
An overview of plasma fractionation
Current range of plasma products and clinical indications
The fractionation of human plasma, although, quantitatively speaking, a minor part of the whole biotechnology industry, is an important activity that ensures the supply of a range of over 25, mostly unique, therapeutic proteins (1-3). The clinical use of plasma-derived medicinal products usually encompasses substitutive (or “augmentation”) intravenous infusion therapy of proteins to treat bleeding, haemostatic, immunological, or metabolic disorders often linked to a congenital or acquired deficiency.
The main plasma-derived therapeutic proteins produced today and their respective clinical indications are presented in Table 1, and briefly described below:
Table 1
| Protein | Clinical use |
|---|---|
| Fibrinogen | Congenital or acquired (postpartum hemorrhage) deficiency |
| Factor II | Factor II deficiency |
| Thrombin (factor IIa) | Component of fibrin sealant as hemostatic or sealing agent |
| Factor V | Factor V deficiency |
| Factor VII | Factor VII deficiency |
| Factor VIII | Hemophilia A |
| Factor IX | Hemophilia B |
| Factor X | Factor X deficiency |
| Factor XI | Hemophilia C (factor XI deficiency) |
| Factor XIII | Factor XIII deficiency |
| Von Willebrand factor | Severe forms (type 3 and in type 2) of Von Willebrand factor deficiency, sometimes in combination with factor VIII administration |
| Factor VIII/Von Willebrand factor | Hemophilia A; severe forms (type 3 and in type 2) of Von Willebrand factor deficiency |
| Prothrombin complex | Treatment of complex liver diseases; warfarin or coumarin derivatives reversal; hemophilia B (in the absence of single factor IC concentrate) |
| Fibrin sealant/fibrin glue (fibrinogen and thrombin) | Topical tissue hemostatic healing and sealing agent for surgical applications |
| Activated prothrombin complex | Hemophilia A with neutralizing anti-FVIII inhibitors (in the absence of other treatment possibly more adapted to patient condition) |
| Antithrombin | Congenital (or acquired) deficiency leading to thrombosis |
| Alpha 1-antitrypsin | Congenital deficiency associated with panacinar lung emphysema |
| C1-esterase inhibitor | Congenital deficiency leading to angioedema |
| Protein C | Congenital deficiency leading to thrombosis |
| Polyvalent IgG (normal) | Prevention of infections in immunodeficient patients; Immune modulation in various immunological disorders |
| Hyperimmune IgG: hepatitis B, hepatitis A, tetanus, rabies, varicella/zoster | Prevention or treatment of infections |
| Anti-Rho (D) | Prevention of haemolytic disease of the newborn |
| Albumin | Volume replacement |
- Coagulation factors currently extracted from human plasma include factor VIII (FVIII), FIX, and the prothrombin complex (a complex fraction comprising FII, FIX, FX, and for some preparations FVII, as well as protein C and protein S). Other coagulation factor preparations routinely produced or under development, by a somewhat limited number of plasma fractionators worldwide, include fibrinogen, von Willebrand factor, FV, FVII, FXI, FX, or FXIII. Most of these proteins are used for intravenous substitutive therapy to compensate for the missing proteins in the blood circulation. Fibrin sealant (also called fibrin glue) is a double component protein preparation that combines a concentrate of fibrinogen (possibly containing also fibronectin and von Willebrand factor among other proteins) and of thrombin; both components are mixed at time of use to generate a fibrin-rich surgical clot that is used, typically for topical applications, to achieve tissue hemostasis, or tissue sealing and healing (5,6).
- Important protease inhibitor or anticoagulant concentrates are available including antithrombin, alpha 1-antitrypsin (also called alpha 1-protease inhibitor), a protein with potent anti-elastase activity), and C1-esterase inhibitor (also called C1-inhibitor).
- The current major plasma protein, in terms of clinical applications and therapeutic importance, is polyvalent normal immunoglobulin (IgG). This large plasma pool preparation contains millions of antibodies reflecting the immunization profile of the donor population to multiple antigens from their living environment. Donors contributing plasma that is used to make “polyvalent” IgG are not selected for having a high titer in a particular antibody. IgG represents an essential therapeutic product for treatment of patients with primary immune deficiencies or with secondary immune deficiencies resulting from disease or disease therapy. They are also used widely for their immunomodulatory properties in treating inflammatory and autoimmune diseases, and other immunological alterations (7). Depending upon patients, specific pathological characteristics, the clinical setting, and the regulatory situation, normal polyvalent IgG are administered intravenously, intramuscularly, or subcutaneously.
