
Fluorescence-guided laparoscopic hepatectomy
Achieving negative tumor margins is critical in oncologic surgeries. This is particularly important in performing complex oncologic resections such as hepatectomy for primary and secondary hepatic malignancies. The achievement of negative resection margins has a direct impact on survival, and complete clearance of the tumor bed is the goal in laparoscopic liver resection for metastases (1). Advances in laparoscopic and robotic platforms have enabled minimally invasive approaches to liver surgeries. While determining tumor margins is difficult in an open setting, it becomes even more challenging in a laparoscopic environment, as traditional methods of direct visual inspection and palpation of the liver are limited.
Tactile feedback from tissue examination with laparoscopic instruments is extremely limited compared to manual manipulation. Laparoscopic liver surgeons are thus faced with relying on discerning subtle tissue irregularities within major anatomic landmarks, using non-specific visual cues. Pre-operative cross sectional imaging, intra-operative ultrasound (IOUS), and intra-operative frozen sections can assist in localizing the lesion, but cannot assist in achieving negative resection margins. Additionally, cross sectional imaging is difficult to translate directly into the operative field and changes with patient positioning. IOUS is limited by a steep learning curve, user variability, a two dimensional image, and a superficial blind area approximately 1 cm under the hepatic capsule (2). Laparoscopic IOUS is challenged with issues in ergonomics of access since trocar site placement limits range of motion.
Enhancement of tumor visualization via fluorescence assists in overcoming these limitations. Indocyanine green (ICG) is a hepatically-metabolized dye traditionally used to estimate cardiac output and hepatic volume (3). The dye binds to plasma proteins and has a peak absorbance at 780 nm and emits fluorescence with a wavelength of approximately 800 nm (4). ICG is preferentially retained in or around biliary malignancies due to impaired biliary excretion of hepatocytes in the affected area (5). Fluorescence-guided surgery (FGS) using ICG was first applied to hepatobiliary surgery by Ishizawa et al. in order to outline biliary structures, but has since been applied to hepatic malignancies (6). Gotoh et al. first described using ICG intra-operatively for hepatocellular carcinoma (HCC) in a series of 10 patients undergoing open hepatectomy. All ten resected lesions had strong fluorescence signals and the use of ICG detected additional lesions in four cases (40%) that were not previously detected by pre-operative imaging or IOUS (7). Ishizawa et al. further described different patterns of ICG fluorescence in HCC and colorectal liver metastases in their series (8). Well-differentiated HCC lesions showed complete fluorescence and moderately differentiated lesions showed partial fluorescence. Poorly-differentiated lesions and colorectal metastases showed no fluorescence, but the surrounding liver tissue fluoresced in a rim-like pattern due to increased cellular density from tumor compression. Using ICG, they were able to detect an additional 8 out of the 63 (13%) HCCs that were not evident on gross inspection.
Laparoscopic surgery is well suited for fluorescence guidance as the operator interacts with the screen image in real-time. Presence or absence of the fluorescence signal gives the surgeon immediate feedback on adequacy of tumor margins. Initial experience with laparoscopic fluorescence-guided hepatectomy was similar to open hepatectomy with ICG. Kawaguchi et al. report the value of ICG fluorescence navigation in laparoscopic liver resections, especially for patients who have had scarring from previous chemotherapy and radiofrequency ablation (9). IOUS was able to identify the lesion to be resected, but was not able to assist in determining areas of recurrent tumor. However the use of ICG imaging displayed a fluorescing border which corresponded to areas of viable cancer cells on histology. In one patient, the use of ICG fluorescence allowed detection of a lesion not detected by pre-operative imaging or IOUS. Kudo et al. report on 17 patients undergoing laparoscopic hepatectomy for HCC, colorectal liver metastases, and uterine cancer (10). Of the 32 lesions resected, 6 were detected by visual inspection with standard laparoscopy while 23 were identified using ICG fluorescence. Similar to the open experience, laparoscopic ICG fluorescence was limited in detection of deeper lesions, >8 mm in their series. They also noted that ICG fluorescence was an especially valuable adjunct to IOUS to assist in setting dissection boundaries prior to parenchymal transection. The technology is a valuable tool to assure negative resection margins in laparoscopic hepatic resections.
