Minimum local anesthetic dose of ropivacaine in real-time ultrasound-guided intraspinal anesthesia for lower extremity surgery: a randomized controlled trial
Introduction
Recently, there has been an increase in interest in the use of ultrasound guided neuraxial blocks (epidural or lumbar anesthesia) (1-5), which provide more accuracy interlaminar spaces location and improves success rate of puncture performance than the conventional surface anatomic landmark guided technique (1,6-9).
Accurate location of interlaminar space plays an important role in intraspinal anesthesia., so ultrasound technique was initially applied to identify landmark structures including transverse process and articular processes (AP) joints et al., in order to achieve a more accuracy interlaminar spaces location than the conventional technique (1). However, accuracy of interlaminar space location is essential but not enough when facing structural deformity of the spine, such as ankylosing spondylitis, lamina osteophytes or superior spinal ligament calcification by age (10). To compensate for this deficiency, a recent study recommended the implementation of real-time ultrasound-guided lumbar puncture, which could improve safety and efficiency (1,6-9). Real-time guidance with constant adaptation makes it more beneficial than only applying it for anatomical location prior to an invasive procedure. Previous studies have reported that advantages including higher success rates, fewer local injuries, less time required for successful puncture performance, fewer needle passes, and lower patient-reported pain scores can be achieved when this approach is adopted (11-13).
Ropivacaine, an amino-amide local anesthetic with structural similarity to bupivacaine and mepivacaine (14), was licensed for intrathecal use by the European Union in 2004 (15). It is a pure S (-) isomer, with a high pKa and low lipid solubility. Because of its physical and chemical properties, ropivacaine produces a marked differential in sensory and motor blockades. Although the early study indicated that intraspinal anesthesia with ropivacaine was less potent than bupivacaine (16), ropivacaine has been widely used for lumbar anesthesia recent years due to less cardiac toxic effects and produces a less intense motor block of shorter duration (17). The minimum local anesthetic dose (MLAD) was defined as the median effective dose (ED50) of intrathecal local anesthetics including bupivacaine, levobupivacaine, ropivacaine, etc. (2).
To date, many publications have investigated the MLAD of ropivacaine in spinal anesthesia for lower extremity surgery and caesarean section, such as Wang and Lee’s studies (3,4). However, in these studies, spinal anesthesia was performed without any assistance from ultrasound.
In consistent with the study of Liu et al. (5), we found that in order to achieve a complete anesthetic sensory block of T10, at least 18 mg of ropivacaine needed to be administered at spinal interspace L4–5 with ultrasound using a paramedian transverse approach, which was more than that in the traditional landmark-based approach (6.43–12.8 mg) (2-4,18). It is clear that ropivacaine dosage is the most decisive factor in the safety and efficiency of spinal anesthesia.
However, to our knowledge, no studies on the ED50 of ropivacaine in ultrasound-guided intraspinal anesthesia have been conducted to date. In clinical practice, both 0.5% and 0.75% ropivacaine are commonly used in intraspinal anesthesia for lower extremity surgery.
Then in this study, we aimed to investigate the MLAD of 0.5% and 0.75% ropivacaine for performing real-time ultrasound-guided intraspinal anesthesia using a paramedian transverse approach for lower extremity surgery.
We present the following article in accordance with the CONSORT reporting checklist (available at http://dx.doi.org/10.21037/atm-20-3805).
Methods
Study design
This prospective, randomized, parallel group, triple-blinded clinical trial was designed in accordance with the CONSORT statement recommendations. Our study (approval number K-2019-06-027) was approved by the Ethics Committee of Fujian Provincial Hospital, Fuzhou, China on 27 June 2019 and registered on the Chinese Clinical Trial Registry website (registration number ChiCTR1900025102). This study was conducted in accordance with the principles set out in the Declaration of Helsinki (as revised in 2013), and the content of the study was thoroughly explained to the participants before written informed consent was obtained.
