Diagnosis and management of von Willebrand disease in the United Kingdom
Introduction
Von Willebrand Disease (VWD) is the commonest inherited bleeding disorder. The condition ranges from a mild to severe bleeding tendency and has a range of recognised subtypes which are associated with qualitative or quantitative defects affecting the von Willebrand factor (VWF) protein (1). Mild cases may present with easy bruising or mucosal bleeding whereas cases with a complete deficiency of VWF exhibit moderate to severe bleeding symptoms including epistaxis, menorrhagia, haemarthroses and postoperative bleeding. The United Kingdom Haemophilia Centre Doctors’ Organisation (UKHCDO) National Haemophilia Database (NHD) collects data on the diagnosis, management and complications of various bleeding disorders present in the UK population and this information is used by the Department of Health as a resource for national service planning relating to bleeding disorders. This UKHCDO NHD data gives an estimated prevalence of clinically significant VWD in the UK of 1 in 6,144, based on the Office for National Statistics UK population in July 2015 (2) with approximately 7% of these cases requiring treatment annually (Table 1). However, wider epidemiological studies indicate that approximately 1 in 100 of the population may have reduced or borderline VWF levels (4-6) and a proportion of these individuals may have a mild bleeding tendency. However, whether this association results in a diagnosis of VWD is debated and these individuals are not accurately reflected on the UKHCDO NHD registry.
Table 1
| von Willebrand disease type | <18 years, VWF IU/dL | >18 years, VWF IU/dL | Total | Treated | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <10 | 10–29 | >30 | N/K | Subtotal | <10 | 10–29 | >30 | N/K | Subtotal | ||||
| Males | |||||||||||||
| 1 | 26 | 155 | 285 | 15 | 481 | 81 | 344 | 641 | 105 | 1,171 | 1,652 | 76 | |
| 2 | 33 | 34 | 8 | 1 | 76 | 90 | 70 | 38 | 3 | 201 | 277 | 65 | |
| 2B | 6 | 10 | 3 | 0 | 19 | 12 | 30 | 15 | 2 | 59 | 78 | 17 | |
| 2M | 19 | 5 | 5 | 0 | 29 | 26 | 27 | 10 | 3 | 66 | 95 | 12 | |
| 2N | 0 | 0 | 2 | 0 | 2 | 3 | 2 | 22 | 6 | 33 | 35 | 1 | |
| 2 Unspecified | 4 | 8 | 6 | 0 | 18 | 11 | 12 | 8 | 1 | 32 | 50 | 5 | |
| 3 | 32 | 32 | 54 | 54 | 86 | 61 | |||||||
| Unreported | 53 | 114 | 244 | 10 | 421 | 158 | 335 | 611 | 58 | 1,162 | 1,583 | 96 | |
| Subtotal males | 3,856 | 333 | |||||||||||
| Females | |||||||||||||
| 1 | 25 | 105 | 238 | 6 | 374 | 126 | 532 | 1,737 | 215 | 2,610 | 2,984 | 101 | |
| 2 | 29 | 22 | 10 | 0 | 61 | 111 | 99 | 66 | 7 | 283 | 344 | 68 | |
| 2B | 3 | 5 | 10 | 0 | 18 | 14 | 43 | 38 | 1 | 96 | 114 | 22 | |
| 2M | 7 | 20 | 8 | 1 | 36 | 48 | 51 | 20 | 7 | 126 | 162 | 28 | |
| 2N | 0 | 1 | 1 | 0 | 2 | 6 | 7 | 48 | 5 | 66 | 68 | 6 | |
| 2 Unspecified | 4 | 1 | 0 | 0 | 5 | 18 | 21 | 20 | 2 | 61 | 66 | 3 | |
| 3 | 18 | 18 | 50 | 50 | 68 | 42 | |||||||
| Unreported | 50 | 137 | 219 | 10 | 416 | 208 | 583 | 1,557 | 172 | 2,520 | 2,963 | 141 | |
| Subtotal females | 6,742 | 411 | |||||||||||
| Grand total—males and females | 10,598 | 744 | |||||||||||
*, Derived from UKHCDO annual report 2015-16 (
Management of VWD in the United Kingdom
The delivery of clinical services to those with an inherited bleeding disorder (IBD), including VWD, is coordinated by the UKHCDO, an association of medical practitioners who work within the Haemophilia Centres of England, Scotland, Northern Ireland or Wales. The direction of the UKHCDO is overseen by an Executive Committee which meets as required. In addition, an Advisory Committee consisting of Adult and Paediatric Haematologists meets with the Executive Committee three times a year. The Executive Committee interacts with the Department of Health on a regular basis to plan required service developments relating to the inherited bleeding disorders. A general meeting open to all the UKHCDO members is held yearly.