- Hyperimmune IgG are made from the plasma of donors, typically vaccinated, identified to express high titer in neutralizing antibodies directed against various antigens such as the D red blood cell antigen, hepatitis B, tetanus, rabies, hepatitis A, or cytomegalovirus.
- Albumin was the first protein to have been extracted from human plasma, and it remains one important protein generated from plasma fractionation. Its clinical use relates to its oncotic as well as detoxification capacity.
- Further updated information on the clinical use of plasma products is available elsewhere (3).
Quality of plasma for fractionation
It is a cornerstone of the safety of pooled industrial plasma products that plasma used for fractionation complies with a set of quality requirements and specifications. Such requirements have to be considered with global perspectives in mind, in particular with regards to the control of the risks of contamination of human blood with emerging infectious agents, as recently evidenced by the Zika virus outbreaks in various regions (8). International and regional guidelines have therefore highlighted the importance of ensuring an epidemiological surveillance of the general population, and of blood/plasma donors, as a mechanism to follow the trends in incidence and prevalence of known and emerging pathogens over time. Such mechanism can facilitate the introduction of appropriate counteracting measures in donors screening and donation testing, when needed, based on case-by-case risk assessment performed for each blood product, and thereby taking into account in particular the implementation of a robust and validated pathogen inactivation processes (1,9).
Quite understandably, the methods used for collecting plasma for fractionation can exert some influence on the efficiency (e.g., yield) and reliability (e.g., absence of proteases) of the fractionation process, and the quality of the end-products. Therefore, the collection of plasma for fractionation should be regarded as an integral part of the manufacture of plasma products. As such, plasma fractionators, as holders of the marketing authorization of products made from collected plasma for fractionation, are (directly if they collect plasma; or indirectly in they obtain plasma from another entity) legally responsible of the compliance of the collection process and quality of plasma with the contractually agreed quality specifications. Plasma fractionators should perform regular audits of blood/plasma collection centers (blood establishments) to verify that plasma collection, as carried out in each collection center or mobile unit, is compliant with the contractual obligations with regards to the identified quality and safety measures. Those include, among others, donor’s screening and deferral criteria, individual donation testing, handling of the blood/plasma donation, quarantine procedures, equipment maintenance and validation, process monitoring, freezing and storage, traceability system and documentation, etc. In some jurisdictions, like European Union and Australia, the specifications in place in collection of plasma for fractionation are compiled in a specific regulatory document called “plasma master file” (10,11). It is widely accepted that such a system initially focused at plasma for fractionation actually results in positive impacts on the quality and safety of all blood components manufactured in the same collection setting (12,13). In that regard, the quality assurance manager within the blood establishment plays a key role in overviewing quality aspects of recovered (14) and source apheresis plasma.
The relevant national regulatory authority should license blood establishments producing plasma for fractionation. The production methods to collect or isolate, test, freeze, store, and ship plasma for fractionation, and ensure traceability, should comply with good manufacturing practices. Readers are invited to consult the WHO “Recommendations for the production, quality control and regulation of plasma for fractionation” (4) and “Guidelines on good manufacturing practices for blood establishments” (15) for further general information of interest at global level (4,15). The publication in this issue by Weinstein addresses specifically the regulatory requirements for the collection of plasma for fractionation in the USA (16), where the majority of the plasma fractionated worldwide is collected, and that from Rossi provides an insight on the French and EU regulatory situation (17).
Volume of plasma fractionated
The current volume of plasma fractionated worldwide is estimated to be between 42 and 45 million litres according to recent estimates by the Marketing Research Bureau. This volume has been steadily increasing over the years since the beginning of the plasma fractionation industry. Most of the plasma (roughly 80%) fractionated is obtained by plasmapheresis, a dedicated procedure that allows isolating only plasma (often called “source plasma”), as the blood cells (most specifically the red blood cells) are returned to the donor. A minor part of the plasma used for fractionation (called “recovered plasma”) is obtained as a by-product of the collection of whole blood that is driven by the clinical needs for red blood cell for transfusion. Both source plasma and recovered plasma are suitable for fractionation as long as the collection process is in compliance with national blood policies, the quality specifications from the fractionator, and the GMP requirements from the relevant regulatory authorities that oversee the blood collection system and are responsible for the marketed plasma products.