Study population
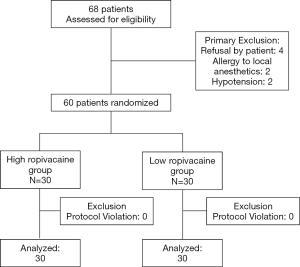
Sixty patients with American Society of Anesthesiologists (ASA) physical status I or II, who were consecutively scheduled for selective lower extremity surgery at the Fujian Provincial Hospital, were enrolled in the study. Exclusion criteria included known local anesthetics or non-steroidal anti-inflammatory drugs allergies, infection near the puncture site, known coagulation disorders, specific cardiovascular diseases, chronic pain, use of pain medication and inability to provide consent. The patients were randomly divided into two different groups: the high ropivacaine group, which received 0.75% ropivacaine, and the low ropivacaine group, which received 0.5% ropivacaine. Randomization was performed with a 1:1 ratio using a computer to generate random number list. Considering the limited duration time for single shot spinal anesthesia using a pencil-point side-hole lumbar puncture needle, this study focused on cases of lower extremity surgery which were expected to last less than 3 h, thus avoiding any complications such as spinal cord injury caused by subarachnoid catheterization.
Medication and procedures
A continuous supply of oxygen (3 L/min) was delivered to the patients via nasal catheter. The patients were monitored according to standard procedures, including by electrocardiography, pulse oximetry, and noninvasive arterial blood pressure measurements. Non-invasive blood pressure was monitored and recorded at three different time points, with the lowest systolic blood pressure (SBP) value considered as the basic blood pressure value and the lowest heart rate in a 15 min time window considered as the basic heart rate. After venous access was established, sodium, potassium, magnesium, calcium, and glucose injections were intravenously administered at 10 mL/kg for 15 min. No medications were administered beforehand. The patients were then placed in the lateral position for spinal anesthesia.
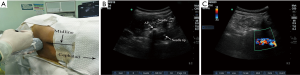
In the high ropivacaine group, a portable colour ultrasound (Edge, SonoSite Company) was used to perform the real-time ultrasound-guided intraspinal anesthesia. A 5–10 MHz curved array transducer was placed in a paramedian sagittal oblique orientation, using sliding and titling scanning techniques to reveal the sacrum and locate the L5–S1 intervertebral space. It was then moved in a cephalad direction to identify the L4/5 intervertebral space, at which point the skin was marked. After the second confirmation, the T12 vertebra was identified by its articulation with the 12th rib and the transducer was moved in a caudad direction to visualize each successive intervertebral space down to L4/5 (10). The transducer was then rotated by 90° in a transverse orientation, with respect to the desired interspaces, and the required needle insertion depth from the skin to the anterior complex was measured using the ultrasound machine’s electronic calipers. After sterilization and laying down towels for surgery, an aseptic protective sheath was wrapped around the probe, and the local anesthetic was delivered specifically to the puncture site. Informed by real-time ultrasound images, a 26 guage spinal needle was guided using the short-axis in-plane technique (Figure 1A,B) described by Liu et al. (19), and the cerebrospinal fluid outflow was used as the criterion to assess whether the puncture needle had effectively reached the subarachnoid space. After confirming free flow of cerebrospinal fluid, the predetermined dose of local anaesthetic was diluted with the cerebrospinal fluid to achieve a final concentration of 0.5% or 0.75%. And then the local anesthetic was injected at a rate of 0.4 mL/s with the orifice of the spinal needle turned cephalad (Figure 1C). After the injection, the lumbar anesthesia needle was removed, the puncture point was covered with a sterile dressing, and the patient was placed back into the supine position.
For the low ropivacaine group, excluding the dilution of the cerebrospinal fluid with 0.5% ropivacaine and injection into the subarachnoid space, the procedures were carried out in a similar fashion as those described for the high ropivacaine group.
Dixon’s up-and-down sequential method was used to determine the subarachnoid medication (20). Based on previous clinical experience, the initial ropivacaine dosage was set to 16 mg for the high ropivacaine group (2,18,21), and 18 mg for the low ropivacaine group (22,23). The dosage interval used for ropivacaine was 1.5 mg (2,23,24). If within 20 min of the subarachnoid injection, the sensory block plane reached T10 and lasted longer than 60 min, a blockade was considered to have been successful; otherwise, it was defined as ‘ineffective’. If a case was ‘effective’, the next case was given a lower dose; if a case was ‘ineffective’, the dose was raised for the next case.