In addition to the coordination role above, specific clinical and research areas are dealt with by relevant UKHCDO working parties, including a VWD-specific working party. Working parties generally meet every four to six months and produce consensus clinical guidelines, conduct research and oversee data collection. In addition, there is a Data Management Working Party that oversees and directs the NHD to ensure data quality and governance. The UKHCDO collates details of any treatment complications or adverse reactions in order to inform future treatment strategies and product safety.
A further body called the Haemophilia Alliance, a national partnership between patients with an IBD and health care professionals involved in the delivery of their care, has now been superseded by the NHS England National Programmes of Care structure, each of which has several Clinical Reference Groups (CRGs) to provide clinical advice and leadership. CRGs consist of clinicians, commissioners, public health experts, patients and carers and advise NHS England on how specialised services should be provided. CRGs lead on the development of clinical commissioning policies, service specifications and quality standards and provide advice on innovation, horizon scanning and service reviews. A key function is to guide work to reduce variation in service standards and to ensure value. In this case, the relevant CRG covers Specialised Blood Disorders. CRGs, through their Patient and Public Voice members, also help ensure that any changes to the commissioning of specialised services involve patients and the public. The devolved nations (Scotland and Wales) have similar arrangements in place, with the Scottish Inherited Bleeding Disorder Network facilitating clinical and other improvements for individuals with inherited bleeding disorders. In Wales, the Welsh Health Specialised Services Committee has an Inherited Bleeding Disorders Advisory Group which produces the relevant IBD service specification.
Overall, the UK has a total of 59 treatment centres where specialist management of IBD is undertaken, organised in a two-tier structure. There are currently 24 Comprehensive Care Haemophilia Centres (CCCs) (3). These centres specialise in the bleeding disorder management of adults, children, or all ages. The remaining 35 centres are designated as Haemophilia Centres (HCs).
CCCs provide multidisciplinary care and co-ordinate delivery of services, providing 24-hour advisory and response service for patients and health care professionals involved in the management of patients with an IBD. Adult and/or paediatric specialist haematologists and nursing staff provide physiotherapy, surgical management for general and specialist procedures including orthopaedics, management of dental work and specialist obstetrics and gynaecological services. Social work support for patients and their families is provided along with access to clinical genetic counselling services. CCCs also offer a 24-hour laboratory service for clotting factor assays and inhibitor screening.
The extent of the service provided within the remaining 35 individual Haemophilia Centres varies depending on available expertise but they offer, as a minimum, a core set of services including 24-hour emergency treatment, provision of adequate supplies of factor concentrates for hospital and home treatment programmes and provision of appropriate clinical advice to patients and their families. Patients with an IBD are encouraged to register with their local haemophilia care centre to ensure appropriate management in case of emergency presentation. Patients may be managed by a combination of services provided between a CCC and Haemophilia Centre within a local or regional multidisciplinary haemophilia network. The relevant CCC or haemophilia network establishes and maintains a comprehensive care programme tailored to each patient based on their needs. Patients are requested to attend for regular review, at least once per year based on the nature and severity of their IBD. An individualised comprehensive care programme details the recommended treatment protocols to be applied under certain clinical situations, in relation to the nature and severity of the patient’s IBD.
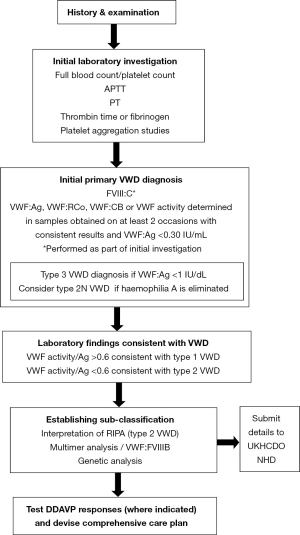
The UKHCDO has developed a guideline, the objective of which is to provide healthcare professionals with appropriate guidance for the diagnosis and management of VWD. Originally published as two separate documents in 2004, the most recent version was issued in 2014 (7). This provides the basis for best practice in the UK and was produced by a panel of UK based medical experts, revised by consensus with the UKHCDO advisory group committee and its content and applicability to the UK clinical setting was then commented on by a sounding board of approximately 50 UK haematologists and the British Committee for Standards in Haematology executive. The major changes to the guideline relate to increased understanding of the genetics of the condition, a relaxation of definition and more focus on how laboratory tests can guide management. The guideline is targeted at all clinical staff, both specialised and generalist haematologists that provide care for patients with VWD. The guideline covers management of the condition with advice on the selection of appropriate pharmacological agents to be used, including DDAVP, tranexamic acid, factor concentrates and the combined oral contraceptive pill. Treatment for specific VWD-related problems and management in pregnancy, surgery and other invasive procedures are given detailed consideration along with guidance on specific management options for the main VWD subtypes. Elements of the document are based on consensus amongst experts where evidence is limited. Figure 1 illustrates a schema for phenotypic and possible genetic laboratory investigation of patients. Table 2 lists the current VWF activity assays and their abbreviations.
