Investigation of antiparkinsonian activity of new imidazole-4,5-dicarboxylic acid derivatives on the experimental model of catalepsy
DOI:
https://doi.org/10.18413/rrpharmacology.9.10006Abstract
Introduction: To study the antiparkinsonian activity of new ligands of the glutamate NMDA receptor complex – 1,2–substituted imidazole-4,5-dicarboxylic acids – on an experimental model of catalepsy caused by haloperidolintraabdominal injections in rats.
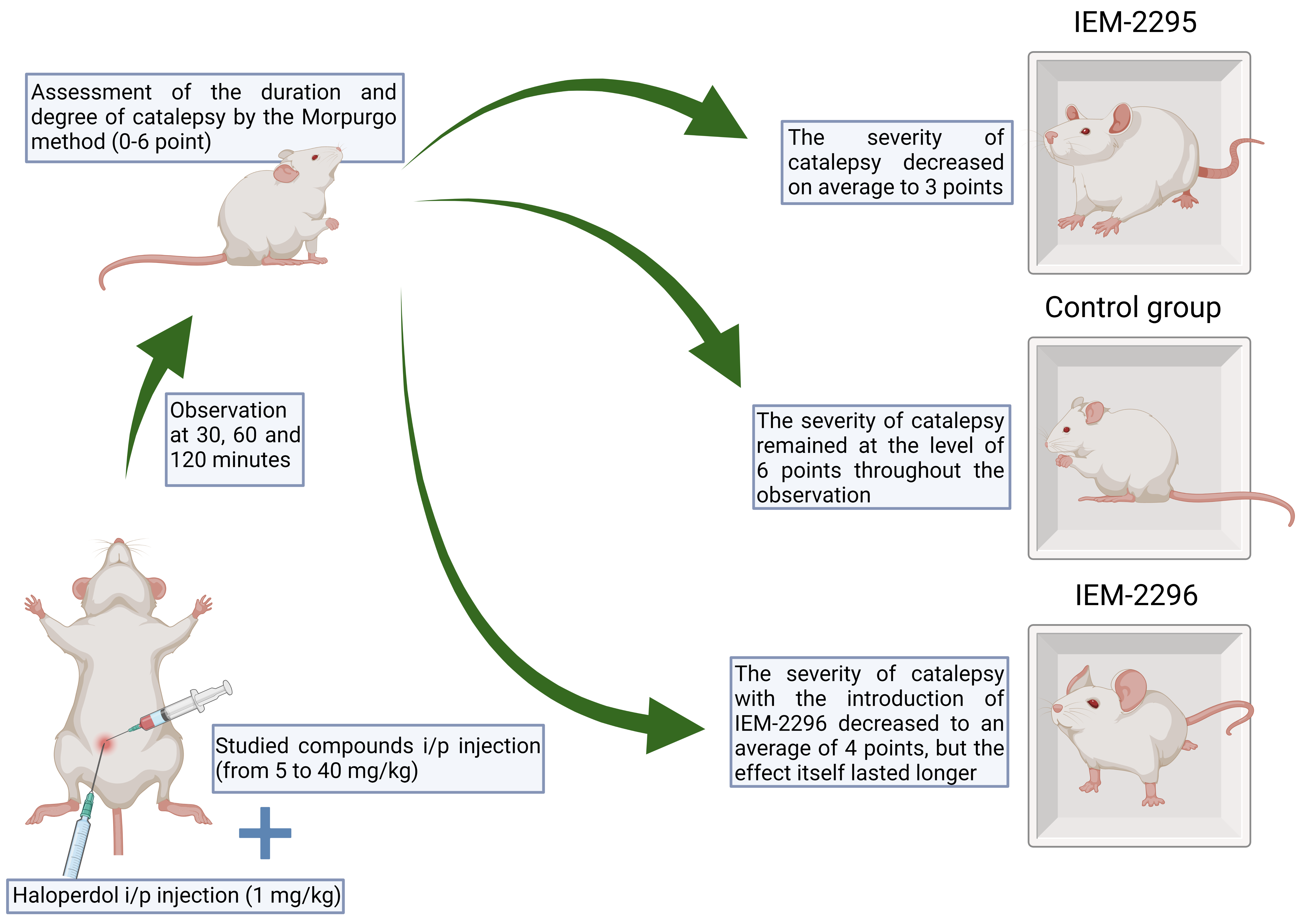
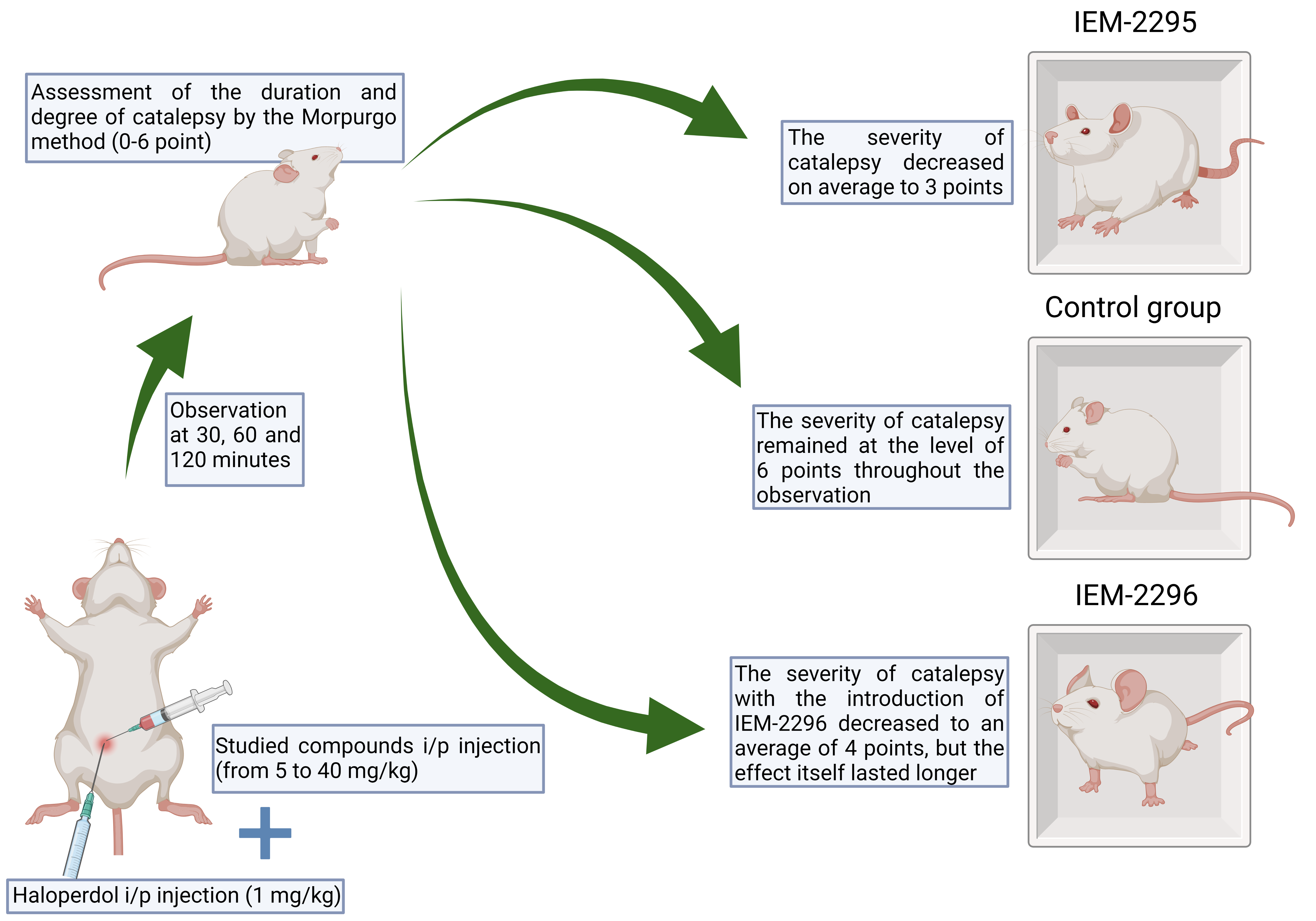
Materials and methods: The experiments were performed on Wistar rats weighing 300-350 g, obtained from the Rappolovo nursery of the Russian Academy of Medical Sciences (Leningrad Region). The animals were kept in standard plastic cages in vivarium conditions with free access to water and food at a temperature of 22±2 °C and in the experiment were divided into several groups (6 animals each). All the experiments were carried out in the autumn-winter period. The animals were kept in accordance with the rules of laboratory practice (GLP), regulatory documents ”Sanitary Rules for the Device, Equipment and Maintenance of Vivarium” and the Order of the Ministry of Health and Social Development of the Russian Federation dated 23.08.2010 No. 708n “On Approval of the Rules of Laboratory Practice”. Imidazole-dicarboxylic acid derivatives (IEM-2295, IEM-2296) were injected intraperitoneally at doses from 5 mg/kg to 40 mg/kg simultaneously with haloperidol at a dose of 1 mg/kg, after which the duration and severity of catalepsy were evaluated after 30, 60, 120 minutes from 0 to 6 points according to the Morpurgo method.