If blood pressure decreased either >25% compared to pre-anesthetic status or <90 mmHg, intravenous ephedrine was delivered accordingly. When the heart rate remained under 55 bpm, 0.5 mg of atropine was intravenously administered. In cases of inadequate analgesia, general anesthesia was used within 30 min of injection.
Assessment and evaluation
Patients’ baseline measurements, including sensory and motor assessments, along with related data from both after surgery and during the recovery period, were collected by a trained research assistant. Spinal anesthesia and all assessments during anesthesia and surgery were performed by the same attending anesthesiologist.
SBP, diastolic blood pressure (DBP), mean arterial pressure, heart rate, oxygen saturation, and respiratory rate were each recorded at the following time points: before anesthesia (T1), 5 min after injection (T2), 10 min after injection (T3), 15 min after injection (T4), and 20 min after injection (T5).
The ice-cube stimulation test was used to measure sensory nerve block. The level of sensory block was assessed every 2 min within the first 20 min after the subarachnoid injection. For cases in which the blockade was considered effective, sensory block was also evaluated every 15 min for 1 h after the subarachnoid injection, until the sensory block had completely resolved. The time required for the sensory block to reach T10 and the length of time for which the sensory block level remained above T10 level were recorded.
Motor block of the lower extremities was evaluated bilaterally using the modified Bromage scale [0–3] according to the following grading system: 0= full flexion of the knees and feet; 1= just able to move the knees; 2= able to move only the feet; 3= unable to move the feet or knees. The time of onset of motor block (the time taken to reach 1 point on the Bromage scale) and, subsequently, the duration of motor block (the time taken for the Bromage score to return to 0 point) were recorded.
The frequency of hypotension, nausea, vomiting, shivering, respiratory depression, ephedrine use, and atropine use was also recorded, as were the resting visual analogue scale (VAS) score, motoring VAS score, respiratory depression, nausea and vomiting, urinary retention, pruritus, infection, headache, epidural hematoma, and nerve injury on the first day after the operation.
Statistical analyses
Our study used Dixon’s up-and-down sequential method (20,25). The initial dosage was 16 mg for the high ropivacaine group with 0.75% ropivacaine (2,18,21), and 18 mg for the low ropivacaine group with 0.5% ropivacaine (22,23). These dosages were informed by previous studies which showed that the average dosages of 0.75% ropivacaine(18) and 0.5% ropivacaine (22) for achieving a sufficient anesthetic effect were 15.5 and 17.5 mg, respectively, with an standard deviation (SD) of 3.1 (18). Meanwhile, the standard error (25) could be anticipated to be
Data are presented as the mean ± SD, median (range), or frequency. Data were analyzed using the Mann-Whitney U (Wilcoxon rank-sum test) and the χ2 or Fisher’s exact test. P values <0.05 were considered statistically significant.
Results
Sixty-eight patients who were consecutively admitted for elective lower extremity surgery between September, 2019 and January, 2020 were recruited for this study. Among them, two were allergic to local anesthetics, two had hypotension, and four were unwilling to participate in the study; all eight of these patients were preoperatively excluded. Ultimately, 60 patients were included and were divided into two groups, with 30 cases in each group. The enrolment statistics are shown in Figure 2. The baseline characteristics including age, sex, height, weight, duration of surgery, and ASA classification were not significantly different between the groups (P>0.05) (Table 1). The findings from analysis of the two groups by Dixon’s up-and-down sequential method are illustrated in Figure 3.

Full table

MLAD of ropivacaine real-time ultrasound-guided intraspinal anesthesia
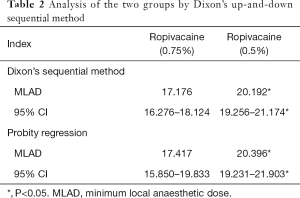
As shown in Table 2, in the high ropivacaine group, the MLAD and its 95% CI were determined to be 17.176 mg (16.276 to 18,124 mg) by Dixon’s up-and-down sequential method and 17.417 mg (15.850 to 19.833 mg) by probity regression. Meanwhile, in low ropivacaine group, the MLAD and its 95% CI were determined to be 20.192 mg (19.256 to 21.174 mg) by Dixon’s up-and-down sequential method and 20.396 mg (19.231 to 21.903 mg) by probity regression.
