Immunotherapy in gastrointestinal cancers
Introduction
Gastrointestinal (GI) cancers comprise of gastric, esophageal, pancreas, hepatobiliary, colorectal and anal cancers. GI cancers to date remain a major cause of cancer related death in the US and well as worldwide. Based on the current cancer statistics, colorectal, pancreatic, gastric and esophageal cancers are third, fourth, fifth and sixth cancer related mortality in the US respectively (1). Treatments with surgery, chemotherapy, radiation therapy, anti-angiogenic therapy have been the mainstay in the treatment of GI cancers, but over the last decade novel therapeutics with immunotherapies such as cytokines, adaptive cell therapies, peptide- protein- or whole-tumor cell or dendritic-cell vaccines and check point inhibitors have been evaluated in clinical trials as immunomodulatory strategies for GI cancers.
T cell signaling, activation and antigen recognition
The anti-tumor immune system is composed of innate and adaptive immunity. Innate immunity forms the first line of defense against tumor cells, whereas adaptive immunity is accountable for long-term immune response (2).
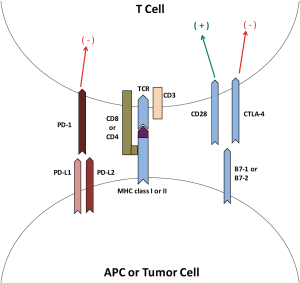
T cells can distinguish a signaling complex comprised of a T cell receptor (TCR) dimer, CD8 or CD4 molecules and CD3 along with the peptide antigens on the antigen presenting cells (APCs) or tumor cells in the presence of class I or class II MHC molecules. CD4+ T cells recognize class II MHC molecules present on the APC [e.g., dendritic cells (DC)] whereas CD8+ T cells recognize class I MHC molecules. The T cell activation process requires co-stimulatory signals which are provided by binding of CD28 on the T cell surface to its ligand B7-1 or B7-2 on the APC or tumor cell, as well as binding of intracellular adhesion molecule (ICAM) and the leukocyte function associated antigen (LFAA) on the APC to their receptors LFA1 and CD2, respectively. This co-stimulatory process is regulated by binding of cytotoxic T lymphocyte antigen-4 (CTLA-4) to B7-1 or B7-2, which acts as a suppressor response also called a co-inhibitory process. This step acts as a ‘checkpoint’ in the immune response cascade preventing the ‘exhaustion’ of T cells (3,4). Figure 1 T gives a schematic representation of T cell signaling, activation and antigen recognition. Other examples of co-stimulatory molecules involved in this process are GITR, OX40 and ICOS. Examples of additional co-inhibitory molecules are programmed death-1 (PD-1), TIM3 and LAG3. PD-1 is an immune check point receptor on the surface of T cell and has two ligands, program death ligand-1 and 2 (PD-L1 and PD-L2). Both CTLA-4 and PD-1 act independently as immune check point inhibitors (5).
Immune escape phenomenon in GI cancers
Immune escape is the ability of the cancer cells to escape immune responses of the host. Many mechanisms of immune escape have been proposed in GI cancers. H. pylori have been identified as one of the environmental factors leading to gastric cancer. Raghavan et al. have proposed a concept of immune modulation by regulatory T cells in H. pylori-induced gastric cancers where regulatory T cells (Tregs) infiltrate the tumor and promote tumor escape from cytotoxic immune responses (6). Many studies have proposed an interesting concept for an immune evasive mechanism whereby gastric adenocarcinoma cells express Fas ligand (FasL) independent of tumor stage that induces Fas receptor-mediated apoptosis of activated lymphocytes, thus overcoming antitumor immune responses (7,8). Similar findings have been found in pancreatic cancer suggesting that FasL induces apoptosis of infiltrating lymphoid cells in the tumor microenvironment, thereby preventing host cytotoxic T cell and natural killer (NK) cell responses (9-11). Dysregulation of FasL has also been proposed as an immune escape method in biliary tract cancer (12-14).
Expression of B7 family members, co-stimulatory molecules on APC or tumor cells, has been shown to be involved in a cancer progression/immune surveillance escape mechanism. Chen et al. has proposed that ICOS-B7H co-stimulatory pathway may be predominant at the site of gastric carcinoma (15). A family of B7 molecules has also been shown to have an impact on the immune escape phenomenon in colorectal cancers (CRC). Sun et al. studied the immunohistochemical expression of B7-H3 pathologic specimens from CRC patients. B7-H3 expression was higher in cancer tissue as compared to normal colorectal tissue, and also correlated with advanced cancer (16). B7-H4 expression has been widely studied in pancreatic cancers (17). Qian et al. have studied the mechanisms of enhanced oncogenicity and inhibition of apoptosis by B7-H4 molecules in pancreatic cancer cells and have proposed that B7-H4 is a cancer promoter and could potentially be a therapeutic target (18). Interestingly, the B7-H1/PD-L1 pathway has been linked to the malignant potential of biliary tract cancers causing immune escape phenomenon by inducing apoptosis of CD8+ tumor infiltrating lymphocytes (19).