Results: The severity of catalepsy with the injection of IEM-2295 decreased on average to 3 points, while in the control group it remained at the level of 6 points throughout the observation. However, the severity of catalepsy with the introduction of IEM-2296 decreased to an average of 4 points, but the effect itself lasted longer than with the introduction of IEM-2295. Thus, it was noted that by the 120th minute of observation, the severity of catalepsy in rats receiving the IEM-2295 compound averaged 5 points, whereas in animals receiving IEM-2296 – 3 points.
Discussion: Basing on the results of our work and similar experiments, we can conclude that the studied compounds, which are not channel blockers, have an active effect on dopaminergic neurotransmission, because of which the symptoms of catalepsy that occur when haloperidol is injected to rats were stopped to one degree or another.
Conclusion: The studied substances exhibit antiparkinsonian activity on an experimental haloperidol model of catalepsy in rats and are promising for development as potential therapies for neurodegenerative diseases. Further study of these compounds and other ligands from the NMDA-blocker group in a wider sample on the catalepsy model, as well as on other models of Parkinsonism, is required.
Graphical Abstract
Graphical Abstract
Keywords:
NMDA receptor antagonists, haloperidol, dopamine, parkinsonismReferences
Aradi SD, Hauser RA (2020) Medical management and prevention of motor complications in Parkinson’s disease. Neurotherapeutics 17(4): 1339–1365. https://doi.org/10.1007/s13311-020-00889-4 [PubMed] [PMC]
Bhattacharya S, Ma Y, Dunn AR, Bradner JM, Scimemi A, Miller GW, Traynelis SF, Wichmann T (2018) NMDA receptor blockade ameliorates abnormalities of spike firing of subthalamic nucleus neurons in a parkinsonian nonhuman primate. Journal of Neuroscience Research 96(7): 1324–1335. https://doi.org/10.1002/jnr.24230 [PubMed] [PMC]
Cárcel L, De la Casa LG (2021) Temporal factors modulate haloperidol-induced conditioned catalepsy. Frontiers in Behavioral Neuroscience 15: 713512. https://doi.org/10.3389/fnbeh.2021.713512 [PubMed] [PMC]
Chia SJ, Tan E-K, Chao Y-X (2020) Historical perspective: Models of Parkinson’s disease. International Journal of Molecular Sciences 21(7): 2464. https://doi.org/10.3390/ijms21072464 [PubMed] [PMC]
Chou KL, Stacy M, Simuni T, Miyasaki J, Oertel WH, Sethi K, Fernandez HH, Stocchi F (2018) The spectrum of “off” in Parkinson’s disease: What have we learned over 40 years? Parkinsonism & Related Disorders 51: 9–16. https://doi.org/10.1016/j.parkreldis.2018.02.001 [PubMed]
Christoffersen CL, Meltzer LT (1995) Evidence for N-methyl-d-aspartate and AMPA subtypes of the glutamate receptor on substantia nigra dopamine neurons: Possible preferential role for N-methyl-d-aspartate receptors. Neuroscience 67(2): 373–381. https://doi.org/10.1016/0306-4522(95)00047-M [PubMed]
Cieślik P, Woźniak M, Tokarski K, Kusek M, Pilc A, Płoska A, Radulska A, Pelikant-Małecka I, Żołnowska B, Sławiński J, Kalinowski L, Wierońska JM (2019) Simultaneous activation of muscarinic and GABAB receptors as a bidirectional target for novel antipsychotics. Behavioural Brain Research 359: 671–685. https://doi.org/10.1016/j.bbr.2018.09.019 [PubMed]
Danysz W, Parsons CG, Kornhuber J, Schmidt WJ, Quack G (1997) Aminoadamantanes as NMDA receptor antagonists and antiparkinsonian agents – preclinical studies. Neuroscience & Biobehavioral Reviews 21(4): 455–468. https://doi.org/10.1016/S0149-7634(96)00037-1 [PubMed]
Dergachev VD, Yakovleva EE, Brusina MA, Bychkov ER, Piotrovskiy LB, Shabanov PD (2021) Antiparkinsonian activity of new N-methyl-D-aspartate receptor ligands in the arecoline hyperkinesis test. Medical Council [Meditsinskiy Sovet] 12: 406–412. https://doi.org/10.21518/2079-701X-2021-12-406-412