Full table
Sensory block in the in two groups
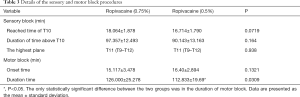
No significant difference was observed between the two groups in terms of sensory block (Table 3). In the high ropivacaine group, the time the sensory block took to reach the T10 level was 18.064±1.878 min, and the length of time for which the sensory block remained above the T10 level was 97.357±12.493 min. T11 (T9–T12) was the highest level reached by the sensory block.
Full table
In the low ropivacaine group, the time the sensory block took to reach the T10 level was 16.714±1.790 min, and the length of time for which the sensory block remained above the T10 level was 90.143±13.163 min. T11 (T9–T12) was the highest level reached by the sensory block.
Motor block in the two groups
The onset time for motor block was 15.117±3.478 min in the high ropivacaine group, and the duration of motor block was 126.000±25.278 min. By contrast, in the low ropivacaine group, the onset time for motor block was 16.400±2.894 min, and the duration of motor block was 112.833±19.693 min. The duration of motor block was significantly different between the two groups (P=0.0309) (Table 2).
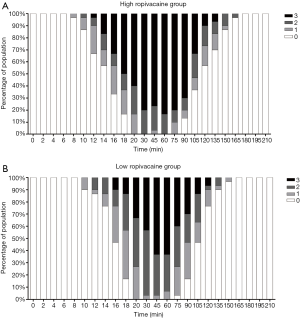
The modified Bromage scale for each group at different times is shown in Figure 4. The degree of motor block in the high ropivacaine group was higher than that in the low ropivacaine group, and the proportion of Bromage scale level 3 scores in the high ropivacaine group versus the low ropivacaine group as follows: at 14 min, 16.7% vs. 0%; at 16 min, 33% vs. 10%; at 18 min, 50% vs. 13.3%; at 20 min, 60% vs. 33.3%; at 30 min, 80% vs. 43.3%; at 45 min, 76.6% vs. 63.3%; at 60 min, 83.3% vs. 63.3%; at 75 min, 80% vs. 40%; at 90 min, 70% vs. 30%; at 105 min, 33.3% vs. 13.3%; at 120 min, 10% vs. 6.67%; and at 135 min, 6.67% vs. 0%. As mentioned above, the durations of level three over 50% in the modified Bromage scale for the two groups were 82 min and 30 min, respectively.
Changes in vital signs in the two groups
The comparison between the SBP and DBP values of the two groups at different times is shown in Figure 5. There were no significant difference observed between the two groups in the data recorded for SBP at the following time points: before anesthesia (T1), 5 min after injection (T2), 10 min after injection (T3), 15 min after injection (T4), and 20 min after injection (T5) (P=0.0705). Moreover, the data for the DBP between the two groups also revealed no significant differences (P=0.6355).
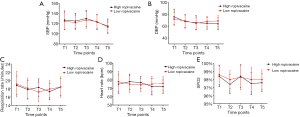
The respiratory rate, heart rate, and pulse oximetry of the patients in the two groups at different time points are shown in Figure 5. Before anesthesia (T1), 5 min after injection (T2), 10 min after injection (T3), 15 min after injection (T4), and 20 min after injection (T5), the differences in the respiratory rate, heart rate, and pulse oximetry were not statistically significantly different between the two groups (P=0.9493 for respiratory rate; P=0.6355 heart rate, and P=0.0614 for pulse oximetry).