A tumor-associated neo-antigen, MUC1 (epithelial mucin glycoprotein), has been a target for immunotherapy in pancreatic cancer. Monti et al. have proposed that tumor-derived MUC1 mucins are immune-inhibitory and responsible for impaired DC maturation and function, in turn impairing innate immunity (20). Mukherjee et al. have shown that MUC1 peptide-based immunization elicits mature MUC1-specific CTLs in peripheral lymphoid organs, which secrete IFN-γ and are cytolytic against MUC1-expressing tumor cells in vitro (21).
Microbiome and immunotherapy
Significant variability is observed in patient responses to immunotherapy, which is not completely understood. Recent work using genetically similar mice suggests that the gut microbiome may play a significant role in response to immunotherapy. A recent study used genetically similar B57BL/6 mice obtained from Jackson Labs (JAX) and Taconic Farms (TAC), subcutaneously inoculated with a melanoma cell line. Upon inoculation, the JAX mouse is found to be more tumor-resistant than the TAC mouse. However, when the two strains are cohoused prior to tumor inoculation, the tumor risk of the TAC mouse is reduced to that of the JAX mouse. This effect is reproducible upon fecal transfer from the JAX mouse to the TAC mouse, suggesting the presence of a tumor-protective effect within JAX mice feces. The degree of tumor protection observed with JAX fecal transfer into TAC mice is equivalent to the effect of treatment of TAC mice with an anti-PD-L1 monoclonal antibody. Impressively, the combination of JAX fecal transfer and anti-PD-L1 treatment resulted in an additive effect in TAC mice, showing the most significant tumor growth suppression. 16S rRNA sequencing of fecal material, in association with CD8+ T cell tumor infiltration, was used to identify which bacterial species in JAX feces were associated with improved anti-tumor immune response. After analysis, Bifidobacterium was the only species associated with improved tumor response, an effect that was recapitulated after oral gavage of TAC mice with Bifidobacterium. The effect of Bifidobacterium was eliminated after CD8+ T cell depletion of host mice, or after heat-inactivation of the bacteria. Furthermore, analysis of spleen and tumor-draining lymph nodes demonstrated increased T cell activation due to enhanced DC function as the likely underlying mechanism (22). Analogous results have been observed in a sarcoma mouse model with the anti-CTLA-4 antibody ipilimumab, however in this case demonstrating a requirement for Bacteroides species. Specifically, anti-tumor efficacy of ipilimumab and activation of tumor-infiltrating lymphocytes (TIL) is lost in germ-free mice and those treated with broad-spectrum antibiotics. Response to anti-CTLA-4 blockade is restored by enteral administration of Bacteroides or by immunization with a Bacteroides vaccine (23). These data suggest an intricate relationship between host microbial flora and response to immunotherapy, and suggest the possibility of manipulating the gut microbiome in conjunction with immunotherapy.
Immune therapies in GI cancers
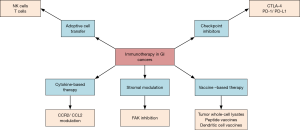
Immune therapies in GI cancers comprise checkpoint inhibitors, vaccine therapies, cytokines and adaptive cell transfer. Currently, checkpoint inhibitors are the most studied in the treatment of GI cancers. Figure 2 represents an overview of immune therapies in GI cancers. Vaccine, cytokine and adaptive cell transfer therapies are not FDA-approved for GI cancers but are being studied in clinical trials.
CRC
PD-1 and CTLA-4 based immunotherapy in CRC
The concept of tumor-specific mutations leading to tumor-associated neo-antigens is fundamental to tumor immunotherapy. A direct prediction of this concept is that more highly mutated tumors will have more tumor-associated neo-antigens, increasing their immunogenicity. Accordingly, more highly mutated tumors (melanoma, lung, GI and head and neck cancers) are the ones which show the greatest responses to immunotherapy (24). Furthermore, subsets of tumors with particularly high mutation frequencies, such as mismatch repair (MMR)-deficient cancers which contain approximately 500-fold more mutations per tumor (25), are predicted to be the most immunogenic and have the greatest responses to immunotherapy.
To date, the PD-1 and CTLA-4 pathways have been the most studied in CRC. The PD-1 pathway is an immune-inhibitory checkpoint pathway that when activated suppresses cytotoxic T cell responses. The PD-1 receptor is expressed on the surface of T cells and has two ligands (PD-L1 and PD-L2), which are predominantly expressed on malignant cells and APCs. Pembrolizumab and nivolumab are fully human IgG4 anti-PD-1 monoclonal antibodies (mAbs) that disrupt the PD-1 pathway. The CTLA-4 pathway serves a similar inhibitory role. CTLA-4 is a cell-surface receptor on T cells that, when engaged by B7-1 or B7-2 on an APC, inhibits T cell activation thereby facilitating tumor immune escape. Ipilimumab (a human IgG1 mAb) was the first approved anti-CTLA-4 mAb and is the prototype member of this class.