Table 2
| Abbreviation of VWF activity | Description |
|---|---|
| VWF:RCo | Ristocetin cofactor activity: all assays that use platelets and ristocetin |
| VWF:GPIbR | All assays that are based on the ristocetin induced binding of VWF to a recombinant WT GP1b fragment |
| VWF:GPIbM | All assays that are based on the spontaneous binding of VWF to a gain-of-function mutant GP1b fragment |
| VWF:CB | All assays that are based on binding to collagen (types I/III) |
| VWF:Ab | All assays that are based on the binding of a monoclonal antibody (mAb) to a VWF A1 domain epitope |
| VWF:RIPA | All assays that are based on ristocetin-induced platelet aggregation |
| VWF:FVIIIB | An assay that measures the level of FVIII that can bind to VWF |
The UK IBD management network
The UKHCDO acts as a focus for the key umbrella groups involved in inherited bleeding disorder care in the UK and represent both healthcare professionals and patients. Specialist nursing forms a key part of the multidisciplinary haemophilia network care programme as nursing staff are normally the initial point of contact for patients interacting with the IBD care system. There is a Haemophilia Nurses Association that holds an annual conference to discuss issues relevant to nursing staff involved in the care of patients with an IBD. The Royal College of Nursing also host a Haematology and Intravenous Therapy Forum for all members interested in blood-related specialist interests.
Physiotherapists involved in the care of IBD patients have a Haemophilia Chartered Physiotherapists Association (HCPA) under the auspices of the Chartered Society of Physiotherapy. They hold an annual study day and general meeting.
As well as the above healthcare professional or coalition groups, the UK has an independent patient-led advocacy group, the Haemophilia Society, which represents people affected by bleeding disorders (8).
The Society provides information and support for people affected by a bleeding disorder and is organised into local groups to form a support network for patients and their families.
Prevalence of VWD in the United Kingdom
The exact prevalence of VWD in the UK remains equivocal given the clinical spectrum of the disorder. However, the UKHCDO administered NHD collates the total number of VWD patients identified within the haemophilia network. The UK had a population of 65,110,000 in 2015 (2) and the number of patients diagnosed with VWD registered on the UKHCDO NHD stands at 10,598 (Table 1). Whilst this gives an overall prevalence of 1 in 6144 representing primarily moderate to severe bleeding tendencies, it will not include the majority of milder type 1 VWD cases. Using a general population estimate of 125 per million individuals exhibiting a VWD related significant bleeding disorder (9), this would indicate that the NHD VWD register should contain approximately 8,125 cases in the current UK population, rather than the 10,598 cases registered on the NHD. The higher prevalence is likely to reflect the inclusion of some milder type 1 cases on the NHD along with a proportion of deceased patients that remain on the register. Although the NHD contains information on the subtype of VWD, this is incomplete, with 6079 individuals assigned to a VWD subtype, including 116 cases with a generic designation of “type 2 VWD,” and the remaining 4519 cases not categorised (Table 1). A generic prevalence of VWD provided by OrphaNet for symptomatic VWD requiring treatment is estimated to be between 1 in 8,500 to 1 in 50,000 (10). The UKHCDO annual report for 2015-16 listed 399 newly registered VWD patients; of these, 36% were male. 55 females and 57 males under 18 years were diagnosed in addition to 145 females and 84 males over 18 years of age (Table 3). There is currently no reliable estimate of the incidence of platelet-type VWD (PT-VWD) in the UK.