Incidence of adverse reactions in two groups
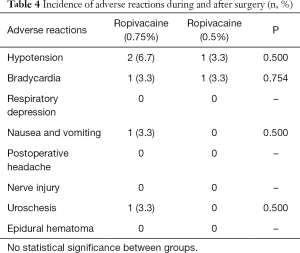
The comparison of the incidence of adverse reactions during and after surgery across the two groups is shown in Table 4. During surgery, 2 (6.7%) patients in the high ropivacaine group and 1 (3.3%) patient in the low ropivacaine group had hypotension; 1 (3.3%) patient in the entire cohort had bradycardia; none of the patients in the cohort had respiratory suppression; and 1 (3.3%) patient in the high ropivacaine group experienced nausea and vomiting. No significant differences between two groups were found in hypotension (P=0.500), bradycardia (P=0.754), respiratory suppression or nausea and vomiting (P=0.500). None of the patients in the study reported postoperative headaches, nerve injuries, or epidural hematoma. Meanwhile, the incidence of urinary retention was 1 (3.3%) in the high ropivacaine group but was absent in the low ropivacaine group, and there was no significant difference (P=0.500)
Full table
Discussion
In this study, we determined the MLAD and the 95% CI of 0.75% and 0.5% ropivacaine and found that the maintenance of motor block in the 0.5% ropivacaine group was greatly reduced compared to the 0.75% ropivacaine group.
For the rapid development of the concept of enhanced recovery after surgery, anesthesiologists have tended to focus on how safe, convenient, and effective anesthesia can be delivered for patients. Spinal anesthesia (subarachnoid block anesthesia) possesses many advantages, such as a rapid onset, satisfactory blockade effect, a higher success rate, lower incidence of complications, and a lower impact on cardiopulmonary function. These are significant advantages, especially for use in older people, who might be affected by various multiple diseases. Therefore, spinal anesthesia has traditionally been favored by clinical anesthesiologists. However, routine intraspinal puncture often fails to achieve success or reduce the occurrence of complications (26-28), which presents a problem that urgently needs to be addressed, especially when the growing number of certain patient populations, such as older or scoliosis patients, are considered.
First proposed in 2009 by Professor Karmakar et al. (1), real-time ultrasound-guided intraspinal anesthesia is a relatively new technique for anesthesia. In the past decade, its use in clinical practice has gradually increased. In this technique, the puncture approach can also be divided into the short-axis in-plane approach, the paracentric approach, and the spinous process lamina oblique axis approach (1). Each of these approaches can greatly improve the success rate and reduce the incidence of complications in the populations for whom puncture is difficult (29). As well as being, by far, the most widely used of all these approaches, the short-axis in-plane approach is also the most practical, and was therefore adopted in our study.
In our previous studies, the dosage of ropivacaine used in this approach was found to be much larger than that used in traditional intraspinal anesthesia. In a conventional subarachnoid block, the dosage of the local anesthetic has a much stronger influence on anesthetic efficacy than other factors such as local anesthetic volume and injection speed (30). Because of the relationship between safety and dosage in real-time ultrasound-guided intraspinal anesthesia, it is necessary to use the appropriate dose of the most frequently used local anesthetic.
Therefore, to determine the most appropriate and safest dosage of local anesthetic and to provide evidence in support of its clinical use, this study compared the MLAD of ropivacaine and the efficacy of real-time ultrasound-guided intraspinal anesthesia by sequential administration for lower extremity surgery across two groups with different concentrations of ropivacaine.
The MLAD is a sensitive index that reflects the intensity of the drug effect, and is located at the maximum of the S-shaped slope of the dose-effect curve, where changes in the dose can incur obvious changes in the effect. In this study, Dixon’s up-and-down sequential method was adopted to test the study subjects in sequence according to the drug concentration or dose as arranged in equal order (31).
Previous studies have shown that the ED50 and 95% CI of ropivacaine for subarachnoid block can vary from 8.41 mg (7.15 to 9.67 mg) to 12.8 mg (12.2 to 13.4 mg) for lower extremity surgery (4). The inconsistency in these results can mainly be attributed to the differences in study designs. The results of the MLAD and 95% CI in this study are higher than those for traditional spinal anesthesia. The MLAD and its 95% CI were determined to be 17.176 mg (16.276 to 18,124 mg) when using 0.75% ropivacaine; and it was 20.192 mg (19.256 to 21.174 mg) for 0.5% ropivacaine. This is can probably be explained by the following reasons: (I) the vertebral space for the puncture site in this study was L4−5, which is lower than the others, meaning the required amount for local anesthetic dosage was higher. (II) The short-axis in-plane approach is far from the midline when compared to the most commonly used paracentric approach. (III) Despite the tip of the needle having been oriented towards the cephalic region, the injection directions of the local anesthetic may have varied.