While tumor fluorescence patterns for HCC, metastatic colorectal adenocarcinoma, and cholangiocarcinoma are well described, other hepatic malignancies are not (11,12). Boogerd et al. in their series of laparoscopic fluorescence-guided hepatectomies, describe additional hepatic malignancies visualized with ICG such as metastatic uveal melanoma and breast cancer (13).
Although there are only 22 patients included in their series, due to the limited availability of surgeons performing laparoscopic hepatectomies using ICG fluorescence guidance during their procedures, Boogerd’s case series is the largest currently describing the experience. Previous studies report on fewer patients and Boogerd’s study broadens limited literature available in this emerging field (9,10,14).
Boogerd et al. additionally compared the sensitivity of pre-operative imaging modalities with ICG-FGS and noted that of the 26 resected malignancies, 20% were missed by CT, 16% by MRI, 38% by inspection, 12% by laparoscopic IOUS, and 8% by ICG fluorescence imaging. Lesions missed by laparoscopic IOUS tended to be more superficial whereas the lesions missed by ICG fluorescence imaging tended to be deeper (>8 mm). When combining the two modalities of laparoscopic IOUS and ICG fluorescence imaging, the sensitivity for tumor detection reached 100%. Other studies have examined the rate of hepatic tumor detection using ICG in an open laparotomy (2). Peloso et al. compared pre-operative CT with IOUS and with ICG fluorescence imaging in patients undergoing open hepatectomy and reported that the combined use of IOUS and ICG fluorescence imaging was useful in identifying lesions, particularly <3 mm. Boogerd’s study is the first to apply these questions of comparative efficacy of modalities used to identify hepatic lesions to the laparoscopic ICG-FGS setting. Their work thoroughly compares ICG fluorescence imaging with other pre-operative and intra-operative imaging modalities in a wide variety of primary and secondary lesions in the liver.
Although ICG has a high sensitivity in contrast enhancement and improved visualization, a major limitation of the dye is that it lacks specificity. Its non-specific plasma protein binding is a drawback. In Boogerd’s study, a positive-predictive value for ICG-FGS was 75% and 70.3% when combined with laparoscopic IUOS. Although the data are not stratified by presence or absence of cirrhosis, 14 of their patients had cirrhosis. In cirrhotic patients, regenerative hepatic nodules are known to take up ICG and fluoresce as discrete nodules, leading to false positives. A positive predictive value of ICG for malignancy in one series was 5.4% in cirrhotic livers vs. 100% in healthy livers (15).
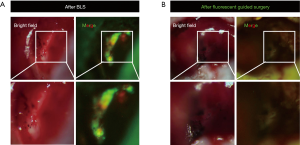
Tumor-targeted fluorescent dyes are being developed to overcome limitations of non-specific ICG binding. Our lab has shown that the use of tumor-specific fluorescent organ labeling in laparoscopy aids in rapid and accurate identification of tumors, especially at the sub-millimeter resolution (16). We have also studied FGS with tumor-specific fluorescence in a colon-cancer liver-metastasis model (17,18). Fluorescence was delivered via an anti-CEA antibody or tumor-specific adenovirus vectors expressing GFP. Both modalities labeled the tumor well and highlighted microscopically-positive margins (Figure 1). Mice undergoing FGS had a significantly lower volume of residual tumor and improved overall and disease-free survival (Figure 2) (17,18). Other tumor specific-fluorescence labeling modalities are being developed such as pH-sensitive probes, activatable cell-penetrating peptides and fluorescent nanoparticles (19-22).

Clinical applications of tumor-specific fluorescent dyes under trials currently include fluorescent folate to label ovarian cancer and fluorescent cetuximab to label head and neck cancers, but data on survival advantage and rate of tumor recurrence are not yet available (23,24). The number of fluorescence labeling platforms will greatly increase in the future along with further data on their impact of oncologic outcomes.