Draoui A, El Hiba O, Aimrane A, El Khiat A, Gamrani H (2020) Parkinson’s disease: From bench to bedside. Revue Neurologique 176(7-8): 543–559. https://doi.org/10.1016/j.neurol.2019.11.002 [PubMed]
Rascol O, Negre-Pages L, Damier P, Delval A, Derkinderen P, Destée A, Fabbri M, Meissner WG, Rachdi A, Tison F, Perez-Lloret S; COPARK Study Group (2020) Utilization patterns of amantadine in parkinson’s disease patients enrolled in the french COPARK study. Drugs & Aging 37(3): 215–223. https://doi.org/10.1007/s40266-019-00740-2 [PubMed]
Fox SH, Katzenschlager R, Lim S-Y, Barton B, de Bie RMA, Seppi K, Coelho M, Sampaio C, on behalf of the Movement Disorder Society Evidence-Based Medicine Committee (2018) International Parkinson and movement disorder society evidence-based medicine review: Update on treatments for the motor symptoms of Parkinson’s disease. Movement Disorders 33(8): 1248–1266. https://doi.org/10.1002/mds.27372 [PubMed]
Groc L, Choquet D (2020) Linking glutamate receptor movements and synapse function. Science 368(6496): eaay4631. https://doi.org/10.1126/science.aay4631 [PubMed]
Haas KT, Compans B, Letellier M, Bartol TM, Grillo-Bosch D, Sejnowski TJ, Sainlos M, Choquet D, Thoumine O, Hosy E (2018) Pre-post synaptic alignment through neuroligin-1 tunes synaptic transmission efficiency. eLife 7: e31755. https://doi.org/10.7554/eLife.31755 [PubMed] [PMC]
Iakovleva EE, Brusina MA, Bychkov ER, Piotrovsky LB, Shabanov PD (2020) Antiparkinsonian activity of new ligands of the glutamate NMDA-receptor complex – imidazole-4,5-dicarboxylic acid derivatives. Bulletin of the Smolensk State Medical Academy [Vestnik Smolenskoi Gosudarstvennoi Meditsinskoi Akademii] 19: 41–47. https://doi.org/10.37903/vsgma.2020.3.5 [in Russian]
Jankovic J, Tan EK (2020) Parkinson’s disease: etiopathogenesis and treatment. Journal of Neurology, Neurosurgery & Psychiatry 91(8): 795–808. https://doi.org/10.1136/jnnp-2019-322338 [PubMed]
Kabra A, Baghel US, Hano C, Martins N, Khalid M, Sharma R (2020) Neuroprotective potential of Myrica esulenta in Haloperidol induced Parkinson’s disease. Journal of Ayurveda and Integrative Medicine 11(4): 448–454. https://doi.org/10.1016/j.jaim.2020.06.007 [PubMed] [PMC]
Kulisevsky J, Oliveira L, Fox SH (2018) Update in therapeutic strategies for Parkinson’s disease. Current Opinion in Neurology 31(4): 439–447. https://doi.org/10.1097/WCO.0000000000000579 [PubMed]
Li B-D, Bi Z-Y, Liu J-F, Si W-J, Shi Q-Q, Xue L-P, Bai J (2017) Adverse effects produced by different drugs used in the treatment of Parkinson’s disease: A mixed treatment comparison. CNS Neuroscience & Therapeutics 23(10): 827–842. https://doi.org/10.1111/cns.12727 [PubMed] [PMC]
Mellone M, Gardoni F (2018) Glutamatergic mechanisms in l-DOPA-induced dyskinesia and therapeutic implications. Journal of Neural Transmission 125(8): 1225–1236. https://doi.org/10.1007/s00702-018-1846-8 [PubMed]
Mironov AN, Bunjatjan ND, Vasil’ev AN, Verstakova OL, Zhuravleva MV, Lepakhin VK (2012) Vol. 1 Guidelines for conducting preclinical research of medicines. Moscow: Grif and K, 944 pp. [in Russian]
Mironova YuS, Zhukova NG, Zhukova IA, Alifirova VM, Izhboldina OP, Latypova AV (2018) Parkinson’s disease and glutamatergic system. Korsakov Journal of Neurology and Psychiatry [Zhurnal Nevrologii i Psikhiatrii im. S.S. Korsakova] 118: 138. https://doi.org/10.17116/jnevro201811851138 [in Russian]
Müller T, Kuhn W, Möhr J-D (2019) Evaluating ADS5102 (amantadine) for the treatment of Parkinson’s disease patients with dyskinesia. Expert Opinion on Pharmacotherapy 20(10): 1181–1187. https://doi.org/10.1080/14656566.2019.1612365 [PubMed]
