-
PDF
- Split View
-
Views
-
Cite
Cite
Anna-Marie Benson, Kevin Winker, Fat-Deposition Strategies Among High-Latitude Passerine Migrants, The Auk, Volume 122, Issue 2, 1 April 2005, Pages 544–557, https://doi.org/10.1093/auk/122.2.544
Close - Share Icon Share
Abstract
We studied fat stores in passerine migrants at a high-latitude site in Fairbanks, Alaska (64°50'N, 147°50'W). We examined fat-deposition strategies during the final (spring) and initial (autumn) stages of long-distance migration, 1992–1998, to (1) improve understanding of geographic fat-deposition patterns by adding a high-latitude perspective; (2) determine whether there are age-related differences in fat-deposition strategies in autumn; and (3) test the “spring fatter” hypothesis of seasonal fat-deposition, which suggests that migrants should carry more fat in spring when they near their breeding areas than in autumn when they depart. Our analyses examined factors affecting daily fat scores during migration and compared between-season differences in fat stores among a total of 18,685 individuals of 16 migrant species. In autumn, adults had higher visible subcutaneous fat scores than immatures in 11 of 16 species. However, in all but two species, those differences were attributable to the effects of overnight low temperature, day length, and time of day, rather than age, probably because of later departures by adults. Fat scores were higher in autumn than in spring in 6 of 16 species, and body-condition indices were higher in autumn in 5 of 16 species. Only one species showed higher fat scores in spring, but that difference was not reflected in a seasonal comparison of body- condition indices. No species arrived with high fat loads in spring, and generally low fat levels in autumn suggest that high-latitude passerine migrants in North America are paying most of the energetic costs of long-distance migration with resources obtained en route to their wintering grounds. Among passerine migrants near these high-latitude breeding grounds, seasonal fat-deposition strategies appear to be responding to energetic needs at the level of daily maintenance, rather than to hypothesized insurance needs in spring or to the forthcoming needs of a long- distance migration in autumn.
Résumé
Stratégies de Constitution de Réserves de Graisse chez des Passereaux Migrants à des Hautes Latitudes
Nous avons étudié les réserves de graisse chez des passereaux migrateurs sur un site situé à une haute latitude à Fairbanks, Alaska (64°50'N, 147°50'W). Nous avons étudié les stratégies de constitution de réserves de graisse lors des haltes finales (printemps) et initiales (automne) survenant au cours de longues migrations entre 1992 et 1998. Les objectifs de cette étude était (1) d'améliorer la compréhension des patrons géographiques pour la constitution de réserves de graisse en considérant la perspective d'une étude à haute latitude; (2) de déterminer s'il y a des différences dans les stratégies de constitution de réserves de graisse en fonction de l'âge au cours de l'automne; et (3) de tester l'hypothèse “spring fatter” (“plus gras au printemps”) de constitution de réserves de graisse saisonnières. Cette hypothèse suggère que les migrateurs devraient posséder plus de graisse au printemps, quand ils sont proches de leurs aires de reproduction, que pendant l'automne quand ils partent. Nos analyses ont examiné les facteurs qui affectent les réserves journalières en graisse au cours de la migration et comparé les différences inter-saisonnières en réserves de graisse parmi un total de 18 685 individus appartenant à 16 espèces migratrices. À l'automne, les adultes avaient des réserves apparentes de graisse plus importantes que les immatures chez 11 des 16 espèces étudiées. Néanmoins, pour toutes les autres espèces (sauf deux), ces différences étaient attribuables aux effets de la basse température au cours de la nuit, de la longueur du jour, et le moment de la journée, plutôt que l'âge, probablement en raison du départ plus tardif des adultes. Les dépôts de graisse étaient plus importants à l'automne pour 5 des 16 espèces. Seule une espèce a montré des dépôts de graisse plus importants au printemps, mais cette différence n'a pas été retrouvée dans une comparaison saisonnière des indices de conditions corporelles. Aucune espèce n'est arrivée avec des réserves de graisse élevées au printemps. Généralement, les faibles niveaux de graisse à l'automne suggèrent que les passereaux migrants à de hautes latitudes en Amérique du Nord assument la plupart des coûts énergétiques encourus au cours des longues migrations grâce à des ressources obtenues lors de leur voyage vers les aires d'hivernage. Parmi les passereaux migrants vers les aires de nidification situées à ces hautes latitudes, les stratégies saisonnières de constitution de réserves de graisse semblent répondre aux besoins énergétiques requis par la demande journalière, plutôt qu'aux besoins complémentaires et hypothétiques au printemps et lors de la longue migration automnale.
