
Esophageal perforation: a retrospective report of outcomes at a single center
Introduction
Esophageal perforation is a rare condition, but it can be life-threatening and is associated with high morbidity and mortality. Myriad possible causes of esophageal perforation exist, but it usually results from iatrogenic, malignant, or spontaneous events (e.g., Boerhaave syndrome). Overall prognosis has improved dramatically over the past 30 years, from a high mortality rate of roughly 30% to a slightly more acceptable rate of 15% (1,2). These changes might be attributed to a number of new interventions in the treatment of esophageal perforation, including gastrointestinal stents, minimally invasive surgical interventions, advances in intensive care, and the increasing use of interventional radiology for drainage.
A recent study focused on evaluation and severity scoring at the time of presentation in patients with esophageal perforation—that is, before any intervention is attempted. Abbas et al. proposed a severity scale called the Pittsburgh Esophageal Perforation Severity Score (PSS) in 2009 (3). Five years later, Schweigert et al. (4) carried out a retrospective, multicenter validation of the Pittsburgh PSS. They proposed a Pittsburgh PSS-based decision tree for management of esophageal perforation, and the first case reports of its clinical use were recently published (5,6).
Gastrointestinal stents have been increasingly used in patients diagnosed with esophageal perforation; however, direct benefits of and specific indications for this tool remain unclear (7). In this single-center review, we evaluated the results of patients treated at a tertiary care facility and assessed the effects of increased use of esophageal stents in this acute setting. We also studied the predictive value of the Pittsburgh PSS in this patient population (3). We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/aoe-20-17).
Methods
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). For the purposes of this study, informed consent was waived, as the research involved no more than minimal risk. The waiver of informed consent did not adversely affect the rights and welfare of the patients. After Institutional Review Board approval from St. Joseph’s Hospital and Medical Center in Phoenix, Arizona (PHXA-17-0308-71-18), a retrospective database for data collection and maintenance was created using REDCap© version 7.2.2 (Vanderbilt University). Patients diagnosed with esophageal perforation were identified using billing information gathered from discharge codes from ICD9: 530.4, 533.9, 862.32; and from ICD10: K22.3, S27.8, T18.198, K91.72. Charts from consecutive cases between May 2014 and September 2017 were reviewed, and patients with objective evidence of esophageal perforation on endoscopy, contrast study, or computed tomography (including patients with pneumomediastinum on computed tomography, but no contrast extravasation) were included. Patients were excluded from study if they were under the age of 18 years, had leakage after previous esophageal resection, or had undergone esophageal stenting for any indication within 1 year of the current presentation. The Pittsburgh PSS was retrospectively calculated for all patients. Table 1 summarizes scoring criteria for the Pittsburgh PSS.
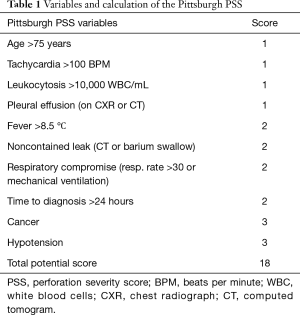
Full table
Statistical analysis was carried out using SPSS v22.0 (IBM, Armonk, NY, USA). Subgroups were compared using t-test for independent means for continuous variables. The Fisher exact and Chi-square tests were applied to categorical variables.
Results
In total, 56 patients with esophageal perforation met inclusion criteria. Thirty-nine patients (69.6%) were men, the mean age of the patients was 60 years, and mean body mass index was 27.1 kg/m2. The most common causes of esophageal perforation were iatrogenic in nature (24/56, 42.9%) followed by Boerhaave syndrome (12/56, 21.4%). Some causes of iatrogenic injury included perforating gastric band, large paraesophageal hernia repair, radiofrequency ablation for atrial fibrillation, and laminar discectomy-related injury. Foreign body ingestions were responsible for perforation in 10 patients (17.9%), almost half of them were food or pill impactions in patients with preexisting esophageal pathologies (e.g., strictures), while the rest were caused by a diverse spectrum of swallowed foreign bodies. In 4 patients (7.1%), perforation resulted from traumatic injury (stab wounds and missile injuries). Perforations originated from malignancy in 3 patients (5.4%), and the cause of injury was undetermined in 3 patients (5.4%). Forty-one patients (73.2%) presented to the first point of care within 24 hours of symptom onset; 6 presented between 24 and 72 hours of symptom onset, and 9 patients (16.1%) presented more than 72 hours after symptom onset.