A recent phase 2 trial was designed to evaluate the efficacy of pembrolizumab in MSI-high and MSI-low colorectal and non-CRC. Patients with previously treated metastatic colorectal or non-CRC were treated with pembrolizumab 10 mg/kg every 2 weeks, stratified by MSI status. All tumor responses were partial, and were only seen in MSI-high tumors. Similarly, tumor biomarker responses were only seen in MSI-high tumors. Within the CRC group, MSI-high tumors treated with pembrolizumab had significantly improved PFS (not reached in the MSI-high group vs. 2.2 months in the MSI-low group) and OS (median OS not reached in the MSI-high group vs. 5 months in the MSI-low group). Furthermore, response to pembrolizumab correlated with higher number of mutations per tumor and higher intratumoral CD8+ CTL density (26). Based on this trial, pembrolizumab obtained.
FDA breakthrough designation in 2015 for use in MSI-high metastatic CRC
Based on the encouraging data above, there are now numerous checkpoint inhibitor-based trials ongoing targeting PD-1 and CTLA-4 (see Table 1). As an example, the CheckMate-142 trial is an ongoing randomized phase 2 study of 59 patients with metastatic CRC evaluating nivolumab monotherapy (3 mg/kg every 2 weeks) vs. the combination nivolumab (3 mg/kg) + ipilimumab (1 mg/kg) both given every 3 weeks. Patients were stratified by microsatellite instability of their tumors into microsatellite unstable (MSI-high) and microsatellite stable (non-MSI-high). The dosing regimen of nivolumab 3 mg/kg and ipilimumab 1 mg/kg every 3 weeks was chosen based on toxicity assessment in a pilot cohort of non-MSI-high patients. Interim results show that a greater percentage of patients treated with nivolumab + ipilimumab have a tumor response (81% vs. 56% of patients) and have superior 6 month PFS (66% vs. 46%). Adverse events were observed in 26% of the patients in the nivolumab + ipilimumab group, compared to 14% of patients in the nivolumab group. The most common adverse events were fatigue, diarrhea and pyrexia (27). Although the final analysis of this trial is awaited, the preliminary data suggest that dual PD-1 and CTLA-4 checkpoint blockade is superior to PD-1 blockade alone.
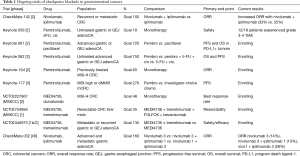
Full table
Given the impressive initial responses to checkpoint inhibitor therapy in MMR-deficient tumors, efforts have been underway to identify molecular features that predict response to immunotherapy, noting that responses are observed in non-MSI-high tumors as well, although at lower frequencies. A method to quantify the innate tumor immune response, the immunoscore, was recently developed by retrospectively analyzing patients treated with standard chemotherapy. The immunoscore was devised by quantifying the density of CTL (CD3+, CD8+) and memory T cells (CD3+, CD45RO+) both in the tumor core and in the invasive margin of the tumor, with scores ranging from 0 (low-level immune infiltrate) to 4 (high-level immune infiltrate). By this method, the majority of tumors with high immunoscores are MSI-high, however a significant number of MSI-low tumors have high immunoscores. Importantly, higher immunoscore correlates with increased density of PD-1 and improved outcome when treated with standard chemotherapy. Moreover, immunoscore more strongly associates with outcome than MSI-status in patients undergoing standard chemotherapy. These data suggest that the superior outcome in high-immunoscore tumors is due to greater natural immune response, and predict that these tumors with higher immunoscores will be more responsive to immunotherapy. Prospective trials using immunotherapy in tumors stratified by immunoscore will be important to verify this approach as a predictive tool (28).
Adoptive cell transfer therapy in CRC
Adoptive cell transfer is a technique whereby tumor-specific immune cells are isolated, expanded ex-vivo, then re-infused into the same host (autologous) or another host (allogeneic) following non-myeloablative lymphocyte conditioning, often using fludarabine/cyclophosphamide. This technique has been undertaken with both NK cells (innate immune system), and CD8+ T cells (adaptive immune system). The antigen specificity of adoptively transferred cells is often unknown. The source of autologous T cells may be either the tumor itself or a sentinel lymph node. A pilot study of adoptive cell transfer of sentinel lymph node-derived autologous CD4+ Th1 cells in stage II–IV CRC was performed with promising results. Of the 9 patients with stage IV CRC in this study, 4 had a complete response, 1 had a partial response, and 4 had stable disease. For the 9 patients with stage IV disease, median OS was 2.6 years, compared to 0.8 years in controls; adverse effects were minimal (29).