Long-distance migration is energetically expensive (Berthold 1975, Blem 1990). During migration, metabolic rates for some passerines can be 6–8x the normal resting rate (Hussell 1969). Those energetic demands are satisfied by fat deposited before and during the migratory period (e.g. Nisbet et al. 1963, Mueller and Berger 1966, Berthold 1975, Cherry 1982, Blem 1990). Although fat deposition has been documented at stopover sites, continental patterns of fat deposition in Nearctic-Neotropic migrants are not well understood. Understanding the geographic and temporal patterns of migratory fat-deposition could elucidate broad patterns of migration strategies and the selective pressures associated with continental migration systems.
Fattening strategies employed during migration vary among species in rate of fat deposition and in amount of fat stored. Strategies may be influenced by body size, foraging habits, season, distances between breeding and nonbreeding ranges, and stopover location in relation to each end of the migratory journey (King et al. 1963; Winker et al. 1992a, b; Winker 1995; Berthold 1996; Sandberg 1996; Pilastro and Spina 1997; Farmer and Wiens 1999; Katti and Price 1999). Independently of migration, fat stores of passerines can also vary in response to daily environmental variables, including temperature and photoperiod (Lehikoinen 1987, Lehikoinen and Hakala 1988, Bednekoff et al. 1994, Bednekoff and Krebs 1995). During migration, many passerines increase fat stores during daylight hours at stopover sites (Cherry 1982, Blem 1990, Winker et al. 1992b, Winker 1995, Morris et al. 1996, Dunn 2002, Jones et al. 2002). Seasonal differences in fat reserves are partly endogenously programmed in circannual patterns (Gwinner 1990). For example, some migrants undergo hyperphagia, the principal mechanism of premigratory fattening, at predictable times in the annual cycle (Berthold 1975).
Here, we examine fat-deposition strategies among passerine migrants just before arrival on and just after departure from high-latitude breeding areas. Our purposes are threefold:
(1) To gain a better understanding of geographic patterns of fat deposition among Nearctic-Neotropic migrants by adding a high-latitude perspective. The fat loads that migrants carry at high latitudes can illuminate how resources are used on and near the breeding grounds to pay the energetic costs of long- distance migration.
(2) To determine whether there are age-related differences in fat-deposition strategies among high-latitude migrants in autumn. In that season, adults tend to depart the breeding grounds later than immatures, probably because of the more extensive prebasic molt that adults undergo at that time (Benson and Winker 2001).
(3) To test the “spring fatter” hypothesis of seasonal fat-deposition, developed from studies at lower latitudes (Winker et al. 1992a, Sandberg 1996, Sandberg and Moore 1996). That hypothesis suggests that migrants should carry more fat in spring when they near their breeding areas than in autumn when they leave those same areas, because in spring, passerine migrants are usually approaching a temporal barrier of resource availability. Given time, resources on their breeding areas will become predictably available. Until resource availability becomes certain, however, carrying extra fat onto the breeding grounds to prevent starvation or to potentially gain reproductive benefits (e.g. through territory defense, gonadal development, or accelerated initiation of breeding) would seem to be advantageous. Fat stores in passerines can provide enough energy to maintain birds during mornings when food is scant (Ketterson and Nolan 1978) and have been estimated to ensure survival for up to four days (Blem 1976). In contrast, autumn migrants are departing their breeding areas, having just completed their prebasic molt (also energetically costly), and they are headed generally southward into areas of longer growing seasons and presumably greater resource predictability.
Given those seasonally different energetic scenarios, and that there are costs to carrying fat (Witter and Cuthill 1993, Sandberg and Moore 1996), the “spring fatter” hypothesis has intuitive appeal. Higher fat stores in spring as compared with other seasons have been documented at many locations in Canada and the United States (King et al. 1963, Biermann and Sealy 1984, Winker et al. 1992a, Dunn 2002). Passerines are also known to arrive at some high-latitude breeding ranges with high fat levels (Sandberg 1996, Fransson and Jakobsson 1998). Testing the “spring fatter” hypothesis requires the study of populations near breeding grounds that lie at the beginning and end of long-distance migrations, far from physical (as opposed to temporal) ecological barriers to migration, such as oceans and deserts, and which themselves require larger fat stores for successful passage.