Table 3
| von Willebrand disease type | <18 years, VWF IU/dL | >18 years, VWF IU/dL | Total | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <10 | 10–29 | >30 | N/K | Subtotal | <10 | 10–29 | >30 | N/K | Subtotal | |||
| Males | ||||||||||||
| 1 | 3 | 9 | 12 | 0 | 24 | 0 | 7 | 9 | 0 | 16 | 40 | |
| 2A | 1 | 4 | 0 | 0 | 5 | 0 | 3 | 2 | 0 | 5 | 10 | |
| 2B | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 2 | 2 | |
| 2M | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 2 | |
| 2N | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 3 | 3 | |
| 2 Unspecified | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 3 | 0 | 0 | 1 | 1 | 1 | |||||||
| Unreported | 8 | 19 | 30 | 0 | 57 | 3 | 13 | 8 | 3 | 27 | 84 | |
| Subtotal males | 142 | |||||||||||
| Females | ||||||||||||
| 1 | 1 | 10 | 10 | 0 | 21 | 0 | 14 | 54 | 1 | 69 | 90 | |
| 2A | 2 | 5 | 0 | 0 | 7 | 2 | 1 | 0 | 0 | 3 | 10 | |
| 2B | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 2 | 2 | |
| 2M | 0 | 2 | 1 | 0 | 3 | 2 | 1 | 0 | 0 | 3 | 6 | |
| 2N | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 2 | 2 | |
| 2 Unspecified | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 3 | 1 | 1 | 1 | 1 | 2 | |||||||
| Unreported | 5 | 24 | 25 | 1 | 55 | 4 | 16 | 67 | 3 | 90 | 145 | |
| Subtotal females | 257 | |||||||||||
| Grand total—males and females | 399 | |||||||||||
*, Derived from UKHCDO annual report 2015-16 (
Diagnostic strategy
Guidance relating to the estimation of baseline VWF level and diagnostic approach is given in the UKHCDO guideline (7). The guideline treats VWD as a bleeding disorder predominantly attributable to reduced levels of VWF activity that is frequently, but not always, associated with a VWF gene defect. The emphasis remains on practical management aspects rather than focusing on VWD subtype molecular classification.
The clinical and laboratory diagnostic process is based on phenotypic evaluation of bleeding and bleeding tendency. This covers examination, history (personal and family), preliminary investigations for a suspected bleeding disorder and the testing strategy to establish a primary diagnosis of VWD. A diagnosis of VWD can be made if VWF activity is less than 0.30 IU/mL in a patient with mucocutaneous bleeding whereas patients with a VWF activity in the range of 0.30–0.5 IU/mL are now regarded as exhibiting primary haemostatic bleeding with “Low VWF” as a risk factor rather than VWD (7). Further information on VWD classification and diagnosis is provided in the GeneReview on VWD (11).
Clinical presentation
Patients suspected of having VWD are identified primarily through their pattern and nature of bleeding. To distinguish between an acquired or inherited disorder, a life-long history of symptoms is expected. For milder cases, evaluation may be based on their response during haemostatic challenges. If this evaluation process is suggestive of VWD, the laboratory analysis algorithm indicated in Figure 1 is followed. The International Society for Thrombosis and Haemostasis bleeding assessment tool (ISTH-BAT) (12) is in use in a number of centres, but is not yet in routine diagnostic use across the UK.
Phenotypic analysis
The initial coagulation screening profile is designed to confirm the presence of a suspected bleeding disorder. This includes a full blood count in order to establish the platelet count and any evidence of anaemia, prothrombin time (PT), activated partial thromboplastin time (APTT), thrombin time or fibrinogen (Figure 1). The use of blood-group specific reference ranges is not recommended (7). Although not part of a VWD-specific screen, a measure of primary haemostasis by use of the Platelet Function Analyser-100® or 200® (Siemens Diagnostics Camberley, UK) can be made, although this has poor sensitivity in milder bleeding disorders.
To establish a primary diagnosis of VWD, VWF:Ag, VWF activity measured as VWF:RCo, VWF:GPIbR or VWF:GPIbM and FVIII:C levels are assayed. It is recommended that VWF collagen binding (VWF:CB) is used to supplement these VWF activity assays in order to improve detection and discrimination of type 2 VWD variants. VWF:Ag and function must be measured in samples obtained on at least two occasions, with consistent results, in order to minimize the risk of misdiagnosis. The ristocetin induced platelet aggregation (RIPA) assay is essential in the diagnosis of type 2B VWD and PT-VWD. If a probable diagnosis of VWD is made following the above tests, sub-typing can be achieved by VWF multimer analysis or, where type 2N VWD is suspected, VWF-FVIII binding studies (VWF:FVIIIB). VWF multimer analysis is not undertaken as part of the primary VWD diagnosis. Given the limited availability of multimer analysis, an alternative approach to distinguish between types 2A and 2M using ratios of VWF: activity and VWF:CB to VWF: Ag is permitted (7).
