State of the art of prostatic arterial embolization for benign prostatic hyperplasia
Introduction
Benign prostatic hyperplasia (BPH) is histologically characterized by proliferation of prostatic cellular elements. Chronic bladder outlet obstruction (BOO) may be a consequence; it leads to urinary retention, urinary infections, hematuria, bladder calculi and renal insufficiency. In male, BPH represents one of the most common reason causing lower urinary tract symptoms (LUTS) (1). In clinical practice, urinary tract infections secondary to BPH are most common causes for urologic consultation.
In presumed BPH diagnosis the first step is still based on digital rectal examination (DRE) to evaluate prostate size and contour. Urinalysis are usually performed to assess the presence of blood, leukocytes, bacteria, protein, or glucose. Prostate-specific antigen (PSA), even if BPH does not cause prostate cancer (PCa) should be screened (2). Moreover, renal function must be investigated in those patients who have elevate post-void residual (PVR) urine volumes (3).
Symptomatology alone is insufficient for diagnosis (4) and patients with suspected large PVR should undergo a bladder ultrasound (US) to determine urine volume and assess for BOO. Trans-rectal US (TRUS) is useful only in selected patients, to determine prostate gland dimensions and volume over the anatomical characteristics of the gland to improve success of minimally invasive treatments or following biopsies on areas incidentally found as suspect for PCa (3,5,6).
During the past years, BPH was considered a surgical disease and patients with moderate or severe LUTS and an abundant PVR with gross hematuria or recurrent urinary tract infection were addressed to radical prostatectomy (RP) or transurethral resection of the prostate (TURP) to reduce symptoms (3). Currently, the wait and watch strategy is recommended for patients referred with mild BPH symptoms [International Prostate Symptom Score (IPSS) ≤7] and for those with moderate/severe (IPSS ≥8) without complications of BPH. Those patients are managed with medical therapy.
Although the TURP is still considered the gold standard for BPH surgical treatment, a morbidity of 20% and several complications are still reported in literature such as ejaculatory dysfunction, erectile dysfunction, urethral strictures, urinary tract infection and post-operative bleeding in same cases requiring transfusion, with an overall retreatment rate of 6% (7).
Moreover, some patients are unfit for surgery based on their comorbidities (8).
For this class of patients and for patients that refuse surgery, novel minimally invasive procedures have been developed, pointing to a safer profile that is fundamental for QoL after treatment and equally effective to surgical techniques, sparing costs with a durable relief of symptoms (9). Among the available minimally invasive procedures (such as intraprostatic injectables, medical devices, and approaches based on tissue ablation) the prostatic artery embolization (PAE) can be considered an emerging technique performed under radiological guidance by interventional radiologists through selective prostatic arteries embolization.
In 2000, the first case of PAE was reported (10). With the goal to reduce recurrent episodes of acute urinary retention (AUR) and persistent gross hematuria in a patient unsuitable for surgery due to its relevant comorbidities PAE was performed. Over the successful management of prostatic bleeding, a great relief in BPH symptoms, including urinary retention, was registered: an unexpected useful side effect (10). Currently, the increased experience with PAE and a conspicuous available literature, have led to more interesting results showing acceptable outcomes in terms of reduced failure rates with some studies that show acceptable IPSS/AUA-SI score at 24 and 36 months post-intervention (11,12). Extensive improvement in technology concerning interventional devices, angiographic suites and guidance software, have reduced complications rate and risk of inadvertent and untargeted embolization that heavily compromise patient outcomes (12-14). These developments have renewed the interest in PAE.
The aim of this review is to define current evidence on feasibility, effectiveness and safety of PAE according with clinical experiences available in literature.
Methods
A systematic literature search was performed on Medline, Scopus and Google Scholar using the syntax “benign prostatic hyperplasia” and “embolization” or embolic agents” and “results” or “indications” or “complications” or “technical success” or “effectiveness and clinical success” for studies published in English from January 2005 to December 2017. All titles and abstracts of studies found in the initial search were then selected to individuate those evaluating patients with BPH that were unsuitable for any other treatment (medical therapy or surgery) and underwent prostatic arterial embolization. Two reviewers (AMI and MP) included all relevant studies. Based on titles and related abstract, duplicated studies, nonhuman studies, studies not concerning prostatic hyperplasia, comments, letters, case reports (<5 patients) and conference abstracts were all excluded. Remaining studies were considered relevant.
Data extraction was performed by two authors (FP and FP) and extracted data were included. A third external reviewer performed final consensus (SAA).
The primary endpoint was to evaluate technical and clinical success and safety of prostatic arterial embolization. The secondary endpoint was the evaluation of the quality of life in terms of symptoms control and erectile function.