The site of esophageal perforation was thoracic in 38 patients (67.9%), cervical in 9 patients (16.1%), and abdominal in 9 patients (16.1%). 52/56 patients (92.9%) underwent computed tomography; however, contrast swallows were only obtained for 23/56 patients (41.1%). Mediastinal air was present in 44.4% of patients [24/54 patients for whom imaging results (computed tomography or native radiograph) were available], and extraluminal contrast was found in 18/23 patients (78.3%) who underwent contrast swallow. Ten/56 patients (17.9%) had pleural effusion evident on imaging. Forty-nine/56 patients (87.5%) underwent endoscopy; most of these endoscopic procedures (43/49, 87.8%) were carried out at the same time as the definitive intervention. Clear, identifiable macroscopic perforation was evident in 44 of the 49 patients who underwent endoscopy (89.8%). The mean and median Pittsburgh PSS of patients in this cohort were 3.7 and 3.5, respectively. Eleven/56 patients (19.6%) were in respiratory distress at the time of presentation, and 5/56 patients (8.9%) required treatment with vasopressors.
In total, 34/56 patients (60.7%) were referred to our institution from another medical facility. Twenty of these 34 were early-referral patients, defined as transfer after suspicion of perforation or after initial radiologic findings indicated a possible perforation. Late-referral patients were defined as those who were transferred to our institution after undergoing invasive intervention, or after more than 24 hours of conservative therapy. Referrals arrived in somewhat worse overall condition represented by Pittsburgh PSS; however, this might be attributed to the time loss in disease course without intervention (Table 2).
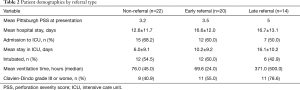
Full table
Treatment approach
The primary treatment approach was conservative management in 8/56 patients (14.3%), stent placement in 8/56 patients (14.3%), and surgical management in 40/56 patients (71.4%; Table 3). Minor lesions with contained leaks warranted conservative treatment, whereas late presentation, injuries of the cervical esophagus, and suspected extensive soiling in the chest indicated surgical exploration. The stent-only approach was used predominantly in patients with thoracic perforations, malignancies, or when an underlying tracheobronchial fistula was suspected.
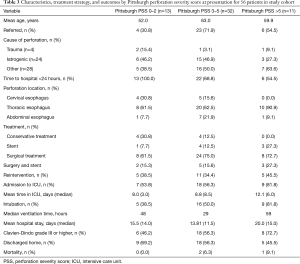
Full table
Conservative management
Of the eight patients who received conservative treatment, 3 had abdominal esophageal perforations and 5 had thoracic esophageal perforations. Four patients in this group had esophageal perforations that were iatrogenic in origin; the mean Pittsburgh PSS in these four patients was 2. Early nil per os regimen and broad-spectrum antibiotics for at least 7 days were the standard of care. No changes in treatment approach were required, and no invasive interventions were deemed necessary. Two/8 patients required brief admission to the intensive care unit (ICU) (1 day each); however, neither patient required intubation. The mean hospital stay in this group was 3.75 days. There was no mortality in this group, and morbidity stayed at or under Clavien-Dindo grade II for all patients. Seven/8 patients were discharged home; 1 was discharged to a skilled nursing facility.
Stent placement
Of the 56 patients in this study, 8 patients received stents as primary treatment. Seven/8 patients in this group had thoracic perforations; 1 had an abdominal perforation. Three perforations were of iatrogenic origin, and 2 of these were malignant in nature. The preferred stent used was a fully covered esophageal stent: EndoMAXXTM (Merit Medical Systems, Inc., South Jordan, UT, USA). Stent size was selected according to manufacturer recommendation, and stents were removed 3 to 4 weeks after deployment. The mean Pittsburgh PSS in patients who received stents was 3.5. Four patients (50.0%) in this group required reintervention: 2 underwent stent repositioning; 2 underwent another invasive approach—both requiring open repair within 1 week of the initial stent placement. One patient ultimately required an esophagectomy a month after initial presentation. Both patients were referred from outside medical facilities and presented to our institution more than 72 hours after initial symptom onset. There were no percutaneous chest tube placements without surgical intervention in this subgroup.