Genetic testing
Genetic diagnosis within the UK is in line with current UKHCDO Haemophilia Genetics Laboratory Network guidelines (13) and genetic analysis should be used where beneficial to clarify diagnosis and aid management (7). Genetic testing has particular utility in type 3 VWD where it can be used to inform family studies in situations where prenatal diagnosis is being considered, although this is only undertaken in rare cases. In type 2 VWD, genetic testing is used in the differential diagnosis of type 2N VWD from haemophilia A. Some laboratories may perform VWF:FVIIIB studies but this assay is not in common use and genetic analysis is required to identify carriers of this recessive condition. Further genetic analysis of type 2 variants may be used to distinguish type 2B and PT-VWD, to distinguish between types 2A, 2B and 2M when specialist phenotypic tests are not available and to confirm a diagnosis of type 2M VWD. Genetic analysis of type 1 VWD is rarely undertaken in the routine diagnostic setting although it may be applied to more severe cases that are likely to have a highly penetrant mutation (14), or occasional compound heterozygous patients. Testing may include next-generation sequencing of a panel of haemostatic genes, Sanger sequencing of a single gene, or dosage analysis to detect large deletions/duplications of an exon or more (13-15).
Treatment strategy
When a diagnosis of VWD has been made, the choice of therapeutic product used in the UK is influenced by VWD subtype, baseline FVIII and VWF levels and the measured response to DDAVP, the nature of the bleeding episode or surgical intervention requiring treatment, the presence or absence of an inhibitor and any potential risks associated with treatment. The UKHCDO guideline (7) provides direction on therapeutic agents utilised within the UK. The treatment of choice, except in type 3 VWD or where it is contraindicated or ineffective, remains 1-deamino-8-D-arginine vasopressin (desmopressin or DDAVP). Patients who may respond to DDAVP undergo a trial infusion with pre, post and fall off sampling in order to establish their response. Where demonstrated to be effective, DDAVP should be used to avoid exposure to blood products. The current UKHCDO guideline has recommendations for performing a DDAVP trial with intravenous, subcutaneous or intranasal formulations and doses, along with times that post-infusion peak levels are achieved (7). DDAVP is particularly useful in type 1 VWD and is also used in types 2A and 2M VWD, although the clinical effect can be variable. Individual responses to DDAVP tend to be consistent and the patient can then be categorised as responsive or not. In patients that do not respond to DDAVP, alternative treatments consist of FVIII/VWF concentrate, tranexamic acid and, in females, oral contraceptives. These alternative treatment options are selected depending on the bleeding in question, and the relative efficacy of the treatment.
Once the VWD subtype and response to DDAVP have been established, a comprehensive care plan for each patient can be created. This plan details appropriate products and dosage to use in response to the severity of bleeding episodes or to cover surgical intervention. Individual patient details and diagnosis are submitted to the NHD and relevant information regarding type and quantity of haemostatic product used (including dose and lot number of each product), adverse reactions and complications is collated on an ongoing basis.
Treatment options
The breakdown of products used and the amount administered in the treatment of VWD in the UK, from April 2015 to March 2016 inclusive, is given in Table 4. Currently (2015-16), Haemate P® (CSL Behring Haywards Heath, UK), (7,625,000 IU), Voncento (CSL Behring Haywards Heath, UK), (5,353,000 IU) and Wilate® (OctaPharma Manchester, UK), (5,710,000 IU) are the most frequently used plasma-derived replacement products (3).