The following parameters were extracted, where available, from the included papers: inclusion criteria, embolic agent, technical success, follow up (FU) time points, complications, prostate volume (PV), PSA, PVR, maximal flow rate (Qmax), IPSS, QoL and International Index of Erectile Function (IIEF).
Indications
Preliminary evidence shown has that PAE may be an effective therapy for the relief of LUTS in patients with BPH, representing a minimally invasive treatment that can replace TURP when not feasible (8).
However, to date, indications and contraindications to PAE have not been fully clarified (15,16). According to most recent guidelines (15), PAE should be contemplated only for highly symptomatic patients with BPH who are not responsive to medical treatment and are unsuitable for surgery or refuse surgery (3,15). In contrast, PAE should be excluded in all other causes of LUTS, such as PCa, prostatitis or urethral strictures, because its efficacy has never been demonstrated (15,17,18).
One of the main limit in defining indications and contraindications of PAE is the lack of evidence from randomized trials, since as of today, PAE has been prospectively evaluated only by single cohort studies (19).
Tables 1-3 reports the studies that we selected from literature and their inclusion criteria.
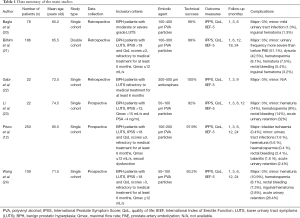
Full table

Full table
Full table
All studies included only symptomatic patients presenting moderate or severe LUTS (12,20-24). Some of them (12,21,23,24), also specified the required grade of LUTS in terms of IPSS: Li defined a threshold score of 12 (23), while Bilhim, Pisco and Wang demanded an IPSS higher than 18, therefore involving only patients with severe LUTS (12,21,24). In addition, most of these studies were designed to comprise only patients who were not responsive to medical treatment for at least 6 months (12,21,22). Further criteria of eligibility were: QoL score >3 (12,21,22) Qmax ≤12 or <15 mL/s (23), PSA <4 ng/mL (23) and sexual dysfunction (12). Notably, no age restrictions were mentioned in any of the published studies (12,20-24).
Thus, despite some heterogeneity, altogether these data underline the agreement between the investigators to consider PAE as a surrogate to TURP for treatment of BPH not amendable to medical therapy.
Procedure technique
Anatomical aspects
The fact that there are no specific anatomical patterns associated with pelvic and prostatic vascular anatomy make the identification and navigation of the prostatic arteries one of the major challenges related to PAE.
However, a few patterns are more generally seen (25).
The main artery that supplies the pelvis is the internal iliac artery (IIA) that supply most of the pelvic viscera, the pelvic walls, the perineum, and the gluteal region (26); the main trunk bifurcates after 3–4 cm in two large branches, one anterior and the other one posterior. Superior gluteal, iliolumbar and lateral sacral arteries generally arise from the posterior branch; superior (SVA) and inferior vesical arteries (IVA), obturator, middle rectal, inferior gluteal, and internal pudendal arteries (IPA) from the anterior. Carnevale et al. (27) created an acronym, PROVISO (Pudendal, Rectal, Obturator, Vesical Inferior and Superior) which can help to memorize the arteries related to prostate vascularization under the ipsilateral Oblique view (last letter of PROVISO) listed in a caudo-cranial sense.
The prostate arteries (PAs) can have multiple origins from the arteries described above but frequently arise as a common trunk and it divides right away in two main branches: the anteromedial for the central gland including the median lobe, and the posterolateral for the peripheral zone and the apex (28,29).
Despite BPH occurs in the central gland, embolization of the anteromedial prostate branch alone is not enough because the deep interconnection between the two branches lead to the necessity to embolize all the ramifications to avoid later revascularization of the central gland (27).
de Assis et al. (29) proposed a vascular anatomical classification of the prostatic arteries into five types.
A knowledge of the normal prostate vascularization and its main variations is mandatory to approach the embolization procedure, and to avoid non-target embolization and cause ischemia to the penis, bladder and rectum. Moreover this assumption is important to avoid loss-time and injury to other vessels due to excessive handling of catheters and wires, more frequent in patients with severe atherosclerosis (27).
Access
PAE procedures are generally performed via transfemoral access (TFA) (12,30,31).
A trans radial approach (TRA), originally developed for percutaneous coronary interventions, has gained interest in recent years (32). Advantages of this approach are reduced bleeding risk, early discharge, patient preference, low cost, and lower risk of morbidity and mortality (32). This method would also allow patients to ambulate immediately post-PAE, which could facilitate urination.
In 2016, Bhatia et al. (33) conducted a retrospective analysis on a total of 64 procedures with the aim to compare safety and feasibility of PAE via TRA and TFA. TRA represented a safe and feasible method to perform PAE; the learning curve of the operator is important.