Minimally-invasive hepatectomy is a promising area where FGS can potentially impact oncologic outcomes. Combining ICG and laparoscopic IOUS enhances surgical detection of lesions, superficial and deep, but are limited by a lack of specificity in determining malignancy. Newly-developing tumor-specific fluorophores will play a major role in addressing this issue. Enhanced contrast helps clearly delineate tumor margins and identify lesions that would otherwise be missed, increasing rates of complete resection.
The goal of FGS, in both laparoscopic and open surgery, is to increase rates of curative cancer surgery. FGS with tumor specific-probes, effected with fluorescent antibodies or viral labeling with a genetic reporter has increased the survival in orthotopic mouse models. A second step after FGS, such as an intra-operative UV irradiation of the resection bed could be used to kill any residual cancer cells (25). Such combined strategies hold great promise for curative cancer surgery.
Acknowledgments
Funding: TM Lwin was funded by the NIH/NCI Award T32CA121938.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Laparoscopic and Endoscopic Surgery. The article did not undergo external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2016.09.04). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Pawlik TM, Scoggins CR, Zorzi D, et al. Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann Surg 2005;241:715-22, discussion 722-4. [Crossref] [PubMed]
- Peloso A, Franchi E, Canepa MC, et al. Combined use of intraoperative ultrasound and indocyanine green fluorescence imaging to detect liver metastases from colorectal cancer. HPB (Oxford) 2013;15:928-34. [Crossref] [PubMed]
- Miller DE, Gleason WL, Mcintosh HD. A comparison of the cardiac output determination by the direct Fick method and the dye-dilution method using indocyanine green dye and a cuvette densitometer. J Lab Clin Med 1962;59:345-50. [PubMed]
- Jonak C, Skvara H, Kunstfeld R, et al. Intradermal indocyanine green for in vivo fluorescence laser scanning microscopy of human skin: a pilot study. PLoS One 2011;6:e23972 [Crossref] [PubMed]
- Schaafsma BE, Mieog JS, Hutteman M, et al. The clinical use of indocyanine green as a near-infrared fluorescent contrast agent for image-guided oncologic surgery. J Surg Oncol 2011;104:323-32. [Crossref] [PubMed]
- Ishizawa T, Bandai Y, Kokudo N. Fluorescent cholangiography using indocyanine green for laparoscopic cholecystectomy: an initial experience. Arch Surg 2009;144:381-2. [Crossref] [PubMed]
- Gotoh K, Yamada T, Ishikawa O, et al. A novel image-guided surgery of hepatocellular carcinoma by indocyanine green fluorescence imaging navigation. J Surg Oncol 2009;100:75-9. [Crossref] [PubMed]
- Ishizawa T, Fukushima N, Shibahara J, et al. Real-time identification of liver cancers by using indocyanine green fluorescent imaging. Cancer 2009;115:2491-504. [Crossref] [PubMed]
- Kawaguchi Y, Nagai M, Nomura Y, et al. Usefulness of indocyanine green-fluorescence imaging during laparoscopic hepatectomy to visualize subcapsular hard-to-identify hepatic malignancy. J Surg Oncol 2015;112:514-6. [Crossref] [PubMed]
- Kudo H, Ishizawa T, Tani K, et al. Visualization of subcapsular hepatic malignancy by indocyanine-green fluorescence imaging during laparoscopic hepatectomy. Surg Endosc 2014;28:2504-8. [Crossref] [PubMed]
- Lim C, Vibert E, Azoulay D, et al. Indocyanine green fluorescence imaging in the surgical management of liver cancers: current facts and future implications. J Visc Surg 2014;151:117-24. [Crossref] [PubMed]