The main differences existing between source and recovered plasmas include the volume collected at each donation (ca. 600–880 versus 100–250 mL, respectively), the maximum frequency (twice a week or 24 times/year versus 4 to 5 times/year, depending upon jurisdictions), and the chemical composition of the citrate-based anticoagulant solution. Source plasma can typically be frozen within minutes after the end of collection whereas recovered plasma freezing is delayed due to the time needed for processing whole blood into its components. Typically, source plasma may contain ca. 10–30% higher level in labile coagulation factor VIII than recovered plasma, but ca. 10–20% lower amount of IgG in relation to the plasma donation frequency (18,19). In a public setting, without the more stringent concerns for cost-effectiveness existing in private for-profit organisations, the cost of apheresis plasma is more than that of recovered plasma, for which cost allocation with red blood cells and platelets is possible (20). This is of importance, since plasma cost is a 20–40% contributor to the cost of production of plasma products, and impacts the dynamics of the plasma fractionation industry (21) and its market (22).
It is estimated that the volume of plasma for fractionation needed to cover clinical needs will continue to increase in the years to come (22), considering that any potential further improvement in the current plasma fractionation technology, in particular in the recovery of the driving proteins (IgG), is not expected to exert noticeable impact on fractionation outcomes in the next few years. Also, it is currently seen as unrealistic to imagine synthetic (cell-derived) production of an IgG product with the polyvalence of the plasma-derived counterpart.
Current fractionation processes
Plasma fractionation is regarded as being a relatively conservative field in a sense that the core methodology used to extract plasma proteins remains today largely based on the cryoprecipitation (23) and ethanol fractionation steps (24) developed over 70 years ago. There are objective reasons, both of technical and regulatory natures that can explain this situation. Albumin and IgG were the first proteins to have been fractionated from human plasma using multiple-step, sequential cold ethanol processes (24,25), whereas it was then later identified that FVIII could be isolated by thawing plasma at cold temperature to generate a cryoprecipitate (26), using a process compatible with albumin and IgG extraction. The widespread industrial implementation of fractionation based on combining cryoprecipitation and cold ethanol fractionation to respond to the clinical needs at the time, has gradually shaped the technology still in use today. New proteins, in lesser clinical demands, have therefore been extracted from side-fractions generated from this initial process scheme, with the objectives to limit the impact on the quality and the recovery of the three driving proteins that were then in clinical use. Nevertheless, over the years, substantial technological evolutions have been seen to address broader clinical needs in a larger diversity of protein therapeutics (3), and in proteins with improved purity and quality levels (27,28). In addition, as illustrated below, the needs to implement dedicated virus inactivation and removal treatments has led to an evolution in the way how therapeutic proteins are now extracted from human plasma (1).
To a large extent, large-scale chromatography has been the major technological approach used to improve the purity profile and recovery of extracted proteins and diversify the portfolio of therapeutic plasma products, thereby also contributing to an improved cost-effectiveness of plasma fractionation. Nowadays, the traditional plasma fractionation technology continues to evolve in a direction where ethanol precipitations steps are being progressively, but not yet totally, abandoned, in the production of intravenous IgGs (29). Ethanol fractionation remains of widespread use among fractionators for albumin extraction. By contrast most coagulation factors (such as factor VIII, IX, and XI, and von Willebrand factor), protease inhibitors, and anticoagulants are extracted using chromatographic procedures (30-32). Further information on the plasma protein purification technologies are available from other sources, and remain valid up to now (1).
Current viral reduction treatments
A set of safety measures
Ensuring the highest possible level of virus safety to human plasma products is the result of the cumulative effect of various GMP-compliant measures (Figure 1). Those encompass the epidemiological control of the population, the informed consent and stringent screening of candidate blood/plasma donors, the testing of virus markers in individual blood/plasma donations, on plasma mini-pools and manufacturing pools, and, last but not least, as the main contributor of virus safety, the implementation of dedicated virus reduction methods (virus inactivation and/or virus removal) during the large-scale production process (1,9). The existence of a traceability system from donors to patients and vice-versa is an additional cornerstone of the pathogen safety of blood products, allowing to perform “look-back” procedures if suspicions of viral transmissions in patients, or viral risks from a donor, were identified (4). The contribution of this “quality multi-pod” (typically refer to the safety tripod) to safeguard industrial plasma products has been well covered in several regulatory documents (4,33) and scientific reviews (9); they highlight the need for constant surveillance of the infectious risks and the appropriateness of the safety system in place to ensure a robust margin of safety.
