. Asian J Neurosurg 2020;15:204-9. [Crossref] [PubMed]
- Kopel J, Sharma P, Warriach I. A solitary fibrous tumor of the kidney. Urol Case Rep 2019;28:101072. [Crossref] [PubMed]
- Matos J, Paparo F, Calcagno T, et al. Solitary fibrous tumor of the prostate. Urology 2020. [Epub ahead of print]. [Crossref] [PubMed]
- Zielińska-Kaźmierska B, Grodecka J, Szyszkowski A. Solitary fibrous tumor of the nasal cavity and paranasal sinuses: A case report. J Oral Biol Craniofac Res 2015;5:112-6. [Crossref] [PubMed]
- Guerrini S, Ricci A, Osman GA, et al. Different clinical and radiological features of solitary fibrous tumor of the pleura: Report of two cases. Lung India 2016;33:72-4. [Crossref] [PubMed]
- Gupta A, Souza CA, Sekhon HS, et al. Solitary fibrous tumour of pleura: CT differentiation of benign and malignant types. Clin Radiol 2017;72:796 e9-e17.
- Rosenberg AE. WHO Classification of Soft Tissue and Bone, fourth edition: summary and commentary. Curr Opin Oncol 2013;25:571-3.
- Thway K, Ng W, Noujaim J, et al. The Current Status of Solitary Fibrous Tumor: Diagnostic Features, Variants, and Genetics. Int J Surg Pathol 2016;24:281-92. [Crossref] [PubMed]
- Savu C, Melinte A, Posea R, et al. Pleural Solitary Fibrous Tumors-A Retrospective Study on 45 Patients. Medicina (Kaunas) 2020;56:185. [Crossref] [PubMed]
- Yagyu H, Hara Y, Murohashi K, et al. Giant Solitary Fibrous Tumor of Pleura Presenting Both Benign and Malignant Features. Am J Case Rep 2019;20:1755-9. [Crossref] [PubMed]
- Xu G, Zhang J. Giant solitary fibrous tumor of the pleura: A case report and literature review. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2016;41:1111-6. [PubMed]
- Hashmi AT, Gupta SS, Saradna A, et al. Solitary Fibrous Tumor of Pleura Invading into the Left Atrium via Pulmonary Vein. Cureus 2018;10:e3473. [PubMed]
- Tan JH, Hsu AA. Challenges in diagnosis and management of giant solitary fibrous tumour of pleura: a case report. BMC Pulm Med 2016;16:114. [Crossref] [PubMed]
- Bar-Haim R, Gavrilov A, Samokhvalov A, et al. Solitary fibrous tumour: a rare tumour of the pleural cavity. BMJ Case Rep 2017;2017:bcr2016217880. [Crossref] [PubMed]
- Raafat E, Karunasiri D, Kamangar N. Solitary fibrous tumour of the pleura presenting as a giant intrathoracic mass. BMJ Case Rep 2017;2017:bcr2016217880. [PubMed]
- Boddaert G, Guiraudet P, Grand B, et al. Solitary fibrous tumors of the pleura: a poorly defined malignancy profile. Ann Thorac Surg 2015;99:1025-31. [Crossref] [PubMed]
- Mongelli F, FitzGerald M, Cafarotti S, et al. Chylothorax after endoscopic ultrasound with fine-needle aspiration causing migrating appearance of a solitary fibrous tumor of the pleura. Ann Thorac Med 2018;13:114-6. [Crossref] [PubMed]
- Liu CC, Wang HW, Li FY, et al. Solitary fibrous tumors of the pleura: clinicopathological characteristics, immunohistochemical profiles, and surgical outcomes with long-term follow-up. Thorac Cardiovasc Surg 2008;56:291-7. [Crossref] [PubMed]
- Boyer-Duck E, Dajer-Fadel WL, Hernandez-Arenas LA, et al. Pierre-Marie-Bamberger syndrome and solitary fibrous tumor: a rare association. Asian Cardiovasc Thorac Ann 2018;26:154-7. [Crossref] [PubMed]
- Kim DW, Na KJ, Yun JS, et al. Doege-potter syndrome: a report of a histologically benign but clinically malignant case. J Cardiothorac Surg 2017;12:64. [Crossref] [PubMed]
- Sorino C, Negri S, Spanevello A, et al. The pleura and the endocrine system. Eur J Intern Med 2020;72:34-7. [Crossref] [PubMed]
- Karki A, Yang J, Chauhan S. Paraneoplastic syndrome associate with solitary fibrous tumor of pleura. Lung India 2018;35:245-7. [Crossref] [PubMed]
- Tamburini N, Fabbri N, Anania G, et al. Synchronous pancreatic and pulmonary metastases from solitary fibrous tumor of the pleura: report of a case. Tumori 2017;103:e9-11. [Crossref] [PubMed]