Methods
Study area
The present research is part of the most intensive standardized mist-netting study ever conducted in subarctic North America. Mist nets were operated by the Alaska Bird Observatory at Creamer's Field Migration Station (CFMS), in Fairbanks, Alaska (64°50'N, 147°50'W), ≈10 km from the confluence of the Chena and Tanana rivers, at 130 m elevation. The Tanana Valley is a well-documented migration corridor (Kessel 1984, Cooper and Ritchie 1995, McIntyre and Ambrose 1999). Encompassing ≈20 ha, CFMS provides a good representation of the common habitat types that occur within the boreal forest of interior Alaska.
Data collection
A standardized netting protocol was used at CFMS from 1992 to 1998, employing an array of 22–50 standard mist nets (30-mm mesh, 2.6 × 12 m). Nets were arranged in a north-south direction, perpendicular to the migration corridor through the Tanana Valley. During spring migration (25 April–15 June), nets were operated daily from 0600 to 1300 hours (Alaska Daylight Savings Time). During autumn migration (15 July–30 September), net-opening times varied with sunrise (e.g. 0600 hours on 15 July and 0730 hours on 30 September), and nets were closed seven hours later. Sampling at the end of each season (10–15 June and 25–30 September) occurred every other day. Birds were banded with U.S. federal bands, and data were collected to determine age (autumn only, using degree of skull ossification), body mass, wing length (chord), and breeding condition (spring only). During autumn, an estimate of the proportion of juvenal plumage on each immature was recorded.
We used ordinally scored subcutaneous fat deposits as an indicator of total body fat, employing an eight-class scoring system modified from Helms and Drury (1960): 0 = no fat observed on furculum or abdomen; 1 = trace of fat on furculum and abdomen; 2 = thin layer of fat on furculum and abdomen; 3 = half of furculum fat-filled, and fat covering most of abdomen; 4 = fat on furculum level with clavicles and slightly mounded on abdomen; 5 = fat slightly bulging on furculum and well mounded on abdomen; 6 = fat greatly bulging in furculum and mounded on abdomen; 7 = large fat pads from furculum and abdomen meet. Using fat-score data is a fairly reliable method for indexing total body fat of passerines if observer bias is limited (Krementz and Pendleton 1990; Rogers 1991, 2003). We minimized observer bias by intensively training field staff on thousands of birds. Although no formal measures of observer bias were recorded, staff frequently compared fat-score estimates to ensure consistency. Additionally, ≈75% of the data were collected by three individuals whose tenure at the bird observatory overlapped by at least two years each, further enhancing standardization.
Data analysis
To ensure independence of records, we used only initial captures of birds. Because our autumn netting period began before the initiation of autumn migration in most species, we excluded individuals that were identifiable at first capture as nonmigrants (i.e. females with incubation patches and first-year birds with >30% of their bodies in juvenal plumage; Benson and Winker 2001). We also excluded all individuals that were molting flight feathers and birds recaptured >7 days after their first capture. Several species breed at our study site and are captured throughout summer banding: Swainson's Thrush, American Robin, Orange-crowned Warbler, Yellowrumped Warbler, Yellow Warbler, Savannah Sparrow, Lincoln's Sparrow, White-crowned Sparrow, and Dark-eyed Junco (scientific names are given in Table 1). For these species, we defined a species-specific migration window, whereby we removed the last 5% of captures during spring migration and the first 5% during autumn migration (Table 1). That date-defined migration window was not applied to species that do not breed at our study site, because of the rapid onset and intense nature of migration at high latitudes. That is, birds captured early in our netting period during autumn or late in the netting period during spring with no evidence of breeding or molting could be migrants. Further, for each species, ≥95% of individuals in the analysis fell into clear distributional peaks for each migration season. Although there is potential for a few “floaters” (birds that are not migrating or breeding) in our data set, their removal would not greatly affect our results, and their inclusion in both seasons might indeed balance the potential effects of inadvertent inclusion.