A recent study demonstrated the ability to use adoptively transferred TIL in metastatic colon cancer to specifically target a tumor-specific driver mutation (KRAS-G12D), a driver mutation that has been historically difficult to target. Briefly, in a single patient with KRAS-G12D-driven mCRC, TIL with antigen specificity to KRAS-G12D was harvested, ex-vivo expanded to approximately 1011 cells, then autologously re-infused. Forty days after infusion, all metastatic lesions had responded, a response that was maintained for 9 months. One isolated lesion displayed progression at the 9-month time point; however, this lesion was found to have loss of expression of the HLA-C allele to which the TIL were specific, thereby allowing for immune escape. This encouraging study demonstrates the feasibility and durability of adoptive T cell transfer in solid tumors (30).
Vaccine-based therapy in CRC
An additional approach to cancer immunotherapy involves vaccine-based strategies, such as tumor whole-cell lysates, peptide vaccines and DC vaccines. Several early phase vaccine-based studies have been conducted in CRC targeting tumor-associated antigens, such as carcinoembryonic antigen (CEA). A recent phase 1/2 trial enrolled 12 patients with non-metastatic CRC who underwent resection followed by 6 cycles of adjuvant 5-FU, who were then treated with tumor cell lysate-pulsed DC (n=6) or CEA-pulsed DC (n=6). Briefly, patients underwent leukapheresis to obtain immature DC, which were then cultured in the presence of the patient’s own tumor cell lysate or in the presence of CEA, with stimulatory cytokines to induce differentiation of DC 2.5×106 cells were then subcutaneously injected on days 0, 14, 48 and 56. After 7 years of follow up, 6/6 patients treated with tumor lysate-pulsed DC were alive, but 3/6 patients treated with CEA-pulsed DC died due to tumor relapse. There were no significant adverse events noted (31). Despite the small sample size of this study, the results are encouraging and warrant further randomized studies. A similar, but larger, study was done evaluating CEA- and MUC1-expressing DC. Overall, 74 patients with mCRC underwent metastasectomy and adjuvant chemotherapy and were then randomly assigned to administration of autologous DC transfected with a CEA- and MUC1-expressing virus, versus administration of cell-free virus with GM-CSF. Overall, there was no significant difference in OS between the two treatment groups, however the two groups pooled has significantly better OS than controls (median OS not reached in vaccine group vs. 44.1 months in control group). Adverse events were mild, including fevers, myalgia and fatigue (32).
Esophageal and gastric cancers
PD-1 and CTLA-4 based immunotherapy in GEJ cancer
PD-L1 is over-expressed in the majority of gastric cancers, but not in normal gastric epithelium, and higher expression of PD-L1 correlates with worse overall survival (33). Studies are currently underway investigating the role of anti-PD1 and anti-CTLA-4 therapies in gastric and GEJ cancer based on the association of their expression with survival. The KEYNOTE-012 trial is a phase 1b trial that evaluated pembrolizumab in 36 patients with PD-L1 positive advanced or metastatic gastric or GEJ adenocarcinoma. Of the 36 enrolled patients that were evaluable, 8 (22%) had a partial response, and a total of six grade 3–4 adverse events were documented by 5 (13%) of the patients, demonstrating that pembrolizumab has a reasonable toxicity profile and encouraging activity (34). The KEYNOTE-059, -061 and -062 trials are ongoing, and will further evaluate pembrolizumab in advanced gastric and GEJ adenocarcinoma. Other trials are currently ongoing evaluating the anti-PD-L1 antibody MEDI4736 (durvalumab) and the anti-CTLA-4 antibodies tremelimumab and ipilimumab (see Table 1).
Adoptive cell transfer therapies in GEJ cancer
Adoptive cell transfer therapies are also being used in gastric and GEJ adenocarcinoma involving both T cells and NK cells. A recent study was performed using autologous ex-vivo cytokine activated and expanded NK cells (CIK) as an adjuvant treatment for gastric cancer. Specifically, 151 patients that had undergone R0 D2 resected gastric cancer for stage IIIA–IV disease where treated with 6 cycles of adjuvant 5-FU, followed by at least 3 cycles of administration of autologous ex-vivo IFN-γ activated NK cells. Among patients with all histologic subtypes of gastric cancer, immunotherapy was associated with borderline improved median OS (48.1 vs. 42.1 months, P=0.071), and significantly increased median DFS (40.4 vs. 34.1 months, P=0.044). Retrospectively stratifying patients by histologic subtype showed that patients with diffuse or mixed-type histology do not benefit from CIK therapy, however patients with intestinal-type tumors treated with CIK therapy have an improved 5-year OS (46.8% vs. 31.4%, P=0.045) and improved 5-year DFS (42.4% vs. 15.7%, P=0.023) (35).