- Ricciuti B, Metro G, Leonardi GC, et al. Malignant giant solitary fibrous tumor of the pleura metastatic to the thyroid gland. Tumori 2016. [Crossref] [PubMed]
- Law MK, Tung YW, Jinc JS. Malignant transformation in solitary fibrous tumor of the pleura. Asian Cardiovasc Thorac Ann 2014;22:981-3. [Crossref] [PubMed]
- Cardinale L, Dalpiaz G, Pulzato I, et al. Computed tomography of solitary fibrous tumor of the pleura abutting the mediastinum: A diagnostic challenge. Lung India 2018;35:121-6. [PubMed]
- Carter BW, Betancourt SL, Shroff GS, et al. MR Imaging of Pleural Neoplasms. Top Magn Reson Imaging 2018;27:73-82. [Crossref] [PubMed]
- Tazeler Z, Tan G, Aslan A, et al. The utility of 18F-FDG PET/CT in solitary fibrous tumors of the pleura. Rev Esp Med Nucl Imagen Mol 2016;35:165-70. [Crossref] [PubMed]
- Dammad T, Duchesne J, Pasnick S. Case report of medical thoracoscopy and endobronchial ultrasound bronchoscopy in the workup of giant solitary fibrous tumor of the pleura. Medicine (Baltimore) 2016;95:e4100. [Crossref] [PubMed]
- Mendez-Sanchez H, Mendez-Vivas W, Vargas-Mendoza GK, et al. Solitary fibrous tumors of the pleura: a clinical-pathological characterization emphasizing changes in lung function. Adv Respir Med 2019;87:247-51. [Crossref] [PubMed]
- Huang SC, Huang HY. Solitary fibrous tumor: An evolving and unifying entity with unsettled issues. Histol Histopathol 2019;34:313-34. [PubMed]
- Zhu R, Pang JY, Yang CM, et al. Solitary Fibrous Tumours/Hemangiopericytomas of the Maters(Meninx):A Clinicopathologic Analysis. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2019;41:512-6. [PubMed]
- Rao N, Colby TV, Falconieri G, et al. Intrapulmonary solitary fibrous tumors: clinicopathologic and immunohistochemical study of 24 cases. Am J Surg Pathol 2013;37:155-66. [Crossref] [PubMed]
- Zhang P, Xiong K, Lv P, et al. Malignant solitary fibrous tumor occurring in the mediastinal pleura showing NAB2ex4-STAT6ex2 fusion and negative STAT6 immunohistochemistry: A case report. Thorac Cancer 2020;11:1344-9. [Crossref] [PubMed]
- Geramizadeh B, Marzban M, Churg A. Role of Immunohistochemistry in the Diagnosis of Solitary Fibrous Tumor, a Review. Iran J Pathol 2016;11:195-203. [PubMed]
- Song Z, Yang F, Zhang Y, et al. Surgical therapy and next-generation sequencing-based genetic alteration analysis of malignant solitary fibrous tumor of the pleura. Onco Targets Ther 2018;11:5227-38. [Crossref] [PubMed]
- Ershadi R, Rahim M, Abbasi M, et al. Giant solitary fibrous tumor of the pleura. J Surg Case Rep 2018;2018:rjy270. [Crossref] [PubMed]
- de Perrot M, Fischer S, Brundler MA, et al. Solitary fibrous tumors of the pleura. Ann Thorac Surg 2002;74:285-93. [Crossref] [PubMed]
- Rocas D, Thivolet-Bejui F, Tronc F, et al. About a case of calcifying fibrous tumor of the pleura. Ann Pathol 2015;35:515-8. [Crossref] [PubMed]
- Attanoos RL, Pugh MR. The Diagnosis of Pleural Tumors Other Than Mesothelioma. Arch Pathol Lab Med 2018;142:902-13. [Crossref] [PubMed]
- Yao MJ, Ding L, Atay SM, et al. A Modern Reaffirmation of Surgery as the Optimal Treatment for Solitary Fibrous Tumors of the Pleura. Ann Thorac Surg 2019;107:941-6. [Crossref] [PubMed]
- Fukai R, Irie Y, Watanabe H. Surgically cured paraneoplastic hypoglycemia associated with solitary fibrous tumor of the pleura: report of two cases. Clin Case Rep 2017;5:1119-22. [Crossref] [PubMed]
- Perrotta F, Cerqua FS, Cammarata A, et al. Integrated therapeutic approach to giant solitary fibrous tumor of the pleura: report of a case and review of the literature. Open Med (Wars) 2016;11:220-5. [Crossref] [PubMed]
- Hatooka S, Shigematsu Y, Nakanishi M, et al. Subxiphoid approach for extracting a giant solitary fibrous tumour of the pleura. Interact Cardiovasc Thorac Surg 2017;25:834-5. [Crossref] [PubMed]