The mean hospital stay for patients who received stenting was 13.4 days. Five patients (62.5%) had outcomes Clavien-Dindo grade IIIa or worse. Five patients were discharged home, 1 was discharged to a skilled nursing facility, 1 was discharged to a long-term acute care hospital, and 1 was discharged to hospice.
Surgical management
The surgical intervention cohort in our study consisted of 40 patients. Five had abdominal perforations, 9 had cervical perforations, and 26 had thoracic perforations. Fifteen perforations were from iatrogenic injury, 10 were from Boerhaave syndrome, and 1 was from a malignancy. There were 23 referrals from outside hospitals in this group, and 16 of these 23 referrals were early referrals—that is, the patients arrived at our institution before they underwent any intervention. The mean Pittsburgh PSS in this group was 3.9. The surgical cohort can be further divided into 3 subgroups; these are detailed in Figure 1.
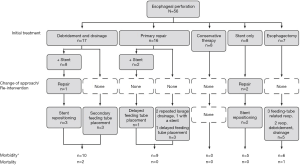
Debridement and drainage
Seventeen patients underwent debridement and drainage; of these, 8 also received stents. Half of the procedures were carried out with video-assisted thoracoscopic surgery. The mean Pittsburgh PSS in this group was 3.7. Nine out of these 17 patients were transferred to our institution from another hospital, including 3 who were referred after therapy was attempted at an outside facility. Seven patients required reintervention in this subgroup: 3 for stent repositioning, 3 for placement of a feeding tube (second surgery), and 1 with extravasation who required laparoscopic exploration, lavage, and repair over the previously deployed stent. Eleven patients in this treatment group were admitted to the intensive care unit, with a mean stay of 5 days, and mean total hospital stay of 16 days. Eight patients were placed on ventilators for a mean duration of 46 hours. Two patients died in this group. The first was an 87-year-old man diagnosed with Boerhaave syndrome. He was admitted with florid sepsis; his hospital course was further complicated by a brainstem stroke. The other was a late referral who developed sepsis and multiple organ failure from the perforation and associated peritonitis. Ten/40 patients (25.0%) in this group had a Clavien-Dindo grade IIIa or worse. Seven patients were discharged home.
Primary repair
Sixteen patients underwent primary repair of their perforation. Ten of these repairs were reinforced with adjacent tissue buttressing. The preferred buttresses were intercostal muscle flaps. The mean Pittsburgh PSS in this group was 3.6. Ten of the 16 patients were referrals, but only 1 was delayed by a therapeutic attempt at the referring facility. One patient had an abdominal perforation, 4 patients had cervical perforations, and 11 patients had thoracic perforations. Two patients received stents at the time of surgery, 4 required reintervention, and 2 required debridement and additional drainage—1 of them with negative-pressure therapy. One patient had delayed feeding tube placement; 1 underwent reoperation and stent deployment. Thirteen of the 16 patients were admitted to the intensive care unit; the mean length of stay was 8.5 days. Twelve needed mechanical ventilation, and the mean duration of ventilation was 52 hours. The mean hospital stay was 14.6 days. Nine/16 patients (56.3%) who underwent primary repair had outcomes Clavien-Dindo grade III or worse. There were no deaths in this group, and 12 patients were discharged home.
Esophagectomy
Seven patients underwent esophagectomy. Four of these patients had been referred from outside institutions. The mean Pittsburgh PSS of patients who required esophagectomy was 4.85. There were no cervical perforations in this group; 2 perforations were abdominal, and 5 were thoracic. Five/7 patients required reintervention: 1 for delayed feeding tube insertion, 1 for wound dehiscence, 2 for additional drainage and open abdominal therapy, and 2 for replacement of a jejunostomy feeding tube. One patient required two reinterventions. Six of the 7 patients were admitted to the intensive care unit; the mean length of stay in that unit was 21.3 days. Each patient who was admitted to the intensive care unit required ventilator support, with a mean duration of 313 hours. The average overall hospital stay in the esophagectomy group was 23.3 days. One patient died, and all patients but one had outcomes Clavien-Dindo grade III or worse.