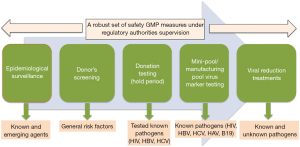
Role of virus reduction
Among all measures in place to build up the safety of industrial plasma products, the implementation of dedicated robust virus reduction treatments has proven to be the key contributor to the viral safety of industrial plasma products against known blood-born as well as emerging viruses (34). This is extremely well illustrated by the fact that robust viral inactivation methods, such as solvent/detergent (S/D) or pasteurisation, have avoided the transmission of highly pathogenic viruses like HCV by coagulation factor concentrates, even before the introduction of anti-HCV or HCV NAT donation testing. The essential role played by virus reduction treatments is further highlighted by the decision made by regulatory authorities, like the US Food and Drug Administration, not to request the testing of plasma for fractionation for viruses like WNV, ZIKV, or DENV. This decision was based on an assessment of the current virus reduction treatment (e.g. S/D, pasteurisation, nanofiltration) implemented in the modern plasma fractionation industry to provide a sufficient margin of virus safety for these viruses (35-39).
Virus reduction methods have, for most of them, been introduced in the 1980’s, with the exception of the pasteurisation process of albumin, which was initially implemented, based on previous experience on serum, to limit the risks of HBV transmission. This treatment was later on found to provide safety against HIV, HCV, and other viruses, together with various ethanol fractionation steps of albumin contributing to removal. Selecting a proper virus reduction strategy of plasma products, typically based on combining “orthogonal” treatment for wider efficacy, is not trivial, as highlighted in the WHO “Guidelines on viral inactivation and removal procedures intended to assure the viral safety of human blood plasma products” (40). Although not updated, these guidelines still provide information on the scientific logic underlying the selection, validation, and large-scale implementation of virus reduction treatments. Viral reduction technologies should be selected based on their robustness to inactivate or remove carefully selected model, ideally “relevant”, viruses, but also with the requirement not to affect the physiological integrity of therapeutic proteins, a crucial consideration to avoid neoantigen generation and to provide good functional recovery of the therapeutic proteins (40).
Virus reduction treatments, combined in a logical manner, should provide synergistic efficiency against lipid-enveloped or non-enveloped viruses. This strategy of combining orthogonal steps is the current basis for ensuring the safety of plasma products against a wide range of viruses. Figure 2 provides an illustration of the sequence of viral reduction treatments that have been developed and applied to coagulation factors in the 1980-1990’s. These successive developments have been much instrumental to avoid the transmission of known viruses as well as the recent viral agents such WNV (35,41), H1N1 (42), DENV (43), SARS coronavirus (44,45), and probably HEV (38), in the absence of donation or manufacturing plasma pool testing. It is aimed that when two dedicated treatments are implemented, both will provide robust efficacy against the most pathogenic enveloped viruses, and at least one against non-enveloped viruses. In plasma fractionation, robustness of virus reduction is usually seen as an experimental demonstration that more than four logs of inactivation or removal in virus infectivity can be reproducibly achieved within the defined process limits, demonstrating limited impact of process variations in critical parameters. The clinical parameters to consider vary based on the treatment applied and may encompass protein content, pH, conductivity, temperature, dose of virus inactivating agents, flow-rate etc. The demonstration of process robustness relies on scientific-based evidence and proper documentation. Proof should be provided that the manufacturer convincingly understands, documents, monitors, and reviews critical parameters possibly affecting virus reduction treatment efficacy (40). Process robustness must be reconsidered and reassessed in view of potential new virus threats affecting the blood and plasma supply, and is under scrutiny by relevant regulatory authorities with a mandate to continuously oversee the safety of plasma products.