Table 4
| Manufacturer | Product | Total units |
|---|---|---|
| Baxalta | Advate | 14,500 |
| BPL | FVIII 8Y | 40,000 |
| CSL Behring | Haemate P | 7,625,150 |
| Voncento | 5,353,350 | |
| Grifols | Alphanate | 780,000 |
| Fanhdi | 4,000 | |
| LFB Biomedicaments | Wilfact/Wilfactin | 555,000 |
| Novo Nordisk | NovoSeven (mg) | 1,956 |
| Octapharma | Octaplex | 500 |
| Wilate | 5,710,000 | |
| Pfizer | ReFacto AF | 29,000 |
| – | Investigational rVWF | * |
*, Anonymised for confidentiality purposes. Units in IU unless otherwise stated. Products containing VWF and FVIII are reported in FVIII units. Derived from UKHCDO annual report 2015-16 (
DDAVP
DDAVP products are registered for both intravenous and intranasal use in the UK and some centres also use subcutaneous infusion. DDAVP is used in the management of types 1, 2A and 2M VWD. It is also used in the treatment of type 2N VWD, but the short half-life of unstabilised FVIII released in to the circulation may limit the haemostatic response. It is no longer considered appropriate to perform a DDAVP trial in type 2B VWD patients due to a combination of a likely (transient) thrombocytopenia and the usually poor therapeutic response. DDAVP is not generally recommended in children under 2 years of age and if, after careful consideration, it is to be used in this group then close monitoring of fluid balance is required (7).
Factor concentrates
Where DDAVP is ineffective or contraindicated, a concentrate containing VWF is the recommended alternative treatment. A range of licensed products are used in the treatment of VWD in the UK and these are listed, along with individual product usage statistics derived from the UKHCDO NHD, in Table 4. The choice of product used is influenced by the VWF:Ag/VWF activity ratio, multimeric structure and the ratio of FVIII to VWF (16). The dosage to be administered for each individual product is based on VWF activity relative to the FVIII:C, calculated in international units (IU) and guidance on this is given with the relevant product data sheet. For commonly used products in the UK, the assayed amount of VWF activity is printed on each vial within a batch. In selecting a treatment product, each of the VWF activity/VWF:Ag ratio, multimer structure and the FVIII/VWF ratio may influence product choice. For treatment of acute bleeding or emergency surgery, a VWF-FVIII concentrate or a combination of high purity FVIII and high purity VWF concentrates should be used (7). UK patients undergoing treatment with concentrates are routinely vaccinated against hepatitis A and B.
Tranexamic acid
In addition to the above agents listed in Table 4, UK clinicians use the antifibrinolytic agent tranexamic acid in the management of VWD. It can be administered orally, either in tablet form or by mouthwash and it is effective in the treatment of oral cavity bleeding. It can also be administered intravenously. It is used alone in the management of epistaxis and menorrhagia or in combination with DDAVP or an appropriate factor concentrate to cover dental extractions and surgery. It is not contra-indicated during pregnancy. For elective procedures, tranexamic acid is administered before treatment commences. General advice on its use in the UK, either in isolation or in combination with other therapeutic products, is given in the UKHCDO guideline (7).
Treatment of VWD associated menorrhagia
Menorrhagia is common in women with VWD. Haemostatic, hormonal and surgical therapies have each been used to help control heavy menstrual bleeding in women with VWD. In the UK, a range of treatment options are available. Orally administered treatments in the form of the combined contraceptive pill or tranexamic acid are considered first and if a poor response is seen, then DDAVP is indicated. This can be self-administered either intranasally or subcutaneously with treatment limited to once per day for 2–3 days on commencement of menstruation. A further treatment option in the form of the progestogen-only intrauterine contraceptive device (Mirena®, Schering Plough Welwyn Garden City, UK) is available. Further information is available through the National Institute for Health and Care Excellence Menorrhagia Guideline (2016) (17). Women who still exhibit menorrhagia despite the above treatments are referred to, and managed in conjunction with, a gynaecologist.
Treatment of VWD during pregnancy and delivery
Guidance on the management of pregnancy and delivery of potentially affected neonates in VWD is detailed in the current UKHCDO guideline (7). The management of VWD during pregnancy is influenced by the subtype. DDAVP, intermediate or high purity VWF concentrates or tranexamic acid can be used during pregnancy, delivery and the puerperium. In the UK, all pregnant women with types 2 and 3 VWD, and those with type 1 VWD whose VWF activity is not likely to normalise, should undergo delivery in an obstetric unit that has ready access to comprehensive neonatal care facilities and close liaison with a CCC or HC. A delivery plan is drawn up jointly between the managing obstetrician and their associated CCC or HC and agreed with the patient. Before conception or during pregnancy, women should be given an opportunity to discuss the inheritance of VWD with a genetic counsellor, and with a paediatric haematologist regarding assessment of the child following delivery.
In many women with type 1 VWD, levels of VWF increase into the normal range as pregnancy progresses. This increase in VWF level may be more variable for type 2 cases, with an increased risk of thrombocytopenia and corresponding bleeding tendency in type 2B VWD cases. Neither VWF or FVIII levels increase in type 3 VWD cases and these require cover with a suitable FVIII/VWF concentrate formulation.