Migration distance, timing of migration, and relationship between fat score and body condition index (ratio of mass to wing chord) of passerine migrants in Fairbanks, Alaska (1992–1998)
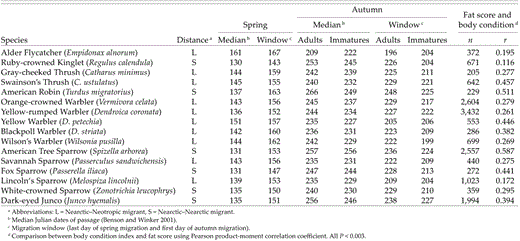
Migration distance, timing of migration, and relationship between fat score and body condition index (ratio of mass to wing chord) of passerine migrants in Fairbanks, Alaska (1992–1998)

Because fat-score data are ordinal, we used Mann-Whitney U-tests to examine differences in fat levels between spring and autumn and, in autumn, between adults and immatures. Although the median is the appropriate measure of central tendency for fat-score data (Hailman 1965), the mean is also presented for comparisons with other studies that reported only means.
In addition to using ordinal fat scores, we calculated an index of body condition using mass (in grams) divided by wing chord (in millimeters). Because factors other than season could affect fat deposition (e.g. temperature, night length, time of capture), a parametric measure (ratio of mass to wing) was required to determine how each factor affected mass gain at this site. In wood warblers, when body mass is standardized for body size, it is a reasonable predictor of an individual's fat content (Rogers and Odum 1964). Because recent studies indicate that fat stores may vary independently of mass gain (e.g. Katti and Price 1999, Piersma et al. 1999, McWilliams and Karasov 2001), we examined the relationship between fat score and body-condition index in autumn (when sample sizes were sufficiently large). The strong positive relationship found in all 16 species (Table 1) legitimizes use of the condition index as a correlate of fat for those populations, and we therefore use both measures in our analyses.
Analysis of covariance (ANCOVA) was used to examine age-related differences in body condition; age was used as a main factor, and time of capture, night length, and daily low temperature (°C) were incorporated as covariates. That is, low temperature on the date the bird was captured (usually the night before), night length on the date the bird was captured (data from the Alaska Climate Research Center; see Acknowledgments), and time of day when the bird was captured were recorded for each individual. We did not include Julian date as a covariate, because it is highly correlated with night length at this location (r = 0.99, P < 0.0001 during autumn); therefore, the effects of night length could be used as a surrogate for Julian date. For species with significant age-related differences in body condition, we tested for year effects using ANCOVA with year and age as main factors and time of day, daily low temperature, and night length as covariates.
Seasonal differences in body-condition indices were evaluated using ANCOVA with season as the main factor and time of capture, daily low temperature, and night length as covariates. For species with significant differences in body condition between seasons, we determined whether those differences were attributable to year effects by using season and year as main factors and time of day, daily low temperature, and night length as covariates in ANCOVA models. Year of capture was not included as a main factor for all species, because we did not have sufficient sample sizes in all years. All analyses were conducted using SPSS (Chicago, Illinois), and each of those tests was conducted on 16 species. The overall rate of making any Type I error (rejection of a true null hypothesis) can be controlled by applying the Dunn-Sidák correction to maintain an overall, study-wide α = 0.05 (Sokal and Rohlf 1995). Using that method, P-values less than 0.0032 are considered significant. That is a conservative approach for independent tests, however, increasing the likelihood of making Type II errors (accepting a false null hypothesis) on individual tests (Sokal and Rohlf 1995). Because we are testing broad hypotheses among multiple species, we chose the conservative approach in general interpretation, but we report individual tests at both levels of α (0.05 for individual independent tests and 0.003 for the overall, study-wide error) to enable species-level comparisons with future studies.
Results
Age-related differences in autumn
Both fat-score and body-condition data were used to examine possible differences in fat loads between adults and immatures in the autumn sample. At the species level, adults of all species had significantly higher mean fat scores than immatures, and that pattern prevailed study-wide, with 11 of 16 species having P < 0.003 (Table 2). Immatures did not have higher fat scores in any species examined, unless P < 0.05 is considered significant, in which case, fat scores of immatures were higher than those of adults in Alder Flycatchers (Table 2).