According to sensory and motor block, previous evidence has indicated that the approximate time of onset for motor block by ropivacaine is 8.3 min (21). Additionally, the ED95 of ropivacaine for the modified Bromage scale was observed to drop to 1 within 5 min of the intrathecal injection (32,33), and its 95% CI, was 5.79 (4.62 to 6.96) mg for caesarean section (32), and 15.75 to 20.96 mg for lower extremity surgery (33).
There was no statistical difference in onset time of for sensory or motor block in our study. However, a shortened sustained duration for motor block was observed in the 0.5% ropivacaine group, which can probably be explained by the relatively low blocking concentrations. A prolonged motor block can cause anxiousness in patients and is considered a risk factor for microthrombus formation. In our study, the modified Bromage scale at different times revealed that the motor blockade effect in the 0.5% ropivacaine group was inferior to that in the 0.75% ropivacaine group, presenting an obvious sensory-motor separation block. Therefore, 0.5% ropivacaine for spinal anesthesia may be more suitable for patients who are in need of early mobilisation to promote rapid recovery. However, it is also worth noting that early regression of motor blocks may lead to insufficient muscle relaxation during lower extremity surgery, which may make the operation more challenging for the surgeon.
As our study shows, the hemodynamic changes across the two experimental groups remained small when procedures were performed and the incidence of complications during and after surgery remained low, with no significant difference observed between the groups. This indicates that a relatively ideal anesthetic protocol, both in terms of safety and efficacy, can be achieved for real-time ultrasound-guided intraspinal anesthesia with either 0.5% or 0.75% ropivacaine. This finding is worthy of promoting in clinical practice.
However, this study has several limitations. First, the value of ED50 is commonly used as a measure of drug's potency, however, it only represents the concentration or dose where 50% of the population exhibit a response. Therefore, ED50 is restricted in clinical practice, to some extent. Second, flexibility is required to adjust the interventions depending on the different types of surgery. Future research should focus on the dose-effect relationships between local anesthetics and real-time ultrasound-guided anesthesia in different populations or for different practical procedures.
Conclusions
As one of the newly developed technologies of recent years, real-time ultrasound-guided intraspinal anesthesia has the advantages of facilitating effective visualization, with a higher success rate and a lower incidence of complications. To the best of our knowledge, our study represents the first time the sequential method has been used to determine the dose-effect dynamics of ropivacaine in real-time ultrasound-guided intraspinal anesthesia for lower extremity surgery. Our results demonstrate that real-time ultrasound-guided intraspinal anesthesia is operational and clinically implementable. Motor block in the lower extremity can be alleviated by 0.5% ropivacaine, which is beneficial for patients’ early recovery of ambulation and rapid post-operative rehabilitation. This study’s MLAD values and their 95% confidence intervals can be referred to or used to determine the accurate intrathecal dosage of ropivacaine in real-time ultrasound-guided intraspinal anesthesia, and are of critical value for lower extremity surgery.
Acknowledgments
I would like to express my sincere appreciation to all of the specialists and professors who have offered me invaluable guidance or help. This includes but is not limited to: Professor Fan, from the Statistics Department of Fujian Medical University, for guidance with statistical analysis; Professor Feng, from the Foreign Language Department of Fujian Medical University, for advice regarding phrases and expressions; and Professor Mei, from the Anaesthesiology Department of Tong-Ji Hospital, for technical help.
Funding: This study received funding from the Medical Education Branch of the Chinese Medical Association, the Medical Education Committee of the Chinese Higher Education Society (2016B-LC033), the Natural Science Foundation of Fujian Province (2018J01246), the Medical Innovation Project of Fujian province (2018-CX-2), high-level hospital foster grants from Fujian Provincial Hospital, Fujian Province, China (grant numbers: 2019HSJJ23 and 2019HSJJ21), the Natural Science Foundation of Fujian Province (2016J01506), and the Training Project for Talents of Fujian Provincial Health Commission (2019-ZQN-1), Provincial special subsidy funds for health care of Fujian Provincial Department of Finance (No. 2020-467).