In women with type 1 or type 2 VWD, VWF levels are checked at booking and at 34–36 weeks gestation. In type 1 cases, vaginal delivery or Caesarean section can proceed if VWF: activity is >0.5 IU/mL. For other relevant subtypes, vaginal delivery or Caesarean section can still proceed if VWF activity is >0.5 IU/mL with a platelet count maintained at >50×109/L. The use of DDAVP is permissible in women during uncomplicated pregnancy and for those requiring cover during childbirth (18). Its use in women with pre-eclampsia is not recommended, nor is repeated administration due to possible hyponatremia in the fetus. Epidural (neuraxial) anaesthesia is not recommended in types 2 and 3 VWD, nor in type 1 VWD where VWF levels have failed to normalise by late pregnancy.
Invasive procedures such as ventouse extraction, rotation forceps and fetal scalp monitoring are avoided in neonates at risk of having significantly reduced VWF activity (7). In families with type 3 VWD, where there is a risk that the fetus may be affected, genetic testing may be offered following appropriate genetic counselling (7). This should be planned in advance in order to characterise the mutation(s) in the family.
Management of surgery including dentistry
Management of surgical procedures in VWD patients is considered straightforward for mild forms of VWD but more severe forms require appropriate liaison between the surgical team, anaesthetist and a specialist haematologist, drawing on the laboratory monitoring facilities provided by the on-site CCC or Haemophilia Centre. Management strategy is determined by the VWD type, the baseline levels of both VWF and FVIII, the patient’s known response to DDAVP, where indicated, and the nature of the invasive procedure to be undertaken (19,20). For major surgery the VWF activity and FVIII should undergo pre-operative correction to greater than or equal to 1.0 IU/mL with either DDAVP or concentrate as required and then sustained above 0.5 IU/mL in the post-operative period. More information on factors to consider relating to major or minor procedures for different subtypes of the disorder are given in the 2014 UKHCDO guideline (7).
Management of inhibitors
Patients with type 3 VWD may develop an inhibitor and fail to respond adequately to replacement factor treatment, 5–10% of patients may develop this complication. Symptoms can include lack/loss of response to infused VWF concentrates or an anaphylactic reaction; this is however, rare. Multi-transfused patients and those with large deletions of an exon or more, nonsense mutations or frameshifts are at the highest risk of these complications. A previous family history of inhibitory antibodies is a further risk factor. The UKHCDO annual report records that there were 6/154 (3.9%) of patients in the register with VWF:Ag ≤1 IU/dL exhibiting a current inhibitor between April 2015–March 2016 (6). Recombinant FVIII, bypassing agents and immune tolerance can provide effective treatment, but there is no clear consensus on management of this small group. The UKHCDO guideline states that in order to confirm a suspected inhibitor in type 3 VWD, measurement of in vivo recovery and survival of VWF should be considered. In cases that do not respond to VWF concentrate or when anaphylaxis occurs, high dose recombinant FVIII infusion, recombinant FVIIa, platelet transfusion and tranexamic acid should be considered (7).
Prophylactic therapies
Prophylaxis is used relatively rarely but it should be considered in the UK for recurrent bleeding in all types of VWD. Prophylaxis has specific application in type 3 VWD patients with haemarthroses, severe epistaxis, pronounced menorrhagia cases, and in VWD with a concomitant risk factor for bleeding. Both intermediate purity FVIII-VWF or high purity VWF concentrates are considered appropriate for prophylaxis. In children with type 3 VWD, prophylaxis is considered when joint bleeding develops (7).
Conclusions
The UK has a well-developed infrastructure for the diagnosis and management of VWD. This is based on a network of treatment centres with specialist expertise relating to bleeding disorders. There is a specific framework for the diagnosis, management and treatment of VWD laid out in relevant expert guidelines and service specification documents that undergo a review/update process. This guidance informs comprehensive management plans tailored to the needs of each patient. Appropriate treatment options are available for all VWD patients and there is a national reporting system which collates product usage and adverse reactions for individual patients, which in turn informs future service planning.
Acknowledgments
Special thanks to the UKHCDO who provided relevant data from the National Haemophilia Database.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Emmanuel J. Favaloro) for the series “Diagnosis and Management of von Willebrand Disease: Diverse Approaches to a Global and Common Bleeding Disorder” published in Annals of Blood. The article has undergone external peer review.