Differences in fat scores between adults and immatures during autumn migration (15 July to 30 September) at Creamer's Field Migration Station in Fairbanks, Alaska (1992–1998)
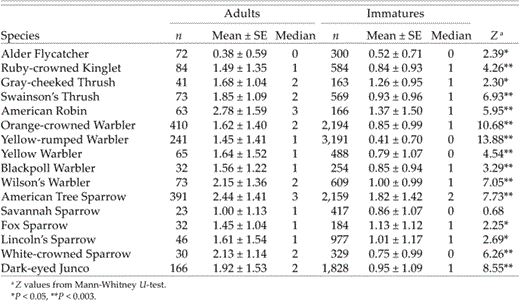
Differences in fat scores between adults and immatures during autumn migration (15 July to 30 September) at Creamer's Field Migration Station in Fairbanks, Alaska (1992–1998)

When time of capture, night length, and overnight low temperature were included in ANCOVA models, age-class differences in body condition (mass/wing) were mostly nonsignificant, with P < 0.003 occurring in only two species (or 4 at α = 0.05; Table 3). In Swainson's Thrushes and Yellow-rumped Warblers, adults possessed higher condition indices when covariates were held constant (Table 3). The ANCOVAs showed no interaction between age and year in Swainson's Thrushes (F = 2.03, df = 6 and 588, P = 0.07) or in Yellow-rumped Warblers (F = 0.14, df = 6 and 3,324, P = 0.98), which suggests that the age-related differences did not vary among years. Time of capture had a significant positive effect on body condition in 11 of 16 species during autumn (Table 3). Negative effects were not found in any species. Overnight low temperature had a significant effect on body condition in just 4 of 16 species (or 6 at α = 0.05), but night length affected body condition in 10 of 16 species (or 13 at α = 0.05; Table 3). In sum, most age-related differences among autumn birds were accounted for by the environmental variables prevailing at time of capture.
Body condition (ratio of mass to wing chord) of adults and immatures during autumn migration (15 July to 20 September) at Creamer's Field Migration Station in Fairbanks, Alaska (1992–1998). ANCOVA models were used with age class as the main factor and time of day (time), daily low temperature, and night length on the date prior to capture as covariates
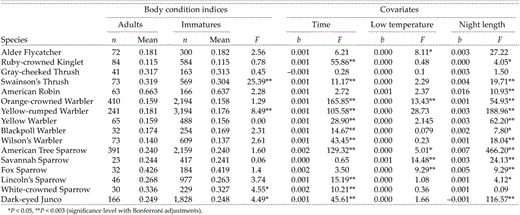
Body condition (ratio of mass to wing chord) of adults and immatures during autumn migration (15 July to 20 September) at Creamer's Field Migration Station in Fairbanks, Alaska (1992–1998). ANCOVA models were used with age class as the main factor and time of day (time), daily low temperature, and night length on the date prior to capture as covariates

Between-season differences
Comparisons of fat scores (adults only) showed that 6 of 16 species had significantly higher fat loads in autumn than in spring (or 10 species at P < 0.05; Table 4). Ruby-crowned Kinglet was the only species with significantly higher fat loads in spring than in autumn (Table 4). Median fat scores for all species were low, ranging from 0 (no fat visible) to 3 (half of furculum filled with fat; Table 4).
Differences in fat scores between spring and autumn (adults only) in passerine migrants at Creamer's Field Migration Station in Fairbanks, Alaska (1992–1998)
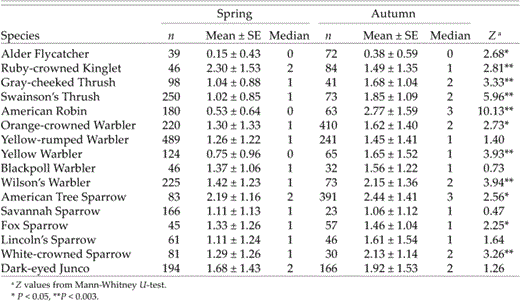
Differences in fat scores between spring and autumn (adults only) in passerine migrants at Creamer's Field Migration Station in Fairbanks, Alaska (1992–1998)

When time, overnight temperature, and night length were included as covariates in ANCOVAs, five species (again, in adults only) showed higher body condition in autumn than in spring: Swainson's Thrush, American Robin, Orange- crowned Warbler, Yellow-rumped Warbler, and Fox Sparrow (Table 5). Two more species, Gray- cheeked Thrush and Yellow Warbler, showed the same pattern at α = 0.05 (Table 4). Our ANCOVA tests for interaction between season and year with time, temperature, and night length held constant showed no significant interaction (after Dunn-Sidák corrections) for any of the following species: Swainson's Thrush (F = 2.76, df = 6 and 262, P = 0.02), American Robin (F = 1.31, df = 6 and 234, P = 0.26), Orange-crowned Warbler (F = 5.74, df = 6 and 630, P = 0.06), Yellow-rumped Warbler (F = 1.45, df = 6 and 141, P = 0.199), and Fox Sparrow (F = 0.54, df = 6 and 73, P = 0.81). Thus, no species had higher body-condition indices in spring when covariates were held constant.