Footnote
Reporting Checklist: The authors have completed the CONSORT reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-3805
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-3805
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-3805). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Our study (approval number K-2019-06-027) was approved by the Ethics Committee of Fujian Provincial Hospital, Fuzhou, China on 27 June 2019 and registered on the Chinese Clinical Trial Registry website (registration number ChiCTR1900025102). This study was conducted in accordance with the principles set out in the Declaration of Helsinki (as revised in 2013), and the content of the study was thoroughly explained to the participants before written informed consent was obtained.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Karmakar MK, Li X, Ho AM, et al. Real-time ultrasound-guided paramedian epidural access: evaluation of a novel in-plane technique. Br J Anaesth 2009;102:845-54. [Crossref] [PubMed]
- Jung J, Kim DH, Son J, et al. Comparative study between local anesthesia and general anesthesia in the treatment of primary spontaneous pneumothorax. Ann Transl Med 2019;7:553. [Crossref] [PubMed]
- Wang W, Li Y, Sun A, et al. Determination of the median effective dose (ED50) of bupivacaine and ropivacaine unilateral spinal anesthesia: Prospective, double blinded, randomized dose-response trial. Bestimmung der mittleren effektiven Dosis (ED50) einer Spinalanästhesie mit Bupivacain und Ropivacain: Eine prospektive, doppelblinde, randomisierte Dosis-Wirkungs-Studie. Anaesthesist 2017;66:936-43. [Crossref] [PubMed]
- Xu T, Zheng J, An XH, et al. Norepinephrine intravenous prophylactic bolus versus rescue bolus to prevent and treat maternal hypotension after combined spinal and epidural anesthesia during cesarean delivery: a sequential dose-finding study. Ann Transl Med 2019;7:451. [Crossref] [PubMed]
- Liu Y, Yang S, Yao W, et al. Minimum effective dose of plain bupivacaine 0.5% for ultrasound-guided spinal anaesthesia using Taylor's approach. Br J Anaesth 2020;124:e230-1. [Crossref] [PubMed]
- Conroy PH, Luyet C, McCartney CJ, et al. Real-time ultrasound-guided spinal anaesthesia: a prospective observational study of a new approach. Anesthesiol Res Pract 2013;2013:525818.
- Brinkmann S, Tang R, Sawka A, et al. Single-operator real-time ultrasound-guided spinal injection using SonixGPS: a case series. Can J Anaesth 2013;60:896-901. [Crossref] [PubMed]
- Lee PJ, Tang R, Sawka A, et al. Brief report: real-time ultrasound-guided spinal anesthesia using Taylor's approach. Anesth Analg 2011;112:1236-8. [Crossref] [PubMed]
- Ghosh S, Madjdpour C, Chin KJ. Ultrasound-guided lumbar central neuraxial block. Bja Education 2015. [Crossref]
- Chin KJ, Karmakar MK, Peng P. Ultrasonography of the adult thoracic and lumbar spine for central neuraxial blockade. Anesthesiology 2011;114:1459-85. [Crossref] [PubMed]
- Gottlieb M, Holladay D, Peksa GD. Ultrasound-assisted Lumbar Punctures: A Systematic Review and Meta-Analysis. Acad Emerg Med 2019;26:85-96. [Crossref] [PubMed]
- Schlotterbeck H, Schaeffer R, Dow W. Ultrasonographic control of the puncture level for lumbar neuraxial block in obstetric anaesthesia. British journal of anaesthesia 2008;100:230-4. [Crossref] [PubMed]
- Whitty R, Moore M, Macarthur A. Identification of the lumbar interspinous spaces: palpation versus ultrasound. Anesth Analg 2008;106:538-40. [Crossref] [PubMed]