Technique
Bilateral PAE is generally accepted as the best choice in terms of clinical results compared to unilateral embolization, due to the deep connections that exist between the PAs. This clinical suspicion has been investigated by Bilhim et al. (8) in a retrospective analysis: poor outcomes were observed in the unilateral group compared to the bilateral one, even if no statistical significance was seen between the two groups.
Bilateral PAE is feasible from a single-sided approach, due to intraprostatic anastomoses and the possibility to cross from one side to the other one (34). This technique may be considered in patients with occluded internal iliac artery on one side.
PAE is generally performed as an inpatient procedure; intravenous ciprofloxacin 400 mg should be administered within 1 hour before the procedure and continued for 7 days with 500 mg twice a day orally (30).
Pre-procedural medication may include oral diclofenac 100 mg/d and famotidine 20 mg twice daily for 2 days before the procedure and the morning of the procedure (35).
The PErFecTED technique
The “PErFecTED technique”, as described by Carnevale et al. (36), led to clinical success as demonstrated with an improvement of lower urinary symptoms and lower recurrence rates.
To the best of our knowledge, the PErFecTED technique has been compared to original PAE in two studies so far. Authors found a superiority in IPSS reduction and Qmax improvement during a FU of 12 months (37).
A second study consisted in retrospectively analysis on 105 consecutive patients who underwent PAE with the two different techniques (38). Of these patients, clinical recurrence at 12 months was statistically higher in the group treated with the original PAE technique.
A Foley catheter is introduced into the bladder and filled with a mixture of iodinated contrast medium (20–30%) and saline solution (36). Vascular access is achieved by the femoral artery. A preliminary internal iliac angiography is performed to evaluate the PAs. Then, an internal vesical artery (IVA) catheterization with an ipsilateral 25–55° oblique view, is obtained. Nitroglycerine or isosorbide mononitrate are vasodilator used to prevent vasospasm and to increase artery size to facilitate microcatheter navigation and distal positioning. When the microcatheter is advanced beyond the collateral branches, the embolization can start. It is preferred to start from the peripheral part of the gland. The recommendation is to embolize with gelatin microspheres slowly (36).
When reached the stasis, the microcatheter should be advanced into the prostatic parenchyma branches for an intraprostatic embolization. The periurethral region of the prostate must be embolized because strictures start from this part of the gland (36).
Cone-beam computed tomography (CBCT)
Digital subtraction angiography (DSA) provides excellent visualization of pelvic vessels, but its low sensitivity for soft-tissue contrast and two-dimensional projection makes it difficult to evaluate complex prostatic vascular anatomy and identify the prostatic arterial supply. CBCT consists in an angiographic unit equipped with a flat-panel detector that can provide volumetric tomographic images. Many authors proved that CBCT can be helpful in endovascular procedures (39-41). In vascular procedures, CBCT permits the assessment of complex vascular anatomy after a single injection of contrast medium in a targeted artery (38).
During PAE, CBCT can be used to localize the prostate, identify PAs and their anatomic variants, improving safety and feasibility of selective embolization (42). For this reason, CBCT must be performed with the catheter into the IIA to evaluate the origin of the PAs. A new CBCT can be performed with the microcatheter in the PA, to avoid non-target embolization (38).
As far as we know, studies have evaluated the usefulness of CBCT. Bagla et al. (43,44) found that CBCT provided information that could probably save the patient from complications or recurrence in 46% of cases.
Wang et al. (38) discovered that CBCT provided more informations than DSA in 64.2% of cases.
The third study by Chiaradia et al. (42) had the goal to evaluate the automatic three-dimensional detection of PAs with the use of CBCT imaging and vessel-tracking software. In all six patients considered in this study, CBCT was useful in the detection of the PAs.
Embolization particles
Dimension of the particles used during PAE vary in the published experience from 50 to 300 to 500 µm (11,23,45,46).
Bilhim et al. (47), performed a comparison between different PVA sizes (80 to 180, 180 to 300 µm). They found out that the larger particles cohort led to a greater reduction in IPSS during the first 6 months, with a nearly statistically significant result.
Goncalves et al. (45) found minor adverse events in patients treated with smaller microspheres. From 3 to 12 months, the smaller particles size revealed a lower regrowth rate in prostate size.
In conclusion, both studies (45,47) have suggested that larger particles tend to perform slightly better, but studies are heterogeneous, and data are yet not enough.
Microcoils
As intraprostatic anastomosis with extraprostatic arteries represents a known cause of nontarget embolization and subsequent ischemic complication of PAE (48), a recent study by Bhatia et al. (49) tried to determine if microcoils during PAE could be a safe adjunctive measure to prevent this complication; in particular, coils were deployed when there was significant reflux or direct filling of distal communications branch arteries between PA and penile, rectal or bladder vasculature. For this reason, the microcatheter need to be advanced or positioned distally enough from these anastomoses. However, given the end-vessel nature of bladder and penile vasculature, there is a risk of ischemia from coil embolization itself and given the small caliber and great tortuosity of this vessel, this method could lead to an increase in procedure and fluoroscopy times.