- Amore D, Rispoli M, Cicalese M, et al. Anterior mediastinal solitary fibrous tumor resection by da Vinci® Surgical System in obese patient. Int J Surg Case Rep 2017;38:163-5. [Crossref] [PubMed]
- Song JY, Kim KH, Kuh JH, et al. Two-stage Surgical Treatment of a Giant Solitary Fibrous Tumor Occupying the Thoracic Cavity. Korean J Thorac Cardiovasc Surg 2018;51:415-8. [Crossref] [PubMed]
- You YH, Liu RT, Zhang Y. A large solitary fibrous tumour of the pleura: a case report and review of the literature. J Int Med Res 2018;46:1672-7. [Crossref] [PubMed]
- Fatimi SH, Inam H, Chagan FK, et al. Solitary fibrous pleural tumor. A rare and challenging case. Int J Surg Case Rep 2020;66:346-9. [Crossref] [PubMed]
- Shanahan B, Redmond KC. Largest known malignant solitary fibrous tumour of the pleura-extended resection warranting cardiopulmonary bypass support. Ir J Med Sci 2019;188:433-5. [Crossref] [PubMed]
- Patel SR, Vachhani P, Moeslein F. Embolic Brain Infarcts: A Rare Fatal Complication of Preoperative Embolization of a Massive Solitary Fibrous Tumor of the Pleura. Cardiovasc Intervent Radiol 2017;40:306-9. [Crossref] [PubMed]
- Kayani B, Sharma A, Sewell MD, et al. A Review of the Surgical Management of Extrathoracic Solitary Fibrous Tumors. Am J Clin Oncol 2018;41:687-94. [Crossref] [PubMed]
- Park MS, Ravi V, Conley A, et al. The role of chemotherapy in advanced solitary fibrous tumors: a retrospective analysis. Clin Sarcoma Res 2013;3:7. [Crossref] [PubMed]
- Patel MD, Damodaran D, Rangole A, et al. Hyperthermic Intrathoracic Chemotherapy (HITHOC) for Pleural Malignancies-Experience from Indian Centers. Indian J Surg Oncol 2019;10:91-8. [Crossref] [PubMed]
- Fiore F, Stoia V, Somma F. Surgical recurrence of solitary fibrous tumor of the pleura treated with microwave (MW) thermoablation: A case report. Thorac Cancer 2020;11:443-6. [Crossref] [PubMed]
- Stacchiotti S, Libertini M, Negri T, et al. Response to chemotherapy of solitary fibrous tumour: a retrospective study. Eur J Cancer 2013;49:2376-83. [Crossref] [PubMed]
- Demicco EG, Wani K, Fox PS, et al. Histologic variability in solitary fibrous tumors reflects angiogenic and growth factor signaling pathway alterations. Hum Pathol 2015;46:1015-26. [Crossref] [PubMed]
- Spagnuolo RD, Brich S, Bozzi F, et al. Sunitinib-induced morpho-functional changes and drug effectiveness in malignant solitary fibrous tumours. Oncotarget 2016;7:45015-26. [Crossref] [PubMed]
- Stacchiotti S, Tortoreto M, Baldi GG, et al. Preclinical and clinical evidence of activity of pazopanib in solitary fibrous tumour. Eur J Cancer 2014;50:3021-8. [Crossref] [PubMed]
- Ogunsakin AA, Hilsenbeck HL, Portnoy DC, et al. Recurrent Severe Hypoinsulinemic Hypoglycemia Responsive to Temozolomide and Bevacizumab in a Patient With Doege-Potter Syndrome. Am J Med Sci 2018;356:181-4. [Crossref] [PubMed]
- de Lemos ML, Kang I, Schaff K. Efficacy of bevacizumab and temozolomide therapy in locally advanced, recurrent, and metastatic malignant solitary fibrous tumour: A population-based analysis. J Oncol Pharm Pract 2019;25:1301-4. [Crossref] [PubMed]
- Boothe JT, Budd GT, Smolkin MB, et al. Durable Near-Complete Response to Anti-PD-1 Checkpoint Immunotherapy in a Refractory Malignant Solitary Fibrous Tumor of the Pleura. Case Rep Oncol 2017;10:998-1005. [Crossref] [PubMed]
- Brock M, Hottinger S, Diebold M, et al. Low tissue levels of miR-125b predict malignancy in solitary fibrous tumors of the pleura. Respir Res 2017;18:43. [Crossref] [PubMed]
- Kovacs T, Waxman J. Recurrence of a malignant solitary fibrous tumor of the pleura 17 years after primary tumor resection - A case report. Respir Med Case Rep 2019;28:100895. [Crossref] [PubMed]
(English Language Editor: J. Chapnick)