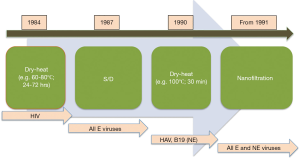
Diversity in viral reduction treatments
The range of virus reduction treatments in place in the plasma fractionation industry is by far the most diverse in use in the whole biological product industry. This diversity has to do with the variety of protein therapeutics generated from the fractionation of human plasma. While virus chemical reduction treatments designed to affect only lipid-based structures, and virus removal methods based on size-exclusion partitioning can be applied in principle to essentially all plasma protein therapeutics, this is not the case for methods based on heat or acid-pH treatments. Drastic treatments may affect the functional activity of some proteins and may require, when possible, substantial adjustments in the conditions implemented (such as the type of stabilizers used during pasteurisation or dry-heat treatment) (9). In that regard it is also interesting to notice that only limited evolution has taken place in the landscape of the range of virus reduction treatments used by the plasma fractionation industry in the last 20 years or so. This probably illustrates the reliability of, and trusts in, the safety margins provided by methods currently in place, as well as reflecting the impact of the tight regulatory systems overlooking plasma products. Implementing new virus reduction methods with new principles of virus clearance, would require substantial validation efforts in order to reach product licensing.
To date, the viral inactivation treatment by S/D, which was developed for industrial applications in the mid-1980’ (46), remains a core technology in use today for the inactivation of lipid-enveloped viruses in essentially all plasma products, apart from albumin largely due to the processing volume. The solvent used is tri (n-butyl) phosphate (TnBP), at final concentrations of 0.3–1% (v/v). The type of detergent selected has evolved, depending upon protein products, from sodium cholate and Tween-80, in the mid-1985’s, to also include Triton X-100, Tween-20, or Triton X-45, all typically used at 0.2–1%. The S/D technology represented a breakthrough in the viral safety of most industrial plasma products, contributing to stopping the transmission of HIV, HBV, and HCV in the late 1980’s, but also, later on, that of other emerging lipid-enveloped viruses such as WNV, DENV, or ZIKV (36). Its major benefit is the lack of alteration of most proteins, apart a possible detrimental impact of detergents like Triton X-100 on the functional activity of serine protease inhibitors and protein S in whole plasma. A limitation, however, is the requirement to implement steps, such as oil extraction and/or ion exchange chromatography, for removing the S/D agents but chromatography can serve concomitantly as a purification steps, as done for several coagulation factor concentrates (27,28,47).
Another important viral inactivation method is pasteurisation which consists in subjecting a protein solution to heat treatment at 60 °C for 10 hours, typically in the presence of stabilizers of the protein function (48). Pasteurisation in the final container, in the presence of caprylate or tryptophanate to stabilize albumin, is the “gold standard” inactivation procedure for albumin. For other pasteurized products, such as concentrates of factor VIII, antithrombin, or alpha 1-antitrypsin, heat treatment takes place during the fractionation process, and stabilizers (amino acids, sugars and/or polyols) used at high doses are removed typically by TFF. Heat-treatment can also be performed on freeze-dried products (in particular coagulation factors) and is known as “dry-heat treatment”. This form of viral inactivation, where products were subjected to 60–68 °C treatment for 24 to 96 hours, was introduced in the 1980’s mostly to inactivate HIV (Figure 2) (9). Over the years, alternative formulas were developed where higher temperatures such as 80 °C for 72 hours, or 100 °C for 30 min, were implemented to inactivate HCV and non-enveloped viruses, like HAV, in coagulation factor concentrates (49).
A low pH (typically at pH4 +/− 0.2) incubation for several hours at ca 30–35 °C has long been applied to IgGs, initially as a means to improve tolerance upon IV infusion in patients, and subsequently recognized as a robust virus inactivation treatment of lipid-enveloped viruses (50). Currently this treatment has been replaced in many situations by S/D or caprylic acid treatment along with a fractionation strategy aiming at improving productivity and IgG purity (29). Whereas low pH incubation does not improve IgG purity, a caprylic acid treatment at low pH contributes to removing non- IgG protein contaminants, therefore increasing IgG purity (51,52). It also helps in removing potentially thrombogenic factors, while being a robust inactivation tool of lipid-enveloped viruses (53-55). Low pH formulation is used for some IgG as additional virus safety step (56) and for final formulation.
Nanofiltration is a dedicated virus removal step where protein solutions are run through specially designed filters with precise nanometer-size exclusion limits in the range of 15 to 50 nm (57). These filters have the capacity to retain viruses while proteins smaller than the pore size can filter through (as an example IgG has a size estimated to be 11–13 nm). The virus exclusion mechanism is independent of the membrane structure of the virus since removal is not based on electric charges or hydrophobicity.