Complications
Complications of PAE are divided in two categories: minor complications that include all the events that don’t require any therapy up to the admission for observation only, and major complications that include therapies requiring hospitalisation up to permanent adverse sequelae and death (50).
Major complications rate is less than 1% (51). The broad range of post-procedural minor events—such as a higher urinary frequency, hematospermia, urinary tract infections and balanitis, haematuria, dysuria, rectal bleeding, AUR, inguinal hematoma, etc.—more often includes self-limiting diseases, with the advantage of restricting transurethral procedure-related complications as bleeding, sexual dysfunction and dilutional hyponatremia (43). The overall incidence is estimated to be around 30% (51).
Mild urethral or perineal pain may occur as post-embolization syndrome and this is not related with PVA particles size using during PEA (47). Patients can experience moderate/severe LUTS after the procedure. The great number of them continues medical therapy, while others undergo to prostatic surgery. Some non-responder patients could repeat PAE 12 months after the first one, but it’s seen that the development of collateral circulation limited the procedural success (21).
The bladder ischemia, rarely described as a post-procedural event, is the main major complication reported until now (51). It is a necrosis and desquamation of the bladder wall that, when localised, requires surgical cystoscopy removal 1 month after PAE without need for bladder reconstruction. It is potentially caused by no-target embolization and emphasize the importance of a correct pre-procedural planning (52). The only other major complication described in the literature is the persistent urinary tract infection requiring hospitalisation for intravenous antibiotics and it seems to be related to the urodynamic study (28)
Complications most frequent observed after PAE are described in Table 1.
Results
PAE is typically evaluated with respect to technical and clinical success. According to Gao et al. (11), a PAE procedure is technically successful when selective prostatic arterial catheterization and embolization on at least one side of the pelvis are achieved. Technical success rates of 92% (22/24 patients) and 1.9% (12/630 patients) are reported by Li and Pisco, respectively (23,53). Cases of failure are mainly related to intra-operative evidence of high tortuosity and atherosclerotic changes of the iliac arteries and may be treated surgically. For the same reason, sometimes PAE can be performed only unilaterally. Pisco et al. have recently suggested a very angled origin of the prostatic artery as another possible cause (53). Anatomic and degenerative vessel-related features are the only reported causes of technical failure.
The clinical outcome of a technically successful PAE procedure describes the perceived or measured improvement of the patient’s clinical conditions. According to Pisco et al. (53), clinical success is achieved when all the three following requirements are met:
- IPSS ≤15 points with a decrease of at least 25% from the baseline score;
- QoL score ≤3 points or a decrease of at least 1 point from baseline;
- No need of any additional medical or surgical therapy after PAE.
In addition to IPSS and QoL, clinical success of PAE is also quantified in terms of Qmax, PV, PVR and IIEF. As the aim of PAE is to cause ischemic necrosis and shrinkage of the prostate gland (54), it is reasonable to assume that clinical improvement goes together with PV reduction and long-term PSA value decrease, in proportion with the extension of infarction area.
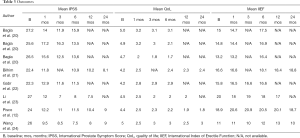
In the studies reviewed (Tables 1-3), patients have been evaluated periodically after PAE presenting promising results. Wang et al. (24) reported data of 105 patients observed for a mean of 24 months. They showed a cumulative rate of clinical success at during 24 months FU. In the same study, 84 patients observed for 24 months, have shown a significative decrease of PV, a consistent and stable increment of Qmax and decrement of PVR (24) . In a wide systematic review by Kuang et al. (18), all data by relative recent studies have been compared to demonstrate a significant improvement of Qmax, IPSS and QoL associated with reduced PV and PVR in a maximum FU of 24 months. Consistent results emerge also from large prostates trials: Bagla et al. (20) have tested PAE effectiveness in three group with increasing prostate size (mean volumes: 37.5, 65.7, 139.4 cm2) during a maximum FU term of 6 months and have demonstrated high and stable rate of clinical success with significant QoL improvement in small prostates as in the larger ones.
Concerning PSA value, several authors (21,23,24) reported a consistent increment at 24 hours after PAE, and then a drop to a significantly lower level than the baseline in the following months almost sustained over time (23,24). Also, Bilhim et al. (21) have found a statistically significant association between higher levels of early PSA and lower IPSS over time, suggesting a potential prognostic role of early PSA value.