A phase 1 pilot study of adoptive T cell transfer was done in patients with esophageal squamous cell carcinoma (recurrent or metastatic), targeting the commonly expressed MAGE-A4 antigen. Previous studies demonstrated that MAGE-A4 antigen is expressed in 52% of esophageal squamous cell carcinoma (36). Briefly, peripheral blood T cells were harvested by apheresis, infected with a retrovirus expressing a plasmid encoding MAGE-A4/HLA-A*24:02 specific TCR α- and β-chains, then stimulated in vitro with MAGE-A4 peptide. Modified T cells (108–109) were re-infused without lymphodepletion conditioning; 2 and 4 weeks after T cell infusion, patients received subcutaneous MAGE-A4 peptide vaccination. The transduced T cells were detectable in peripheral blood for approximately 2 months in 7/10 patients, and retained ex vivo reactivity to MAGE-A4. The tumor responses were mixed, but with minimal toxicity, this study demonstrates the feasibility of the approach (37).
Hepatocellular carcinoma
PD-1 and CTLA-4 based immunotherapy in HCC
HCC is a challenging malignancy due to frequently advanced disease at diagnosis and lack of highly effective therapy. Sorafenib is the only therapy approved to treat advanced HCC. Compared to placebo, sorafenib increases median OS from 7.9 to 10.7 months; it obtained FDA approval in 2007 for treatment of advanced HCC based on this data (38). Due to the modest benefit with sorafenib, there has been increasing interest in immunotherapy as a treatment modality for HCC. The phase 1/2 CheckMate-040 study was performed to evaluate the safety and preliminary efficacy of nivolumab (0.1–10 mg/kg) in advanced HCC. Of the 51 patients enrolled, 73% had previously received sorafenib, and 76% had extra-hepatic disease. The disease control rate (in 48/51 evaluable patients) was 64%, with a median OS of 15.1 months. 20% of patients experienced grade 3–4 treatment-related adverse events (39). Despite the small number of patients, these data compare favorably to sorafenib. Given these promising results, CheckMate-459, a phase 3 randomized trial of nivolumab vs. sorafenib as first-line treatment of advanced HCC, is currently ongoing (40).
The first study of an anti-CTLA-4 agent in HCC used tremelimumab in patients with HCC due to hepatitis C genotype 1b. Toxicity and anti-viral response was assessed in 20 patients; tumor response was assessed in 17 patients. Tremelimumab was administered at 90 mg/kg every 90 days. The overall disease control rate was 76%, with the most common grade ≥3 treatment-related adverse event being AST elevation (45% of patients), but was transient and was largely limited to cycle 1. The median OS was 8.2 months, similar to that seen with sorafenib (41).
Vaccine-based therapy in HCC
Several small studies have been done in HCC using cytokines and adoptive cell transfer, including tumor lysate-pulsed DC. However, these studies have seen limited success. A novel vaccine-based approach is currently underway in the European Union (EU); the HEPAVAC consortium. Briefly, the HEPAVAC consortium is developing a multi-target/multi-epitope peptide vaccine based on common MHC class I and class II tumor-associated antigens expressed on HCC. Ideally, the vaccine will be used in a non-patient-specific manner with the option of a subsequent patient-specific vaccination (42). The HEPAVAC vaccine clinical trial was expected to start near the end of 2016.
Pancreatic cancer
Checkpoint inhibitor-based approaches in PDAC
There are no large studies of PD-1 or CTLA-4 based therapies in PDAC. However, there are numerous studies currently underway investigating anti-PD-1 agents (nivolumab and pembrolizumab) and anti-CTLA-4 agents (ipilimumab and tremelimumab) alone or combined with chemotherapy and radiation. A novel approach in PDAC addresses the desmoplastic stroma that creates a barrier to effective therapies. This approach relies on manipulation on the focal adhesion kinase (FAK) pathway using the small molecule FAK inhibitors VS-4718 or defactinib. In the KPC mouse model, the addition of the small molecule FAK inhibitor VS-4718 increased response to anti-PD1 and anti-CTLA4 therapy combined with gemcitabine and significantly prolonged survival in the mouse (43). Based on this work, clinical trials using FAK inhibitors are currently underway. Additional approaches in PDAC include oncolytic viruses (such as Reolysin) with specificity for RAS-activated tumors.
Vaccine trails in PDAC
Vaccine trials in pancreatic cancer have shown exciting results in this difficult to treat disease. The two most promising vaccines in PDAC are the GVAX vaccine and CRS-207, often used together. GVAX is a pancreatic cancer cell line that expresses GM-CSF, while CRS-207 is a live-attenuated Listeria monocytogenes strain that expresses mesothelin. In combination, the two therapies induce T cell immunity against pancreatic cancer antigens such as mesothelin. The phase 2b Eclipse trial of GVAX + CRS-207 showed promising interim results in metastatic pancreatic cancer (44), however, the final results are negative (data not yet published). Despite the negative results of Eclipse, the STELLAR trial is ongoing, randomizing patients with metastatic pancreatic cancer who have received one prior chemotherapy regimen to GVAX + CRS-207 ± nivolumab.