Comparison of approaches
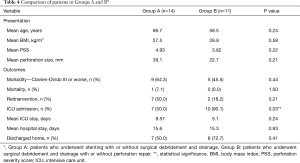
Given the diversity of the entire cohort, comparison of all treatment approaches would not be possible. However, some subgroups share similar demographic and presentation characteristics, and therefore can be compared. In order to assess the effect of endoluminal stents, we compared two subgroups of the cohort. Only direct admissions to the emergency department or early referral patients (with no previous therapeutic approaches to disarrange outcomes) with thoracic perforations were included in the two subgroups. We excluded patients who underwent conservative therapy or esophagectomy, as these scenarios do not involve the use of stents.
Group A consisted of patients who underwent stenting with or without surgical debridement and drainage. Patients in Group B, however, underwent surgical debridement and drainage with or without perforation repair. The two groups were homogenous in age, body mass index, perforation size, and Pittsburgh PSS at the time of presentation.
No statistically significant differences were observed between Groups A and B in terms of mortality (P>0.99); morbidity of Grade III or worse on the Clavien-Dindo classification (P=0.43); reintervention rate (P=0.21); length of hospital stay (P=0.93) or whether the patients were discharged home or to a specialized care facility (P=0.41). However, there was a difference in admission rate to the ICU (P=0.03), but not in length of stay in the ICU (P=0.24). See Table 4 for details.
Full table
Other outcomes
Perforation size was recorded in 29 of the 56 patients who made up the entire study cohort. In these 29 patients, the mean and median perforation sizes were 32.1 and 20 mm, respectively. Overall morbidity weighted by Clavien-Dindo classification for grade III or higher was 57.2%. In total, 6/56 patients (10.7%) developed a leak. Sepsis developed in 9/56 patients (16.1%), with 8 progressing to multiple organ failure. Acute kidney failure developed in 11 patients (19.6%), while pneumonia occurred in 7 (12.5%). Overall mortality within 1 month was 5.4% (3 patients).
Follow-up information was available for 39 patients within 1 month of discharge. Nineteen/39 patients (48.7%) described being self-reliant. Three of the 39 patients (7.7%) were on nil per os diet at the time of the first follow-up, and 10/39 (25.6%) were on a liquid-only diet. Twenty-one/39 patients (53.8%) reported being able to eat soft foods with no symptoms. Four/39 patients (10.3%) reported dysphagia, and 20/39 (51.3%) still had a feeding tube in place at the time of the first follow-up visit.
Discussion
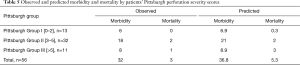
In our cohort of 56 patients, the observed mortality (3/56, 5.4%) was congruent with the predicted mortality (5.3 patients, 9.5%) based on Pittsburgh PSS (P=0.46; Table 5). Morbidity was comparable to the predicted values. Higher Pittsburgh PSS seemed to be associated with longer stay in the ICU, increased ventilation time, and higher morbidity; however, these differences were not statistically significant. All therapeutic approaches were carried out in a manner similar to the treatment algorithm proposed by Schweigert et al. (4). The PSS was calculated at the time of presentation to our facility, not at the first line of care in case of referrals. Abbas et al. (3) did not discuss the number of referrals in their original study; the difference in proportion of referrals between the two cohorts might explain the small differences in mean Pittsburgh PSS and mortality to some degree. Overall, the Pittsburgh PSS accurately predicted mortality/morbidity and would have adequately guided treatment approaches.
Full table
Etiology
Esophageal perforation has a very diverse etiology, but its primary causes are iatrogenic injury, Boerhaave syndrome, and malignancy, which is reflected in our patient population. Iatrogenic injury most often occurs during an endoscopic procedure, and surgical procedures around the gastroesophageal junction may also cause perforations that go unrecognized intraoperatively. More and more unique causes of iatrogenic perforation are being published. In our cohort, we observed a somewhat lower prevalence of malignant perforation (i.e., 5.4%) compared to the prevalence reported in the literature (roughly 15%) (1,8,9).
Site of perforation
The distribution of perforation sites is similar to the distribution described in the literature—that is, the majority of perforations are thoracic, and the rest are divided equally between cervical and abdominal perforations. We observed a high proportion of surgical intervention in the cervical perforation group, but that group had no deaths. Cervical perforations are usually sealed by the muscles of the neck, and cervical exploration has lower morbidity; therefore, cervical perforations are associated with a lower threshold to intervention than thoracic or abdominal explorations (10).