- van der Vorst JR, Schaafsma BE, Hutteman M, et al. Near-infrared fluorescence-guided resection of colorectal liver metastases. Cancer 2013;119:3411-8. [Crossref] [PubMed]
- Boogerd LS, Handgraaf HJ, Lam HD, et al. Laparoscopic detection and resection of occult liver tumors of multiple cancer types using real-time near-infrared fluorescence guidance. Surg Endosc 2016; [Epub ahead of print]. [Crossref] [PubMed]
- Sakoda M, Ueno S, Iino S, et al. Anatomical laparoscopic hepatectomy for hepatocellular carcinoma using indocyanine green fluorescence imaging. J Laparoendosc Adv Surg Tech A 2014;24:878-82. [Crossref] [PubMed]
- Tanaka T, Takatsuki M, Hidaka M, et al. Is a fluorescence navigation system with indocyanine green effective enough to detect liver malignancies? J Hepatobiliary Pancreat Sci 2014;21:199-204. [Crossref] [PubMed]
- Tran Cao HS, Kaushal S, Metildi CA, et al. Tumor-specific fluorescence antibody imaging enables accurate staging laparoscopy in an orthotopic model of pancreatic cancer. Hepatogastroenterology 2012;59:1994-9. [PubMed]
- Murakami T, Hiroshima Y, Zhang Y, et al. Improved disease-free survival and overall survival after fluorescence-guided surgery of liver metastasis in an orthotopic nude mouse model. J Surg Oncol 2015;112:119-24. [Crossref] [PubMed]
- Yano S, Takehara K, Miwa S, et al. Improved resection and outcome of colon-cancer liver metastasis with fluorescence-guided surgery using in situ GFP labeling with a telomerase-dependent adenovirus in an orthotopic mouse model. PLoS One 2016;11:e0148760 [Crossref] [PubMed]
- Xiong H, Kos P, Yan Y, et al. Activatable Water-Soluble Probes Enhance Tumor Imaging by Responding to Dysregulated pH and Exhibiting High Tumor-to-Liver Fluorescence Emission Contrast. Bioconjug Chem 2016;27:1737-44. [Crossref] [PubMed]
- Metildi CA, Felsen CN, Savariar EN, et al. Ratiometric activatable cell-penetrating peptides label pancreatic cancer, enabling fluorescence-guided surgery, which reduces metastases and recurrence in orthotopic mouse models. Ann Surg Oncol 2015;22:2082-7. [Crossref] [PubMed]
- Herrera VL, Colby AH, Tan GA, et al. Evaluation of expansile nanoparticle tumor localization and efficacy in a cancer stem cell-derived model of pancreatic peritoneal carcinomatosis. Nanomedicine (Lond) 2016;11:1001-15. [Crossref] [PubMed]
- KleinJan GH. Hybrid radioguided occult lesion localization (hybrid ROLL) of (18)F-FDG-avid lesions using the hybrid tracer indocyanine green-(99m)Tc-nanocolloid. Rev Esp Med Nucl Imagen Mol 2016;35:292-7. [Crossref] [PubMed]
- Hoogstins CE, Tummers QR, Gaarenstroom KN, et al. A Novel Tumor-Specific Agent for Intraoperative Near-Infrared Fluorescence Imaging: A Translational Study in Healthy Volunteers and Patients with Ovarian Cancer. Clin Cancer Res 2016;22:2929-38. [Crossref] [PubMed]
- Warram JM, de Boer E, van Dam GM, et al. Fluorescence imaging to localize head and neck squamous cell carcinoma for enhanced pathological assessment. J Pathol Clin Res 2016;2:104-12. [Crossref] [PubMed]
- Hiroshima Y, Maawy A, Zhang Y, et al. Fluorescence-guided surgery in combination with UVC irradiation cures metastatic human pancreatic cancer in orthotopic mouse models. PLoS One 2014;9:e99977 [Crossref] [PubMed]
Cite this article as: Lwin TM, Sicklick JK, Hoffman RM, Bouvet M. Fluorescence-guided laparoscopic hepatectomy. Ann Laparosc Endosc Surg 2016;1:10.