Technological perspectives and conclusions
It is a pragmatic position to believe that the human plasma fractionation industry will continue to be needed to supply essential plasma products, including, for some time, those, like coagulation factors VIII (58) and IX, that can also be prepared by recombinant technology (59), since they can exhibit some specific therapeutic advantages for some patients and have been found in two clinical studies to exhibit a lower propensity to induce inhibitors in previously untreated patients (60,61). In addition, human plasma and its fractionation can serve as a cost-effective discovery tool for the purification and clinical evaluation of novel protein therapies. The following candidates have been proposed, and some are now under development: factors II, V, X, XII, plasminogen, plasmin, high density lipoprotein (HDL), haptoglobin, hemopexin, alpha 1-microglobulin, ceruloplasmin, factor H, and alpha 2-macroglobulin (30,62). The plasma products have reached a high level of virus safety but it remains important to maintain extreme vigilance to maintain the safety margin and avoid the infectious risks that could be associated with any potential emerging pathogens entering the plasma pool in the future.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Blood for the series “Plasma Fractionation” published in Annals of Blood. The article has undergone external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aob.2018.05.03). The series “Plasma Fractionation” was commissioned by the editorial office without any funding or sponsorship. Thierry Burnouf served as an unpaid Guest Editor of the series and serves as an unpaid editorial board member of Annals of Blood from Feb 2017 to Feb 2020.
Ethical Statement: The author is accountable for all aspects of the manuscript and ensure that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Burnouf T. Modern plasma fractionation. Transfus Med Rev 2007;21:101-17. [Crossref] [PubMed]
- Bertolini J, Hayes T. Plasma for Fractionation. Production of Plasma Proteins for Therapeutic Use 2012:423-36.
- Strengers PF. Evidence-based clinical indications of plasma products and future prospects. Ann Blood 2017;2:20. [Crossref]
- WHO. Recommendations for the production, quality control and regulation of plasma for fractionation. Available online: http://www.who.int/bloodproducts. Geneva: World Health Organization, 2005.
- Jackson MR. Fibrin sealants in surgical practice: an overview. Am J Surg 2001;182:S1-S7. [Crossref] [PubMed]
- Radosevich M, Goubran HI, Burnouf T. Fibrin sealant: scientific rationale, production methods, properties, and current clinical use. Vox Sang 1997;72:133-43. [Crossref] [PubMed]
- Lemieux R, Bazin R, Neron S. Therapeutic intravenous immunoglobulins. Mol Immunol 2005;42:839-48. [Crossref] [PubMed]
- Franchini M, Velati C. Blood safety and zoonotic emerging pathogens: now it’s the turn of Zika virus! Blood Transfus 2016;14:93-4. [PubMed]
- Burnouf T, Radosevich M. Reducing the risk of infection from plasma products: specific preventative strategies. Blood Rev 2000;14:94-110. [Crossref] [PubMed]
- Velthove KJ, Over J, Abbink K, et al. Viral safety of human plasma–derived medicinal products: Impact of regulation requirements. Transfus Med Rev 2013;27:179-83. [Crossref] [PubMed]
- Calizzani G, Vaglio S, Candura F, et al. The evolution of the regulatory framework for the plasma and plasma-derived medicinal products system in Italy. Blood Transfus 2013;11:s6. [PubMed]
- Cheraghali AM, Abolghasemi H. Plasma fractionation, a useful means to improve national transfusion system and blood safety: Iran experience. Haemophilia 2009;15:487-93. [Crossref] [PubMed]
- Farrugia A, Evers T, Falcou PF, et al. Plasma fractionation issues. Biologicals 2009;37:88-93. [Crossref] [PubMed]
- Pai SC. Role of the quality assurance person in the production of recovered plasma for fractionation. Ann Blood 2018;3:25. [Crossref]
- WHO. Annex 4 WHO guidelines on good manufacturing practices for blood establishments. WHO Technical Report Series 2011;961:148-214.