Treatment options
Historically speaking, an uncontained esophageal perforation meant open exploration, debridement, lavage, and drainage with esophageal repair. It often also required buttressing with a well-vascularized adjacent structure. However, it has become clear that a significant proportion of contained perforations can be treated with conservative therapy.
The emerging use of esophageal stents for malignant disease has resulted in greater availability and expertise with esophageal stents in recent years. Nearly all gastrointestinal stents have been approved for use in malignant obstructions; the use of stents in benign disease is considered off-label use. However, this off-label use is rapidly gaining traction in esophageal treatment centers around the world. Although the actual outcomes of stenting as a therapeutic approach are not yet defined, it is clear that stenting is not necessarily associated with decreased reintervention rate. This experience is based on the high reported rates of stent repositioning procedures and restenting. However, these repositioning procedures are less invasive than reoperation (7,9,10). Upon comparison of thoracic esophageal perforation subgroups, stenting did not produce favorable outcomes outside the scope of ICU admission in our cohort. Some experts maintain that open repair is the best choice in any patient with an uncontained leak. Without doubt, direct inspection of contamination and damage can be fundamental in selecting the correct treatment modality.
Decision-making approach
Upon retrospective evaluation, all clinical decisions made in our cohort were surprisingly in close concordance with the Pittsburgh PSS based decision tree proposed by Schweigert et al. (4).
Injury to the cervical esophagus warrants surgical intervention, as exploration is associated with low mortality (11). Moving distally, the proportion of nonoperative management rises (31.6% for thoracic injury; 44.4% for abdominal injury). Aside from perforation site, preoperative factors that support primary repair as the first-line treatment (instead of using a stent or other combination of therapies) are ill-defined (1).
Placing a stent in the cervical esophagus is generally contraindicated due to the high migration rate of stents placed in this region, as well as patient discomfort and associated morbidity. Special stents developed for the cervical section of the esophagus have only recently become available (12). A similar problem arises with stents placed at the esophagogastric junction; high probability of reflux and reflux-associated aspiration are expected when deploying stents that traverse this anatomical barrier (13,14). These guidelines are reflected in our practice as well—no cervical stents were deployed, and only one patient underwent stenting for an esophageal perforation below the diaphragm.
Early definitive intervention still plays a major role in final outcomes. Our outcomes suggest that not only time to the first intervention is critical in optimizing outcomes, but so is having the resources to carry out definitive treatment (Table 2). Therefore, early transfer to a tertiary care center with esophageal expertise for definitive care of esophageal perforation is critical (15).
Prognosis
The Pittsburgh PSS might be helpful when esophageal perforations are encountered in a medical facility where elective esophageal surgery is uncommon. A Pittsburgh PSS of 3 or higher might encourage the provider to initiate a transfer to a larger, more experienced center, if feasible. Schweigert’s decision tree (4), which is based on the Pittsburgh PSS, is even easier to interpret. If there is a chance that a patient might need escalation of care to a highly invasive, specialized procedure unavailable at the present site of care, the patient should be transferred right away. Definitive intervention within 24 hours of symptom onset yields much lower morbidity and mortality rates (16). This idea is almost universally accepted and has made its way into the Pittsburgh PSS; however, recently some have questioned its viability and have proposed that the threshold be extended (8,17).
The most obvious limitation of our study is its retrospective nature. Although the size of this cohort is larger than described in most single-center reviews, the overall number does not allow for statistical comparison among subgroups.
Persson et al. (10) recently voiced concern about the astonishingly low scientific level of evidence surrounding esophageal perforation. Randomized prospective clinical trials are still lacking; however, given the nature of the disease, it is unlikely that such trials could be organized.
Esophageal perforations continue to carry significant morbidity and mortality. Many therapeutic options are available, and a select group of patients can be managed with endoscopic stent therapy alone. However, not infrequently there is a need for change in course and utilization of alternate treatments; therefore, hypervigilance until the condition has been clinically resolved is mandatory. Optimal care can be provided where expertise in all the above therapeutic modalities is readily available.