Differences in adult body condition (ratio of mass to wing chord) between spring (25 April to 15 June) and autumn migration (15 July to 20 September) at Creamer's Field Migration Station in Fairbanks, Alaska (1992–1998). ANCOVA models were used with age class as main factors and time of capture (time), daily low temperature, and night length on the date of capture as covariables
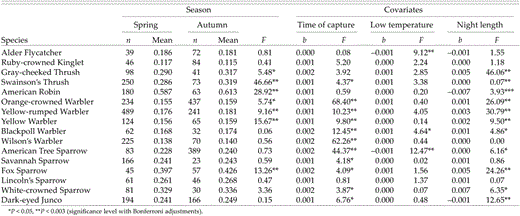
Differences in adult body condition (ratio of mass to wing chord) between spring (25 April to 15 June) and autumn migration (15 July to 20 September) at Creamer's Field Migration Station in Fairbanks, Alaska (1992–1998). ANCOVA models were used with age class as main factors and time of capture (time), daily low temperature, and night length on the date of capture as covariables

We also present results for seasonal differences in body condition when immatures are included in the autumn sample (Table 6). Inclusion of immatures in analyses, though making our study more directly comparable to those in other regions that have not separated age groups, introduces additional variation that produces different species-level results. The larger sample sizes of immatures increases power to detect small differences in body-condition indices and is the most likely explanation for differing results. When immatures are included in these analyses, Alder Flycatcher, Wilson's Warbler, and Savannah Sparrow show higher spring condition indices; whereas Orange-crowned Warbler, Yellow-rumped Warbler, Yellow Warbler, and American Tree Sparrow have higher autumn condition indices.
Differences in body condition (ratio of mass to wing chord) between spring (25 April to 15 June) and autumn migration (15 July to 20 September) at Creamer's Field Migration Station in Fairbanks, Alaska (1992–1998). ANCOVA models were used with season as main factors and time of capture (time), daily low temperature, and night length on the date of capture as covariables. This table includes adults and immatures in the autumn sample, unlike Table 5
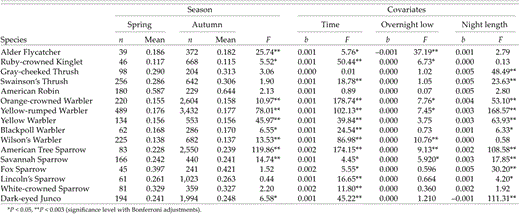
Differences in body condition (ratio of mass to wing chord) between spring (25 April to 15 June) and autumn migration (15 July to 20 September) at Creamer's Field Migration Station in Fairbanks, Alaska (1992–1998). ANCOVA models were used with season as main factors and time of capture (time), daily low temperature, and night length on the date of capture as covariables. This table includes adults and immatures in the autumn sample, unlike Table 5

Analysis of adults only (which possess fewer complicating variables present in autumn, such as molt and foraging inexperience; Table 5) enable us to address more cleanly the role of ambient environmental factors in relation to fat deposition, and we therefore focus on adult- only results below.
Discussion
Most species examined at our study site carried relatively low fat loads upon arrival in interior Alaska in spring (Table 2). King et al. (1965) found similar results in an intensive study of White-crowned Sparrows in central Alaska. Those findings are contrary to results from the subarctic of Scandinavia, which showed that some migrants arrived with high fat levels in spring (Sandberg 1996, Fransson and Jakobsson 1998). King et al. (1965) suggested that White- crowned Sparrows may not restore the fat depleted during the final migratory flight in spring, and that perhaps the tendency to maintain increased reserves diminishes as migration progresses in spring in northwestern North America.
Although hyperphagia is well documented during and prior to migration (Berthold 1975), it is not known whether it persists until birds reach their breeding grounds. Our data suggest that, in interior Alaska, passerines tend to follow the pattern that King et al. (1965) described for the White-crowned Sparrow, and that Dunn (2002) described for several species at different locations in Canada: migrants are depositing only the fat they need for migration and daily energetic requirements; little or no “insurance” fat is being deposited; and there is a correspondingly lower potential demand placed on resources at stopover sites used just before arrival on breeding grounds. Another consideration, however, is that the amount of energy stored during spring migration is dependent on resource availability along the migration route—a bird cannot store fat for insurance if food is not readily available. Perhaps stopover sites during the final stages of spring migration to interior Alaska do not provide sufficient resources to enable fat deposition beyond what is necessary to fuel the final stages of migration.