- Weinstein M. Regulation of plasma for fractionation in the United States. Ann Blood 2018;3:3. [Crossref]
- Rossi F. The Organization of Transfusion and Fractionation in France and its Regulation. Ann Blood 2018; In Press. [Crossref]
- Burkhardt T, Rothe R, Moog R. Immunoglobulin G levels during collection of large volume plasma for fractionation. Transfus Apher Sci 2017;56:417-20. [Crossref] [PubMed]
- Laub R, Baurin S, Timmerman D, et al. Specific protein content of pools of plasma for fractionation from different sources: impact of frequency of donations. Vox Sang 2010;99:220-31. [Crossref] [PubMed]
- Eandi M, Gandini G, Povero M, et al. Plasma for fractionation in a public setting: cost analysis from the perspective of the third-party payer. Blood Transfus 2015;13:37-45. [PubMed]
- Farrugia A, Scaramuccia D. The dynamics of contract plasma fractionation. Biologicals 2017;46:159-67. [Crossref] [PubMed]
- Hotchko M, Robert P. Recent market status and trends of fractionated plasma products. Ann Blood 2018;3:19. [Crossref]
- Pool JG. Cryoprecipitate in the treatment of hemophilia. Calif Med 1970;113:66-7. [PubMed]
- Cohn E, Strong L, Hughes W, et al. Preparation and properties of serum and plasma proteins. IV. A system for the separation into fractions of the protein and lipoprotein components of biological tissues and fluids. J Am Chem Soc 1946;68:459-75. [Crossref] [PubMed]
- Kistler P, Nitschmann H. Large-scale production of human plasma fractions. Eight years experience with the alcohol fractionation procedure of Nitschmann, Kistler and Lergier. Vox Sang 1962;7:414-24. [Crossref] [PubMed]
- Farrugia A. Factor VIII manufactured from plasma—the ups and downs, and the up again: a personal journey—part 1: history of the development of plasma-derived factor VIII therapies. Ann Blood 2018;3:17. [Crossref]
- Burnouf T, Burnouf-Radosevich M, Huart J, et al. A Highly Purified Factor VIII: c Concentrate Prepared from Cryoprecipitate by Ion-Exchange Chromatography. Vox Sang 1991;60:8-15. [Crossref] [PubMed]
- Burnouf T, Michalski C, Goudemand M, et al. Properties of a Highly Punified Human Plasma Factor IX: c Therapeutic Concentrate Prepared by Conventional Chromatography. Vox Sang 1989;57:225-32. [PubMed]
- Radosevich M, Burnouf T. Intravenous immunoglobulin G: trends in production methods, quality control and quality assurance. Vox Sang 2010;98:12-28. [Crossref] [PubMed]
- Burnouf T. New approaches for manufacturing plasma derivatives. ISBT Science Series 2014;9:160-7. [Crossref]
- Burnouf T. Integration of chromatography with traditional plasma protein fractionation methods. Bioseparation 1991;1:383-96.
- Burnouf T. Chromatography in plasma fractionation: benefits and future trends. J Chromatogr B Biomed Appl 1995;664:3-15. [Crossref] [PubMed]
- CPMP. Note for guidance on plasma -derived medicinal products. CPMP/BWP/269/95 rev.4. Available online: http://www.emea.eu.int . 2009.
- Kreil TR. Building blocks of the viral safety margins of industrial plasma products. Ann Blood 2018;3:14. [Crossref]
- Kreil TR. West Nile virus: Recent experience with the model virus approach. Dev Biol (Basel) 2004;118:101-5. [PubMed]
- Dichtelmuller HO, Biesert L, Fabbrizzi F, et al. Robustness of solvent/detergent treatment of plasma derivatives: a data collection from Plasma Protein Therapeutics Association member companies. Transfusion 2009;49:1931-43. [Crossref] [PubMed]
- Farcet MR, Kreil TR. Zika virus is not thermostable: very effective virus inactivation during heat treatment (pasteurization) of human serum albumin. Transfusion 2017;57:797-801. [Crossref] [PubMed]
- Farcet MR, Lackner C, Antoine G, et al. Hepatitis E virus and the safety of plasma products: investigations into the reduction capacity of manufacturing processes. Transfusion 2016;56:383-91. [Crossref] [PubMed]
- Leydold SM, Farcet MR, Kindermann J, et al. Chikungunya virus and the safety of plasma products. Transfusion 2012;52:2122-30. [Crossref] [PubMed]
- WHO. Guidelines on viral inactivation and removal procedures intended to assure the viral safety of human blood plasma products. Available online: www.who.int/bloodproducts. Geneva: 2003.