Indoleamine 2,3 dioxygenase (IDO) in PDAC
IDO metabolizes tryptophan to the intermediate kynurenine, which is further metabolized, ultimately leading to production of NAD and ATP, with coincident depletion of tryptophan. Activation of this pathway leads to suppression of CTL via cell cycle arrest or anergy, and activates Tregs, overall facilitating tumor immune escape. IDO expression in lymphocytes is induced by inflammatory signals, including LPS and IFN-γ, in the tumor microenvironment. Additionally, IDO expression is inducible in tumor cells and APCs. Indoximod is a small molecule oral IDO inhibitor currently under evaluation in several clinical trials (45). Notably, a multi-institution phase 2 trial of indoximod in combination with gemcitabine/abraxane is currently underway in metastatic pancreatic cancer, with interim results presented at ASCO 2016 showing an objective response rate of 37% in 30 patients (NCT02077881) (46).
CCR2 modulation in PDAC
The CCR2/CCL2 chemokine pathway plays a critical role in recruiting immunosuppressive monocytes/macrophages into the pancreatic cancer stroma. Pancreatic cancer secretes CCL2 to a greater extent than normal pancreas, which facilitates migration of CCR2+ blood and bone marrow monocytes into the pancreatic tumor stroma. In resected pancreatic cancer, a shift in CCR2+ monocyte distribution from bone marrow to blood (i.e., a higher ratio of peripheral blood: bone marrow CCR2+ monocytes) predicts worse survival. Therefore, inhibition of the CCR2/CCL2 signaling pathway is predicted to improve innate anti-tumor immune responses by attenuating the immunosuppressive TME and thereby improve survival (47). In a phase 1b trial of borderline resectable and locally advanced PDAC, the small molecule CCR2 inhibitor PF-04136309, in combination with FOLFIRINOX, was safe and tolerable, and displayed an objective tumor response of 49% (16/33 patients), compared to zero of five patients treated with FOLFIRINOX alone. As expected, the addition of PF-04136309 resulted in a re-distribution of CCR2-positive monocytes from the peripheral blood to the bone marrow compartment (the opposite of that seen with FOLFIRINOX alone) and an approximate 4-fold decrease in the number of tumor-associated macrophages (TAM), a 2-fold increase in the number of CD4+ and CD8+ T cells, and nearly a 6-fold decrease in the number of Tregs in tumors compared to FOLFIRINOX alone (48). This work lays the foundation for further investigation into CCR2 modulation in pancreatic cancer.
Conclusions
The initial observation suggesting that the immune system may play a role in modulating cancers was made nearly 125 years ago (49). Over the last two decades, our understanding of the basic mechanisms underlying immune modulation has greatly improved, allowing for the development of multiple exciting therapeutic approaches for immunomodulation of tumors. The challenges moving forward are not only to fine-tune these various immune-based approaches, but also learn how to appropriately integrate them with molecularly targeted agents (such as TKIs, angiogenesis inhibitors, etc.) as well as cytotoxic chemotherapy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin 2014;64:9-29. [Crossref] [PubMed]
- Medzhitov R, Janeway C Jr. Innate immunity. N Engl J Med 2000;343:338-44. [Crossref] [PubMed]
- Delves PJ, Roitt IM. The immune system. Second of two parts. N Engl J Med 2000;343:108-17. [Crossref] [PubMed]
- Fooksman DR. Organizing MHC Class II Presentation. Front Immunol 2014;5:158. [Crossref] [PubMed]
- Carter LL, Carreno BM. Cytotoxic T-lymphocyte antigen-4 and programmed death-1 function as negative regulators of lymphocyte activation. Immunol Res 2003;28:49-59. [Crossref] [PubMed]
- Raghavan S, Quiding-Jarbrink M. Immune modulation by regulatory T cells in Helicobacter pylori-associated diseases. Endocr Metab Immune Disord Drug Targets 2012;12:71-85. [Crossref] [PubMed]