Conflicts of Interest: The series “Diagnosis and Management of von Willebrand Disease: Diverse Approaches to a Global and Common Bleeding Disorder” was commissioned by the editorial office without any funding or sponsorship. Anne Goodeve receives funding from CSL-Behring to support the von Willebrand mutation database (https://grenada.lumc.nl/LOVD2/VWF/home.php?select_db=VWF). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the manuscript and ensure that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sadler JE, Budde U, Eikenboom JC, et al. Update on the pathophysiology and classification of von Willebrand disease: a report of the Subcommittee on von Willebrand Factor. J Thromb Haemost 2006;4:2103-14. [Crossref] [PubMed]
- Office for National Statistics UK population data for 2015-16 Available online: http://www.statistics.gov.uk/cci/nugget.asp?id=6
- UKHCDO annual report 2015-16. Available online: http://www.ukhcdo.org/wp-content/uploads/2017/03/Bleeding-Disorder-Statistics-for-April-2015-to-March-2016-for-UKHCDO-Website.pdf
- Rodeghiero F, Castaman G, Dini E. Epidemiological investigation of the prevalence of von Willebrand's disease. Blood 1987;69:454-459. [PubMed]
- Werner EJ, Broxson EH, Tucker EL, et al. Prevalence of von Willebrand disease in children: a multiethnic study. J Pediatr 1993;123:893-8. [Crossref] [PubMed]
- Sanders YV, Fijnvandraat K, Boender J, et al. Bleeding spectrum in children with moderate or severe von Willebrand disease: Relevance of pediatric-specific bleeding. Am J Hematol 2015;90:1142-8. [Crossref] [PubMed]
- Laffan MA, Lester W, O'Donnell JS, et al. The diagnosis and management of von Willebrand disease: a United Kingdom Haemophilia Centre Doctors Organization guideline approved by the British Committee for Standards in Haematology. Br J Haematol 2014;167:453-65. [Crossref] [PubMed]
-
UK Haemophilia Society - Sadler JE, Gralnick HR. Commentary: a new classification for von Willebrand disease. Blood 1994;84:676-9. [PubMed]
- Prevalence of symptomatic VWD Available online: www.orpha.net/consor/cgi-bin/OC_Exp.php?Expert=903
- Goodeve, AC, James P. GeneReviews, von Willebrand disease (2017). Available online: https://www.ncbi.nlm.nih.gov/books/NBK7014/
- International Society on Thrombosis and Haemostasis Bleeding Assessment Tool, Available online: https://c.ymcdn.com/sites/www.isth.org/resource/resmgr/ssc/isth-ssc_bleeding_assessment.pdf. Accessed 31 Oct 2017.
- Keeney S, Bowen D, Cumming A, et al. UK Haemophilia Centre Doctors' Organisation (UKHCDO). The molecular analysis of von Willebrand disease: a guideline from the UK Haemophilia Centre Doctors' Organisation Haemophilia Genetics Laboratory Network. Haemophilia 2008;14:1099-111. [Crossref] [PubMed]
- Peake I, Goodeve A. Type 1 von Willebrand disease. J Thromb Haemost 2007;5:7-11. [Crossref] [PubMed]
- Swystun LL, James PD. Genetic diagnosis in hemophilia and von Willebrand disease. Blood Rev 2017;31:47-56. [Crossref] [PubMed]
- Keeling D, Tait C, Makris M. Guideline on the selection and use of therapeutic products to treat haemophilia and other hereditary bleeding disorders. A United Kingdom Haemophilia Center Doctors' Organisation (UKHCDO) guideline approved by the British Committee for Standards in Haematology. Haemophilia 2008;14:671-84. [Crossref] [PubMed]
- Heavy menstrual bleeding: assessment and management. National Institute for Health and Care Excellence (2016), Available online: https://www.nice.org.uk/guidance/cg44
- Castaman G, Tosetto A, Rodeghiero F. Pregnancy and delivery in women with von Willebrand's disease and different von Willebrand factor mutations. Haematologica 2010;95:963-9. [Crossref] [PubMed]
- James AH, Eikenboom J, Federici AB. State of the art: von Willebrand disease. Haemophilia 2016;22:54-9. [Crossref] [PubMed]
- Heijdra JM, Cnossen MH, Leebeek FWG. Current and emerging options for the management of inherited von Willebrand disease. Drugs 2017;77:1531-47. [Crossref] [PubMed]
Cite this article as: Keeney S, Goodeve A. Diagnosis and management of von Willebrand disease in the United Kingdom. Ann Blood 2018;3:29.