Finally, just a minority part of studies includes complete data on IIEF score and no significant difference in erectile function at 1, 3 or 6 months after PAE is shown (51). However, in a recent study by Pisco et al. IIEF score improved in 21.9% of patients, probably due to the discontinuation of medication for BPH that may affect sexual function (53).
Clinical failure affects a minor number of patients. Pisco et al. have reported different results: they have registered clinical failure in 43 patients of 238 (18.1%) during the first month after PAE (unilateral PAE in 12 patients, bilateral PAE in 25 patients, incomplete PAE in 6 patients) without recognizing technical reason for failure or a direct relationship with PV reduction (52). Bilhim et al. (8) have studied the difference between patients treated unilaterally or bilaterally showing a better but not statistically significative outcome in case of bilateral treatment. Finally, Gao et al. (11) have presented 5 cases of failure of 54 patients (9.3%), 4 bilaterally and 1 unilaterally treated.
Some authors finding PAE as a possible solution to manage BPH in poor surgical candidates at substantial risk. Recently, Rampoldi et al. (30) selected a court of 43 patients unsuitable for surgery. PAE have been performed in 41 patients and a significant improvement of the QoL was assessed for 33 of them (80%). Bhatia et al. (55) selected a population of 30 catheter-dependent patients with large PV. PAE allowed to 26 patients (86.7%) to remove catheter at a mean time of 18.2 days and these results have been confirmed at 6 and 12 months of FU. All these patients have continued to void independently and have not required reintroduction of a urethral catheter with a net improvement of QoL. Finally, consistent results have been reported by Carnevale et al. (56) performing PAE on an analogous court of 11 patients: 10 patients removed catheter at a mean of 12.1 days and have shown a consequent improvement in terms of QoL.
Discussion
Regarding the results published, PAE represents an emerging as a viable non-surgical treatment for LUTS caused by BPH.
On the basis of the data published, IPSS as well as QoL improve in the first 12 months of FU.
In literature, the only randomized controlled trial published comparing PAE and TURP (11), concluded that both procedures led to significant clinical improvements. However, the advantages of the PAE procedure must be evaluated against the potential for technical and clinical failures in a minority of patients surgically treated. PAE was associated with shorter hospital stays compared with TURP.
Knowledge of the arterial anatomy is essential for an effective and a safe embolization, avoiding complications related to no-target embolization to surrounding organs (bladder, rectum, and penis). Prostatic arterial anatomy is highly variable; in most cases PAs arise from the internal pudendal artery or they present a common origin with the SVA or from the common anterior gluteal-pudendal trunk. But in other cases, PAs arise from different arteries (branches of the internal iliac artery) (25-29).
Rotational angiography and CBCT represent a valid help for identifying the PAs and its origin (38,43,44).
The concept that smaller-sized particles may penetrate more distal inducing greater ischemia is well known. BPH develops primarily in the peri-urethral region, therefore embolization of this area leads to improvement of symptoms and clinical success (24,47).
Bilateral PAE produces better results than that unilateral (24).
On the basis of the results analyzed, PAE may be considered effective for the treatment of LUTS secondary to BPH. In Tables 2 and 3 objective results (PV and PVR) and symptoms like Qmax, IPSS, and QoL are reported (Tables 2,3) (12,20-24).
No major complications were observed in most published series; Pisco et al. (12) reported bladder ischemia in 0.4% of cases. The incidence of minor complications (i.e., transient hematuria, hematospermia, and rectal bleeding) after PAE is acceptable (12,20-24). The AUR after PAE was described; it was attributed to the edema in the periurethral area after embolization. AUR was managed conservatively with a bladder catheter and antibiotics for about 1 week (12).
Limitations of this review include a lack of direct comparison of clinical outcomes and complications to TURP. As reported above, only one was a randomized control trial that compared PAE to TURP (11). The outcomes in the remaining articles could not be directly compared to TURP.
In most studies, the lack of standardized methods of reporting complications represent another limitation. The risk should be an overlap of data between studies from the same researchers.
Another limitation is the lack of long-term FU; patient outcomes demonstrated an improvement in the short term; at the moment, no data about the recurrence rate of the symptomatology are available.
Conclusions
With the data available to date, PAE is an effective treatment for BPH in short-to-intermediate FU period.