Nuzzo T, Punzo D, Devoto P, Rosini E, Paciotti S, Sacchi S, Li Q, Thiolat M-L, Véga C, Carella M, Carta M, Gardoni F, Calabresi P, Pollegioni L, Bezard E, Parnetti L, Errico F, Usiello A (2019) The levels of the NMDA receptor co-agonist D-serine are reduced in the substantia nigra of MPTP-lesioned macaques and in the cerebrospinal fluid of Parkinson’s disease patients. Scientific Reports 9(1): 8898. https://doi.org/10.1038/s41598-019-45419-1 [PubMed] [PMC]
Perez-Lloret S, Rascol O (2018) Efficacy and safety of amantadine for the treatment of l-DOPA-induced dyskinesia. Journal of Neural Transmission 125(8): 1237–1250. https://doi.org/10.1007/s00702-018-1869-1 [PubMed]
Poewe W, Seppi K, Tanner CM, Halliday GM, Brundin P, Volkmann J, Schrag A-E, Lang AE (2017) Parkinson's disease. Nature Reviews Disease Primers 3: 17013. https://doi.org/10.1038/nrdp.2017.13 [PubMed]
Ramírez-Jarquín UN, Shahani N, Pryor W, Usiello A, Subramaniam S (2020) The mammalian target of rapamycin (mTOR) kinase mediates haloperidol-induced cataleptic behavior. Translational Psychiatry 10(1): 336. https://doi.org/10.1038/s41398-020-01014-x [PubMed] [PMC]
Schwab RS (1969) Amantadine in the treatment of Parkinson’s disease. JAMA: The Journal of the American Medical Association 208(7): 1168. https://doi.org/10.1001/jama.1969.03160070046011 [PubMed]
Standaert DG, Testa CM, Young AB, Penney JB (1994) Organization of N-methyl-D-aspartate glutamate receptor gene expression in the basal ganglia of the rat. The Journal of Comparative Neurology 343(1): 1–16. https://doi.org/10.1002/cne.903430102 [PubMed]
Szydlowska K, Tymianski M (2010) Calcium, ischemia and excitotoxicity. Cell Calcium 47(2): 122–129. https://doi.org/10.1016/j.ceca.2010.01.003 [PubMed]
Tarakad A (2020) Clinical rating scales and quantitative assessments of movement disorders. Neurologic Clinics 38(2): 231–254. https://doi.org/10.1016/j.ncl.2019.12.001 [PubMed]
Ugale V, Dhote A, Narwade R, Khadse S, Reddy PN, Shirkhedkar A (2021) GluN2B/N-methyl-D-aspartate receptor antagonists: advances in design, synthesis, and pharmacological evaluation studies. CNS & Neurological Disorders – Drug Targets 20(9): 822–862. https://doi.org/10.2174/1871527320666210309141627 [PubMed]
Vanle B, Olcott W, Jimenez J, Bashmi L, Danovitch I, IsHak WW (2018) NMDA antagonists for treating the non-motor symptoms in Parkinson’s disease. Translational Psychiatry 8(1): 117. https://doi.org/10.1038/s41398-018-0162-2[PubMed] [PMC]
Vecchia DD, Kanazawa LKS, Wendler E, Hocayen P de AS, Vital MABF, Takahashi RN, Da Cunha C, Miyoshi E, Andreatini R (2021) Ketamine reversed short-term memory impairment and depressive-like behavior in animal model of Parkinson’s disease. Brain Research Bulletin 168: 63–73. https://doi.org/10.1016/j.brainresbull.2020.12.011 [PubMed]
Vieira M, Yong XLH, Roche KW, Anggono V (2020) Regulation of NMDA glutamate receptor functions by the GluN2 subunits. Journal of Neurochemistry 154(2): 121–143. https://doi.org/10.1111/jnc.14970 [PubMed] [PMC]
Wang R, Reddy PH (2017) Role of glutamate and NMDA receptors in Alzheimer’s disease. Journal of Alzheimer’s Disease 57(4): 1041–1048. https://doi.org/10.3233/JAD-160763 [PubMed] [PMC]
Wolosker H, Balu DT, Coyle JT (2016) The rise and fall of the d-serine-mediated gliotransmission hypothesis. Trends in Neurosciences 39(11): 712–721. https://doi.org/10.1016/j.tins.2016.09.007 [PubMed] [PMC]
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Research Results in Pharmacology

This work is licensed under a Creative Commons Attribution 4.0 International License.
 Русский
Русский
 English
English