- Akerman B, Hellberg IB, Trossvik C. Primary evaluation of the local anaesthetic properties of the amino amide agent ropivacaine (LEA 103). Acta Anaesthesiol Scand 1988;32:571-8. [Crossref] [PubMed]
- Wille M. Intrathecal use of ropivacaine: a review. Acta Anaesthesiologica Belgica 2004;55:251. [PubMed]
- Malinovsky JM. Intrathecal Anesthesia: Ropivacaine Versus Bupivacaine. Anesth Analg 2000;91:1457-60. [Crossref] [PubMed]
- Milligan KR. Recent advances in local anaesthetics for spinal anaesthesia. Eur J Anaesthesiol 2004;21:837-47. [Crossref] [PubMed]
- Sell A, Olkkola KT, Jalonen J, et al. Minimum effective local anaesthetic dose of isobaric levobupivacaine and ropivacaine administered via a spinal catheter for hip replacement surgery. Br J Anaesth 2005;94:239-42. [Crossref] [PubMed]
- Liu Y, Qian W, Ke XJ, et al. Real-time Ultrasound-guided Spinal Anesthesia Using a New Paramedian Transverse Approach. Curr Med Sci 2018;38:910-3. [Crossref] [PubMed]
- Dixon WJ. Staircase bioassay: the up-and-down method. Neurosci Biobehav Rev 1991;15:47-50. [Crossref] [PubMed]
- Bin Z. The Dose-response Relationship of Three Local Anesthetics After Intrathecal Injection for Elderly Patients. Guangzhou First Municipal People’s Hospital; Guangzhou Medical College; 2010.
- McNamee DA, McClelland AM, Scott S, et al. Spinal anaesthesia: comparison of plain ropivacaine 5 mg ml(-1) with bupivacaine 5 mg ml(-1) for major orthopaedic surgery. Br J Anaesth 2002;89:702-6. [Crossref] [PubMed]
- Michalek-Sauberer A, Kozek-Langenecker SA, Heinzl H. Median effective local anesthetic doses of plain bupivacaine and ropivacaine for spinal anesthesia administered via a spinal catheter for brachytherapy of the lower abdomen. Reg Anesth Pain Med 2008;33:4-9. [Crossref] [PubMed]
- Johnston DF, Sondekoppam RV, Giffin R, et al. Determination of ED50 and ED95 of 0.5% Ropivacaine in Adductor Canal Block to Produce Quadriceps Weakness: A Dose-Finding Study. Reg Anesth Pain Med 2017;42:731-6. [Crossref] [PubMed]
- Dixon WJ. The Up-and-Down Method for Small Samples. J Am Stat Assoc 1965;60:967-78. [Crossref]
- Harrison DA, Langham BT. Spinal anaesthesia for urological surgery. A survey of failure rate, postdural puncture headache and patient satisfaction. Anaesthesia 1992;47:902-3. [Crossref] [PubMed]
- Horlocker TT, McGregor DG, Matsushige DK. A retrospective review of 4767 consecutive spinal anesthetics: central nervous system complications. Perioperative Outcomes Group. Anesth Analg 1997;84:578-84. [PubMed]
- Auroy Y, Narchi P, Messiah A. Serious complications related to regional anesthesia: results of a prospective survey in France. Anesthesiology 1997;87:479-86. [Crossref] [PubMed]
- Perlas A, Chaparro L, Chin K. Lumbar Neuraxial Ultrasound for Spinal and Epidural Anesthesia: A Systematic Review and Meta-Analysis. Reg Anesth Pain Med 2016;41:251-60. [Crossref] [PubMed]
- Tarkkila P, Huhtala J, Tuominen M. Home-readiness after spinal anaesthesia with small doses of hyperbaric 0.5% bupivacaine. Anaesthesia 1997;52:1157-60. [Crossref] [PubMed]
- Pace NL, Stylianou MP. Advances in and limitations of up-and-down methodology: a précis of clinical use, study design, and dose estimation in anesthesia research. Anesthesiology 2007;107:144-52. [Crossref] [PubMed]
- Camorcia M, Capogna G, Lyons G, et al. Epidural test dose with levobupivacaine and ropivacaine: determination of ED(50) motor block after spinal administration. Br J Anaesth 2004;92:850-3. [Crossref] [PubMed]
- Chen MQ, Chen C, Fang W. Determination of the median effective dose (ED50) of spinal plain ropivacaine for motor block in adults. Anaesthesist 2016;65:353-8. [Crossref] [PubMed]