Prospective controlled multicenter trials with longer FU periods will be required for validation of PAE. Moreover, larger randomized control trials with comparison to TURP are required.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Vuichoud C, Loughlin KR. Benign prostatic hyperplasia: epidemiology, economics and evaluation. Can J Urol 2015;22 Suppl 1:1-6. [PubMed]
- Barry MJ. Clinical practice. Prostate-specific-antigen testing for early diagnosis of prostate cancer. N Engl J Med 2001;344:1373-7. [Crossref] [PubMed]
- McVary KT, Roehrborn CG, Avins AL, et al. Update on AUA guideline on the management of benign prostatic hyperplasia. J Urol 2011;185:1793-803. [Crossref] [PubMed]
- D'Silva KA, Dahm P, Wong CL. Does this man with lower urinary tract symptoms have bladder outlet obstruction?: The Rational Clinical Examination: a systematic review. JAMA 2014;312:535-42. [Crossref] [PubMed]
- Ahmed HU, El-Shater Bosaily A, Brown LC, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet 2017;389:815-22. [Crossref] [PubMed]
- Pesapane F, Patella F, Fumarola EM, et al. Intravoxel Incoherent Motion (IVIM) Diffusion Weighted Imaging (DWI) in the Periferic Prostate Cancer Detection and Stratification. Med Oncol 2017;34:35. [Crossref] [PubMed]
- Abrams P. Re: Update on AUA guideline on the management of benign prostatic hyperplasia: K. T. McVary, C. G. Roehrborn, A. L. Avins, M. J. Barry, R. C. Bruskewitz, R. F. Donnell, H. E. Foster, Jr., C. M. Gonzalez, S. A. Kaplan, D. F. Penson, J. C. Ulchaker and J. T. Wei J Urol 2011; 185: 1793-1803. J Urol 2012;187:358-9; author reply 9. [Crossref] [PubMed]
- Bilhim T, Pisco J, Rio Tinto H, et al. Unilateral versus bilateral prostatic arterial embolization for lower urinary tract symptoms in patients with prostate enlargement. Cardiovasc Intervent Radiol 2013;36:403-11. [Crossref] [PubMed]
- Kluivers KB, Riphagen I, Vierhout ME, et al. Systematic review on recovery specific quality-of-life instruments. Surgery 2008;143:206-15. [Crossref] [PubMed]
- DeMeritt JS, Elmasri FF, Esposito MP, et al. Relief of benign prostatic hyperplasia-related bladder outlet obstruction after transarterial polyvinyl alcohol prostate embolization. J Vasc Interv Radiol 2000;11:767-70. [Crossref] [PubMed]
- Gao YA, Huang Y, Zhang R, et al. Benign prostatic hyperplasia: prostatic arterial embolization versus transurethral resection of the prostate--a prospective, randomized, and controlled clinical trial. Radiology 2014;270:920-8. [Crossref] [PubMed]
- Pisco JM, Rio Tinto H, Campos Pinheiro L, et al. Embolisation of prostatic arteries as treatment of moderate to severe lower urinary symptoms (LUTS) secondary to benign hyperplasia: results of short- and mid-term follow-up. Eur Radiol 2013;23:2561-72. [Crossref] [PubMed]
- Pesapane F, Nezami N, Patella F, et al. New concepts in embolotherapy of HCC. Med Oncol 2017;34:58. [Crossref] [PubMed]
- Tay KJ, Schulman AA, Sze C, et al. New advances in focal therapy for early stage prostate cancer. Expert Rev Anticancer Ther 2017;17:737-43. [Crossref] [PubMed]
- McWilliams JP, Kuo MD, Rose SC, et al. Society of Interventional Radiology position statement: prostate artery embolization for treatment of benign disease of the prostate. J Vasc Interv Radiol 2014;25:1349-51. [Crossref] [PubMed]
- A Pereira J, Bilhim T, Duarte M, et al. Patient selection and counseling before prostatic arterial embolization. Tech Vasc Interv Radiol 2012;15:270-5.