- Remington KM, Trejo SR, Buczynski G, et al. Inactivation of West Nile virus, vaccinia virus and viral surrogates for relevant and emergent viral pathogens in plasma-derived products. Vox Sang 2004;87:10-8. [Crossref] [PubMed]
- Jeong EK, Sung HM, Kim IS. Inactivation and removal of influenza A virus H1N1 during the manufacture of plasma derivatives. Biologicals 2010;38:652-7. [Crossref] [PubMed]
- Xie YW, Chan PK, Szeto CK, et al. Clearance of dengue virus in the plasma-derived therapeutic proteins. Transfusion 2008;48:1342-7. [Crossref] [PubMed]
- Yunoki M, Urayama T, Yamamoto I, et al. Heat sensitivity of a SARS-associated coronavirus introduced into plasma products. Vox Sang 2004;87:302-3. [Crossref] [PubMed]
- Rabenau HF, Biesert L, Schmidt T, et al. SARS-coronavirus (SARS-CoV) and the safety of a solvent/detergent (S/D) treated immunoglobulin preparation. Biologicals 2005;33:95-9. [Crossref] [PubMed]
- Horowitz MS, Rooks C, Horowitz B, et al. Virus safety of solvent/detergent-treated antihaemophilic factor concentrate. Lancet 1988;2:186-9. [Crossref] [PubMed]
- Burnouf-Radosevich M, Burnouf T. A therapeutic, highly purified factor XI concentrate from human plasma. Transfusion 1992;32:861-7. [Crossref] [PubMed]
- Nowak T, Niedrig M, Bernhardt D, et al. Inactivation of HIV, HBV, HCV related viruses and other viruses in human plasma derivatives by pasteurisation. Dev Biol Stand 1993;81:169-76. [PubMed]
- Kim IS, Choi YW, Kang Y, et al. Dry-heat treatment process for enhancing viral safety of an antihemophilic factor VIII concentrate prepared from human plasma. J Microbiol Biotechnol 2008;18:997-1003. [PubMed]
- Louie RE, Galloway CJ, Dumas ML, et al. Inactivation of hepatitis C virus in low pH intravenous immunoglobulin. Biologicals 1994;22:13-9. [Crossref] [PubMed]
- Wu YW, Champagne J, Toueille M, et al. Dedicated removal of immunoglobulin (Ig)A, IgM, and Factor (F)XI/activated FXI from human plasma IgG. Transfusion 2014;54:169-78. [Crossref] [PubMed]
- Lebing W, Remington KM, Schreiner C, et al. Properties of a new intravenous immunoglobulin (IGIV-C, 10%) produced by virus inactivation with caprylate and column chromatography. Vox Sang 2003;84:193-201. [Crossref] [PubMed]
- Dichtelmuller H, Rudnick D, Kloft M. Inactivation of lipid enveloped viruses by octanoic acid treatment of immunoglobulin solution. Biologicals 2002;30:135-42. [Crossref] [PubMed]
- Korneyeva M, Hotta J, Lebing W, et al. Enveloped virus inactivation by caprylate: A robust alternative to solvent-detergent treatment in plasma derived intermediates. Biologicals 2002;30:153-62. [Crossref] [PubMed]
- El-Ekiaby M, Vargas M, Sayed M, et al. Minipool caprylic acid fractionation of plasma using disposable equipment: a practical method to enhance immunoglobulin supply in developing countries. PLoS Negl Trop Dis 2015;9:e0003501 [Crossref] [PubMed]
- Poelsler G, Berting A, Kindermann J, et al. A new liquid intravenous immunoglobulin with three dedicated virus reduction steps: virus and prion reduction capacity. Vox Sang 2008;94:184-92. [Crossref] [PubMed]
- Burnouf T, Radosevich M. Nanofiltration of plasma-derived biopharmaceutical products. Haemophilia 2003;9:24-37. [Crossref] [PubMed]
- Arcieri R, Calizzani G, Candura F, et al. The increased demand for plasma-derived factor VIII in Italy. Blood Transfus 2017;15:279-80. [PubMed]
- Burnouf T. Recombinant plasma proteins. Vox Sang 2011;100:68-83. [Crossref] [PubMed]
- Peyvandi F, Mannucci PM, Garagiola I, et al. A Randomized Trial of Factor VIII and Neutralizing Antibodies in Hemophilia A. N Engl J Med 2016;374:2054-64. [Crossref] [PubMed]
- Calvez T, Chambost H, d'Oiron R, et al. Analyses of the FranceCoag cohort support differences in immunogenicity among one plasma-derived and two recombinant factor VIII brands in boys with severe hemophilia A. Haematologica 2018;103:179-89. [Crossref] [PubMed]
- Marketing Research Bureau. Novel plasma proteins: scientific and commercialization potential in the United States. Orange, Co, USA: Marketing Research Bureau, 2013.
Cite this article as: Burnouf T. An overview of plasma fractionation. Ann Blood 2018;3:33.