Acknowledgments
An abstract pertaining to this paper was presented at the 16th World Congress of The International Society for Diseases of the Esophagus, Sep. 16–19, 2018 Vienna. The authors are grateful to Clare Sonntag, who provided expert editorial assistance.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/aoe-20-17
Data Sharing Statement: Available at http://dx.doi.org/10.21037/aoe-20-17
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoe-20-17). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board approval of St. Joseph’s Hospital and Medical Center in Phoenix, Arizona (PHXA-17-0308-71-18). For the purposes of this study, informed consent was waived, as the research involved no more than minimal risk.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ali JT, Rice RD, David EA, et al. Perforated esophageal intervention focus (PERF) study: a multi-center examination of contemporary treatment. Dis Esophagus 2017;30:1-8. [Crossref] [PubMed]
- Attar S, Hankins JR, Suter CM, et al. Esophageal perforation: a therapeutic challenge. Ann Thorac Surg 1990;50:45-9; discussion 50-1. [Crossref] [PubMed]
- Abbas G, Schuchert MJ, Pettiford BL, et al. Contemporaneous management of esophageal perforation. Surgery 2009;146:749-55; discussion 755-6. [Crossref] [PubMed]
- Schweigert M, Sousa HS, Solymosi N, et al. Spotlight on esophageal perforation: A multinational study using the Pittsburgh esophageal perforation severity scoring system. J Thorac Cardiovasc Surg 2016;151:1002-9. [Crossref] [PubMed]
- Antonoff MB. Validation of the Pittsburgh esophageal perforation severity score: Further impetus for a prospective study. J Thorac Cardiovasc Surg 2016;151:1012-3. [Crossref] [PubMed]
- Giulini L, Dubecz A, Schweigert M, et al. Traumatic oesophageal perforation: a successful management based on the Pittsburgh Perforation Severity Score. Eur J Cardiothorac Surg 2019;55:792-4. [Crossref] [PubMed]
- Thornblade LW, Cheng AM, Wood DE, et al. A Nationwide Rise in the Use of Stents for Benign Esophageal Perforation. Ann Thorac Surg 2017;104:227-33. [Crossref] [PubMed]
- Bhatia P, Fortin D, Inculet RI, et al. Current concepts in the management of esophageal perforations: a twenty-seven year Canadian experience. Ann Thorac Surg 2011;92:209-15. [Crossref] [PubMed]
- Sudarshan M, Elharram M, Spicer J, et al. Management of esophageal perforation in the endoscopic era: Is operative repair still relevant? Surgery 2016;160:1104-10. [Crossref] [PubMed]
- Persson S, Rouvelas I, Irino T, et al. Outcomes following the main treatment options in patients with a leaking esophagus: a systematic literature review. Dis Esophagus 2017;30:1-10. [Crossref] [PubMed]
- Biffl WL, Moore EE, Feliciano DV, et al. Western Trauma Association Critical Decisions in Trauma: Diagnosis and management of esophageal injuries. J Trauma Acute Care Surg 2015;79:1089-95. [Crossref] [PubMed]
- Shim CS. Esophageal stent for cervical esophagus and esophagogastric junction. Clin Endosc 2012;45:235-9. [Crossref] [PubMed]
- Sato H, Ishida K, Sasaki S, et al. Regulating migration of esophageal stents - management using a Sengstaken-Blakemore tube: A case report and review of literature. World J Gastroenterol 2018;24:3192-7. [Crossref] [PubMed]
- Vermeulen BD, Siersema PD. Esophageal Stenting in Clinical Practice: an Overview. Curr Treat Options Gastroenterol 2018;16:260-73. [Crossref] [PubMed]
- Kaman L, Iqbal J, Kundil B, et al. Management of Esophageal Perforation in Adults. Gastroenterology Res 2010;3:235-44. [PubMed]
- Keeling WB, Miller DL, Lam GT, et al. Low mortality after treatment for esophageal perforation: a single-center experience. Ann Thorac Surg 2010;90:1669-73; discussion 1673. [Crossref] [PubMed]
- Wu JT, Mattox KL, Wall MJ Jr. Esophageal perforations: new perspectives and treatment paradigms. J Trauma 2007;63:1173-84. [Crossref] [PubMed]
Cite this article as: Kovács B, Masuda T, Bremner RM, Smith MA, Huang JL, Hashimi AS, Patel C, Ahmed S, Mittal SK. Esophageal perforation: a retrospective report of outcomes at a single center. Ann Esophagus 2021;4:2.