- Bennett MW, O'Connell J, O'Sullivan G C, et al. Expression of Fas ligand by human gastric adenocarcinomas: a potential mechanism of immune escape in stomach cancer. Gut 1999;44:156-62. [Crossref] [PubMed]
- Zheng HC, Sun JM, Wei ZL, et al. Expression of Fas ligand and caspase-3 contributes to formation of immune escape in gastric cancer. World J Gastroenterol 2003;9:1415-20. [Crossref] [PubMed]
- Elnemr A, Ohta T, Yachie A, et al. Human pancreatic cancer cells express non-functional Fas receptors and counterattack lymphocytes by expressing Fas ligand; a potential mechanism for immune escape. Int J Oncol 2001;18:33-9. [PubMed]
- Ohta T, Elnemr A, Kitagawa H, et al. Fas ligand expression in human pancreatic cancer. Oncol Rep 2004;12:749-54. [PubMed]
- von Bernstorff W, Spanjaard RA, Chan AK, et al. Pancreatic cancer cells can evade immune surveillance via nonfunctional Fas (APO-1/CD95) receptors and aberrant expression of functional Fas ligand. Surgery 1999;125:73-84. [Crossref] [PubMed]
- Shimonishi T, Isse K, Shibata F, et al. Up-regulation of fas ligand at early stages and down-regulation of Fas at progressed stages of intrahepatic cholangiocarcinoma reflect evasion from immune surveillance. Hepatology 2000;32:761-9. [Crossref] [PubMed]
- Li Z, Wang J, Tang C, et al. Regulative effect of IFN-gamma on the Fas/Fas L system of cholangiocarcinoma cells. Zhonghua Yu Fang Yi Xue Za Zhi 2002;36:495-8. [PubMed]
- Que FG, Phan VA, Phan VH, et al. Cholangiocarcinomas express Fas ligand and disable the Fas receptor. Hepatology 1999;30:1398-404. [Crossref] [PubMed]
- Chen XL, Cao XD, Kang AJ, et al. In situ expression and significance of B7 costimulatory molecules within tissues of human gastric carcinoma. World J Gastroenterol 2003;9:1370-3. [Crossref] [PubMed]
- Sun J, Chen LJ, Zhang GB, et al. Clinical significance and regulation of the costimulatory molecule B7-H3 in human colorectal carcinoma. Cancer Immunol Immunother 2010;59:1163-71. [Crossref] [PubMed]
- Wang L, Ma Q, Chen X, et al. Clinical significance of B7-H1 and B7-1 expressions in pancreatic carcinoma. World J Surg 2010;34:1059-65. [Crossref] [PubMed]
- Qian Y, Hong B, Shen L, et al. B7-H4 enhances oncogenicity and inhibits apoptosis in pancreatic cancer cells. Cell Tissue Res 2013;353:139-51. [Crossref] [PubMed]
- Ye Y, Zhou L, Xie X, et al. Interaction of B7-H1 on intrahepatic cholangiocarcinoma cells with PD-1 on tumor-infiltrating T cells as a mechanism of immune evasion. J Surg Oncol 2009;100:500-4. [Crossref] [PubMed]
- Monti P, Leone BE, Zerbi A, et al. Tumor-derived MUC1 mucins interact with differentiating monocytes and induce IL-10highIL-12low regulatory dendritic cell. J Immunol 2004;172:7341-9. [Crossref] [PubMed]
- Mukherjee P, Ginardi AR, Madsen CS, et al. MUC1-specific CTLs are non-functional within a pancreatic tumor microenvironment. Glycoconj J 2001;18:931-42. [Crossref] [PubMed]
- Sivan A, Corrales L, Hubert N, et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science 2015;350:1084-9. [Crossref] [PubMed]
- Vetizou M, Pitt JM, Daillere R, et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 2015;350:1079-84. [Crossref] [PubMed]
- Alexandrov LB, Nik-Zainal S, Wedge DC, et al. Signatures of mutational processes in human cancer. Nature 2013;500:415-21. [Crossref] [PubMed]
- Lee V, Murphy A, Le DT, et al. Mismatch Repair Deficiency and Response to Immune Checkpoint Blockade. Oncologist 2016;21:1200-11. [Crossref] [PubMed]
- Le DT, Uram JN, Wang H, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med 2015;372:2509-20. [Crossref] [PubMed]
- Michael J. Overman SK, Raymond S, et al. Nivolumab ± ipilimumab in treatment (tx) of patients (pts) with metastatic colorectal cancer (mCRC) with and without high microsatellite instability (MSI-H): CheckMate-142 interim results. J Clin Oncol 2016;34:abstr 3501.