A similar situation prevailed in autumn. The generally low levels of fat carried by migrants at that time of year (Table 2) suggest that they must be paying most of the energetic costs of their long-distance migrations with resources obtained en route to their wintering grounds.
Age-related differences in autumn fattening
Adults had significantly higher fat stores than immatures in 11 of 16 species. Our results are similar to those of many previous studies that have documented adults that were heavier than immature birds at migratory stopover sites (Morris et al. 1996, Woodrey and Moore 1997, Jones et al. 2002). However, at our study site, adults departed significantly later than immatures in many of those species (Benson and Winker 2001), and higher fat loads in adults could be attributed to longer nights and colder temperatures later in the season in all but two species (Table 3). Adult Swainson's Thrushes and Yellow-rumped Warblers had higher mean body-condition indices than immatures in autumn, and the differences could not be explained by daily environmental variables. Thus, although most species do not show age- related differences in autumn fattening, some do. We cannot determine whether that reflects genuine species-level variation in autumn fat- deposition strategies between age classes or simply superior performance in adults of some species because of experience.
“Spring fatter” hypothesis
Only 1 of the 16 species in our study showed higher relative fat loads in spring than in autumn (Ruby-crowned Kinglet), and that difference was not reflected in the between-season comparison of body condition (Tables 4 and 5). In a direct comparison of fat loads alone, the opposite situation appeared to prevail: many species seemed to carry heavier fat loads in autumn. However, when environmental variables prevailing at time of capture were analyzed as covariates, those differences disappeared in all but five species. King et al. (1965) interpreted an increase in fat deposition among White-crowned Sparrows prior to migration from central Alaska as premigratory fattening; however, he did not evaluate the influence of daily variables known to affect fat storage. Lower overnight temperatures and increased night length in autumn accounted for most of the autumnal increase in fat and body condition that we observed. We could not examine variance in food availability, but that factor may also influence fat stores (Lima 1986, Houston and McNamara 1993, Pravosudov and Grubb 1997), and it could not be ruled out as a possible mechanism for the higher autumn fat stores found among 5 of the 16 species. Thus, again, although this may indicate some species-level variation in fat-deposition strategies among migrants at this latitude, local factors alone could be responsible for that variation.
The hypothesis that migrants should carry higher levels of fat onto their breeding grounds in spring for insurance against unpredictable resources or to possibly increase reproductive success is not valid for the species studied at this site, which clearly suggests that the hypothesized benefits of such fat deposition are outweighed by the costs (whether temporal or energetic). It also suggests that, as King et al. (1965) alluded, hyperphagia may not persist during the final stages of high-latitude migration in spring in northwestern North America. Just as importantly, however, the species examined did not have high fat levels in either season. At this latitude, most of the variation in passerine fat-deposition between seasons and between age classes in autumn appears to be explained by environmental variables prevailing when capture occurred: time of day, overnight low temperatures, and night length. Our results indicate that passerine migrants near their high- latitude breeding grounds in Alaska have fat- deposition strategies similar to those of many species at stopover sites across Canada (Dunn 2002). Seasonal fat-deposition strategies appear to be responding to energetic needs at the level of daily maintenance, rather than to hypothesized insurance needs in spring or to the forthcoming needs of a long-distance migration in autumn.
Acknowledgments
We thank the many volunteers, interns, and staff who have made contributions to the data collection process at the Alaska Bird Observatory (ABO); N. D. DeWitt, T. H. Pogson, and S. K. Springer, in particular, banded thousands of birds here. Members of ABO funded this research, together with large contributions from ABR Inc. Environmental Research and Services, Alaska Department of Fish and Game, ARCO Alaska, the Bureau of Land Management, Exxon Company USA, the Skaggs Foundation, and the U.S. Fish and Wildlife Service. A.-M.B. also thanks the Wilson Ornithological Society for the Paul A. Stewart Award. E. C. Murphy and E. A. Rexstad offered statistical advice and improved the manuscript with their insightful comments. Thanks to M. Katti, E. Dunn, and two anonymous reviewers for helpful reviews. The Alaska Climate Research Center data we used is available at climate.gi.alaska.edu/FaiData/Fai0592.html.
Literature Cited