- Burnett AL, Wein AJ. Benign prostatic hyperplasia in primary care: what you need to know. J Urol 2006;175:S19-24. [Crossref] [PubMed]
- Kuang M, Vu A, Athreya S. A Systematic Review of Prostatic Artery Embolization in the Treatment of Symptomatic Benign Prostatic Hyperplasia. Cardiovasc Intervent Radiol 2017;40:655-63. [Crossref] [PubMed]
- Bhatia S. Meta-Analysis of Prostatic Artery Embolization for Benign Prostatic Hyperplasia-Review of 12-Month Outcomes Data. J Vasc Interv Radiol 2017;28:772. [Crossref] [PubMed]
- Bagla S, Smirniotopoulos JB, Orlando JC, et al. Comparative Analysis of Prostate Volume as a Predictor of Outcome in Prostate Artery Embolization. J Vasc Interv Radiol 2015;26:1832-8. [Crossref] [PubMed]
- Bilhim T, Pisco J, Pereira JA, et al. Predictors of Clinical Outcome after Prostate Artery Embolization with Spherical and Nonspherical Polyvinyl Alcohol Particles in Patients with Benign Prostatic Hyperplasia. Radiology 2016;281:289-300. [Crossref] [PubMed]
- Gabr AH, Gabr MF, Elmohamady BN, et al. Prostatic Artery Embolization: A Promising Technique in the Treatment of High-Risk Patients with Benign Prostatic Hyperplasia. Urol Int 2016;97:320-4. [Crossref] [PubMed]
- Li Q, Duan F, Wang MQ, et al. Prostatic Arterial Embolization with Small Sized Particles for the Treatment of Lower Urinary Tract Symptoms Due to Large Benign Prostatic Hyperplasia: Preliminary Results. Chin Med J (Engl) 2015;128:2072-7. [Crossref] [PubMed]
- Wang MQ, Guo LP, Zhang GD, et al. Prostatic arterial embolization for the treatment of lower urinary tract symptoms due to large (>80 mL) benign prostatic hyperplasia: results of midterm follow-up from Chinese population. BMC Urol 2015;15:33. [Crossref] [PubMed]
- Bilhim T, Tinto HR, Fernandes L, et al. Radiological anatomy of prostatic arteries. Tech Vasc Interv Radiol 2012;15:276-85. [Crossref] [PubMed]
- Bilhim T, Pereira JA, Fernandes L, et al. Angiographic anatomy of the male pelvic arteries. AJR Am J Roentgenol 2014;203:W373-82. [Crossref] [PubMed]
- Carnevale FC, Soares GR, de Assis AM, et al. Anatomical Variants in Prostate Artery Embolization: A Pictorial Essay. Cardiovasc Intervent Radiol 2017;40:1321-37. [Crossref] [PubMed]
- de Assis AM, Moreira AM, de Paula Rodrigues VC, et al. Prostatic artery embolization for treatment of benign prostatic hyperplasia in patients with prostates > 90 g: a prospective single-center study. J Vasc Interv Radiol 2015;26:87-93. [Crossref] [PubMed]
- de Assis AM, Moreira AM, de Paula Rodrigues VC, et al. Pelvic Arterial Anatomy Relevant to Prostatic Artery Embolisation and Proposal for Angiographic Classification. Cardiovasc Intervent Radiol 2015;38:855-61. [Crossref] [PubMed]
- Rampoldi A, Barbosa F, Secco S, et al. Prostatic Artery Embolization as an Alternative to Indwelling Bladder Catheterization to Manage Benign Prostatic Hyperplasia in Poor Surgical Candidates. Cardiovasc Intervent Radiol 2017;40:530-6. [Crossref] [PubMed]
- Carnevale FC, Moreira AM, Harward SH, et al. Recurrence of Lower Urinary Tract Symptoms Following Prostate Artery Embolization for Benign Hyperplasia: Single Center Experience Comparing Two Techniques. Cardiovasc Intervent Radiol 2017;40:366-74. [Crossref] [PubMed]
- Anjum I, Khan MA, Aadil M, et al. Transradial vs. Transfemoral Approach in Cardiac Catheterization: A Literature Review. Cureus 2017;9:e1309. [PubMed]
- Bhatia S, Harward SH, Sinha VK, et al. Prostate Artery Embolization via Transradial or Transulnar versus Transfemoral Arterial Access: Technical Results. J Vasc Interv Radiol 2017;28:898-905. [Crossref] [PubMed]
- Amouyal G, Pellerin O, Del Giudice C, et al. Bilateral Arterial Embolization of the Prostate Through a Single Prostatic Artery: A Case Series. Cardiovasc Intervent Radiol 2017;40:780-7. [Crossref] [PubMed]
- Yu SCH, Cho C, Hung E, et al. Case-Control Study of Intra-arterial Verapamil for Intraprostatic Anastomoses to Extraprostatic Arteries in Prostatic Artery Embolization for Benign Prostatic Hypertrophy. J Vasc Interv Radiol 2017;28:1167-76. [Crossref] [PubMed]
- Carnevale FC, Moreira AM, Antunes AA. The "PErFecTED technique": proximal embolization first, then embolize distal for benign prostatic hyperplasia. Cardiovasc Intervent Radiol 2014;37:1602-5. [Crossref] [PubMed]