- Mlecnik B, Bindea G, Angell HK, et al. Integrative Analyses of Colorectal Cancer Show Immunoscore Is a Stronger Predictor of Patient Survival Than Microsatellite Instability. Immunity 2016;44:698-711. [Crossref] [PubMed]
- Karlsson M, Marits P, Dahl K, et al. Pilot study of sentinel-node-based adoptive immunotherapy in advanced colorectal cancer. Ann Surg Oncol 2010;17:1747-57. [Crossref] [PubMed]
- Tran E, Robbins PF, Lu YC, et al. T-Cell Transfer Therapy Targeting Mutant KRAS in Cancer. N Engl J Med 2016;375:2255-62. [Crossref] [PubMed]
- Hunyadi J, Andras C, Szabo I, et al. Autologous dendritic cell based adoptive immunotherapy of patients with colorectal cancer-A phase I-II study. Pathol Oncol Res 2014;20:357-65. [Crossref] [PubMed]
- Morse MA, Niedzwiecki D, Marshall JL, et al. A randomized phase II study of immunization with DC modified with poxvectors encoding CEA and MUC1 compared with the same poxvectors plus GM-CSF for resected metastatic colorectal cancer. Ann Surg 2013;258:879-86. [Crossref] [PubMed]
- Hou J, Yu Z, Xiang R, et al. Correlation between infiltration of FOXP3+ regulatory T cells and expression of B7-H1 in the tumor tissues of gastric cancer. Exp Mol Pathol 2014;96:284-91. [Crossref] [PubMed]
- Muro K, Chung HC, Shankaran V, et al. Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): a multicentre, open-label, phase 1b trial. Lancet Oncol 2016;17:717-26. [Crossref] [PubMed]
- Shi L, Zhou Q, Wu J, et al. Efficacy of adjuvant immunotherapy with cytokine-induced killer cells in patients with locally advanced gastric cancer. Cancer Immunol Immunother 2012;61:2251-9. [Crossref] [PubMed]
- Akcakanat A, Kanda T, Tanabe T, et al. Heterogeneous expression of GAGE, NY-ESO-1, MAGE-A and SSX proteins in esophageal cancer: Implications for immunotherapy. Int J Cancer 2006;118:123-8. [Crossref] [PubMed]
- Kageyama S, Ikeda H, Miyahara Y, et al. Adoptive Transfer of MAGE-A4 T-cell Receptor Gene-Transduced Lymphocytes in Patients with Recurrent Esophageal Cancer. Clin Cancer Res 2015;21:2268-77. [Crossref] [PubMed]
- Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378-90. [Crossref] [PubMed]
- El-Khoueiry AB, Sangro B, Cheung YC, et al. Phase I/II safety and antitumor activity of nivolumab (nivo) in patients (pts) with advanced hepatocellular carcinoma (HCC): Interim analysis of the CheckMate-040 dose escalation study. J Clin Oncol 2016;34:abstr 4012.
- Sangro B, Park JW, Dela Cruz CM, et al. A randomized, multicenter, phase 3 study of nivolumab vs sorafenib as first-line treatment in patients (pts) with advanced hepatocellular carcinoma (HCC): CheckMate-459. J Clin Oncol 2016;34:abstr TPS4147.
- Sangro B, Gomez-Martin C, de la Mata M, et al. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J Hepatol 2013;59:81-8. [Crossref] [PubMed]
- Buonaguro L, Consortium H. Developments in cancer vaccines for hepatocellular carcinoma. Cancer Immunol Immunother 2016;65:93-9. [Crossref] [PubMed]
- Jiang H, Hegde S, Knolhoff BL, et al. Targeting focal adhesion kinase renders pancreatic cancers responsive to checkpoint immunotherapy. Nat Med 2016;22:851-60. [Crossref] [PubMed]
- Le DT, Wang-Gillam A, Picozzi V, et al. Safety and survival with GVAX pancreas prime and Listeria Monocytogenes-expressing mesothelin (CRS-207) boost vaccines for metastatic pancreatic cancer. J Clin Oncol 2015;33:1325-33. [Crossref] [PubMed]
- Zhai L, Spranger S, Binder DC, et al. Molecular Pathways: Targeting IDO1 and Other Tryptophan Dioxygenases for Cancer Immunotherapy. Clin Cancer Res 2015;21:5427-33. [Crossref] [PubMed]
- Bahary N, Garrido-Laguna I, Wang-Gillam A, et al. Results of the phase Ib portion of a phase I/II trial of the indoleamine 2,3-dioxygenase pathway (IDO) inhibitor indoximod plus gemcitabine/nab-paclitaxel for the treatment of metastatic pancreatic cancer. J Clin Oncol 2016;34:abstr 452.
- Sanford DE, Belt BA, Panni RZ, et al. Inflammatory monocyte mobilization decreases patient survival in pancreatic cancer: a role for targeting the CCL2/CCR2 axis. Clin Cancer Res 2013;19:3404-15. [Crossref] [PubMed]
- Nywening TM, Wang-Gillam A, Sanford DE, et al. Targeting tumour-associated macrophages with CCR2 inhibition in combination with FOLFIRINOX in patients with borderline resectable and locally advanced pancreatic cancer: a single-centre, open-label, dose-finding, non-randomised, phase 1b trial. Lancet Oncol 2016;17:651-62. [Crossref] [PubMed]
- Starnes CO. Coley's toxins in perspective. Nature 1992;357:11-2. [Crossref] [PubMed]