- Carnevale FC, Iscaife A, Yoshinaga EM, et al. Transurethral Resection of the Prostate (TURP) Versus Original and PErFecTED Prostate Artery Embolization (PAE) Due to Benign Prostatic Hyperplasia (BPH): Preliminary Results of a Single Center, Prospective, Urodynamic-Controlled Analysis. Cardiovasc Intervent Radiol 2016;39:44-52. [Crossref] [PubMed]
- Wang MQ, Duan F, Yuan K, et al. Benign Prostatic Hyperplasia: Cone-Beam CT in Conjunction with DSA for Identifying Prostatic Arterial Anatomy. Radiology 2017;282:271-80. [Crossref] [PubMed]
- Tacher V, Radaelli A, Lin M, et al. How I do it: Cone-beam CT during transarterial chemoembolization for liver cancer. Radiology 2015;274:320-34. [Crossref] [PubMed]
- Lucatelli P, Corona M, Argiro R, et al. Impact of 3D Rotational Angiography on Liver Embolization Procedures: Review of Technique and Applications. Cardiovasc Intervent Radiol 2015;38:523-35. [Crossref] [PubMed]
- Ierardi AM, Pesapane F, Rivolta N, et al. Type 2 endoleaks in endovascular aortic repair: cone beam CT and automatic vessel detection to guide the embolization. Acta Radiol 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Chiaradia M, Radaelli A, Campeggi A, et al. Automatic three-dimensional detection of prostatic arteries using cone-beam CT during prostatic arterial embolization. J Vasc Interv Radiol 2015;26:413-7. [Crossref] [PubMed]
- Bagla S, Martin CP, van Breda A, et al. Early results from a United States trial of prostatic artery embolization in the treatment of benign prostatic hyperplasia. J Vasc Interv Radiol 2014;25:47-52. [Crossref] [PubMed]
- Bagla S, Rholl KS, Sterling KM, et al. Utility of cone-beam CT imaging in prostatic artery embolization. J Vasc Interv Radiol 2013;24:1603-7. [Crossref] [PubMed]
- Goncalves OM, Carnevale FC, Moreira AM, et al. Comparative Study Using 100-300 Versus 300-500 mum Microspheres for Symptomatic Patients Due to Enlarged-BPH Prostates. Cardiovasc Intervent Radiol 2016;39:1372-8. [Crossref] [PubMed]
- Carnevale FC, Antunes AA, da Motta Leal Filho JM, et al. Prostatic artery embolization as a primary treatment for benign prostatic hyperplasia: preliminary results in two patients. Cardiovasc Intervent Radiol 2010;33:355-61. [Crossref] [PubMed]
- Bilhim T, Pisco J, Campos Pinheiro L, et al. Does polyvinyl alcohol particle size change the outcome of prostatic arterial embolization for benign prostatic hyperplasia? Results from a single-center randomized prospective study. J Vasc Interv Radiol 2013;24:1595-602.e1. [Crossref] [PubMed]
- Golzarian J, Antunes AA, Bilhim T, et al. Prostatic artery embolization to treat lower urinary tract symptoms related to benign prostatic hyperplasia and bleeding in patients with prostate cancer: proceedings from a multidisciplinary research consensus panel. J Vasc Interv Radiol 2014;25:665-74. [Crossref] [PubMed]
- Bhatia S, Sinha V, Bordegaray M, et al. Role of Coil Embolization during Prostatic Artery Embolization: Incidence, Indications, and Safety Profile. J Vasc Interv Radiol 2017;28:656-64.e3. [Crossref] [PubMed]
- Sacks D, McClenny TE, Cardella JF, et al. Society of Interventional Radiology clinical practice guidelines. J Vasc Interv Radiol 2003;14:S199-202. [Crossref] [PubMed]
- Uflacker A, Haskal ZJ, Bilhim T, et al. Meta-Analysis of Prostatic Artery Embolization for Benign Prostatic Hyperplasia. J Vasc Interv Radiol 2016;27:1686-97.e8. [Crossref] [PubMed]
- Pisco J, Campos Pinheiro L, Bilhim T, et al. Prostatic arterial embolization for benign prostatic hyperplasia: short- and intermediate-term results. Radiology 2013;266:668-77. [Crossref] [PubMed]
- Pisco JM, Bilhim T, Pinheiro LC, et al. Medium- and Long-Term Outcome of Prostate Artery Embolization for Patients with Benign Prostatic Hyperplasia: Results in 630 Patients. J Vasc Interv Radiol 2016;27:1115-22. [Crossref] [PubMed]
- Camara-Lopes G, Mattedi R, Antunes AA, et al. The histology of prostate tissue following prostatic artery embolization for the treatment of benign prostatic hyperplasia. Int Braz J Urol 2013;39:222-7. [Crossref] [PubMed]
- Bhatia S, Sinha VK, Kava BR, et al. Efficacy of Prostatic Artery Embolization for Catheter-Dependent Patients with Large Prostate Sizes and High Comorbidity Scores. J Vasc Interv Radiol 2018;29:78-84.e1. [Crossref] [PubMed]
- Carnevale FC, da Motta-Leal-Filho JM, Antunes AA, et al. Quality of life and clinical symptom improvement support prostatic artery embolization for patients with acute urinary retention caused by benign prostatic hyperplasia. J Vasc Interv Radiol 2013;24:535-42. [Crossref] [PubMed]


