-
PDF
- Split View
-
Views
-
Cite
Cite
Chris D. Jiggins, Ecological Speciation in Mimetic Butterflies, BioScience, Volume 58, Issue 6, June 2008, Pages 541–548, https://doi.org/10.1641/B580610
Close - Share Icon Share
Abstract
There has been a recent revival of interest in the role of ecology in speciation. The wing patterns of Heliconius butterflies are signals to predators as well as mates, and can cause strong reproductive isolation between populations. Reproductive isolation has been studied in some detail between the sympatric species Heliconius melpomene and Heliconius cydno, and in reviewing this work I show that habitat isolation and color pattern preference are by far the most important factors causing speciation. The surprising observation that genes for mate preference and color pattern are genetically associated implies divergence in sympatry or resulting from sexual selection. Color pattern is therefore an example of an ecological trait that contributes to speciation through pleiotropic effects on mate choice, although phylogenetic evidence shows that it is only one of many factors responsible for speciation in mimetic butterflies.
Arecent renaissance of interest in the role of ecology in speciation has led to a change of emphasis in the literature, away from stochastic changes in isolated populations and toward adaptive change. A primary reason for this shift has been the detailed ecological study of a number of closely related species or population pairs, in which reproductive isolation has evolved as a side effect of adaptive divergence. Widely cited examples include sticklebacks, Darwin's finches, Rhagoletis fruit flies, stick insects, and mimetic Heliconius butterflies (Grant 1986, Feder 1998, Mallet et al. 1998, Schluter 1998, Nosil 2004). Each of these examples is unique in its own way, but they all share traits that are under divergent ecological selection and that also cause reproductive isolation of some form. In other words, divergence in ecological adaptation leads directly to reproductive isolation between divergent populations.
This interaction between ecological traits and reproductive isolation effectively bypasses many of the theoretical objections to sympatric speciation (Gavrilets 2004, Jiggins et al. 2004a). The main problem of sympatric speciation is that genetic associations between genes controlling species characteristics—such as niche adaptation and other forms of reproductive isolation—are broken down by hybridization (Felsenstein 1981). Niche adaptation itself often generates a degree of isolation between divergent forms, but probably leads to speciation only when the divergent niche-adapted forms also tend to mate assortatively. Thus, the problem is often how genes for mate choice and niche adaptation become associated with one another. On the other hand, if the same gene is responsible for several such traits, then associations between them are sustained, and sympatric speciation becomes more straightforward. An important empirical question needs to be answered: how common is it for reproductive isolation to be enhanced as a side effect (also known as a pleiotropic effect) of ecological adaptation?
The color patterns of butterflies are a compelling example of a trait involved in both ecological adaptation and mate choice. Most of the 17,500 butterfly species can be readily recognized by their wing color patterns. It has long been suggested that such divergence could be more than coincidental, and that color-pattern change could play a direct causative role in speciation (Bates 1862, Vane-Wright 1978). In support of this idea is a considerable body of evidence showing a role for color pattern in mate recognition across a wide diversity of butterfly species (Crane 1955, Stride 1956, 1957, 1958, Brower 1959, Silberglied and Taylor 1973, Silberglied 1979, Fordyce et al. 2002, Costanzo and Monteiro 2007). Notably, in lycaenid butterflies, even minute details of color pattern have been shown to play a role in mate recognition (Fordyce et al. 2002).
In addition to mate selection, wing patterns are also involved in ecological adaptation, through signaling to predators and thermoregulation (Nijhout 1991, Heinrich 1993). Many species are cryptic, adapted to blend into their background and avoid predation either through disruptive coloration or mimicry of the inedible environment. Others are warningly colored to advertise noxiousness, often acquired by sequestration of toxic plant chemicals eaten by the larvae. Thus, adaptation to novel habitats or niches can lead to selection for divergence in wing pattern, with associated changes in mate recognition. Butterfly wing patterns therefore are an example of the kind of trait in which assortative mating is likely to be a common side effect of ecological adaptation.
In addition, mimicry is widespread among butterflies; it can be either deceptive, whereby edible species mimic distasteful models, or mutualistic, whereby several distasteful species converge on a common pattern in order to share the cost of educating predators. These are known as Batesian and Müllerian mimicry, respectively, after Henry Walter Bates and Fritz Müller (Bates 1862, Müller 1879). At first sight, it is not obvious how mimicry, a theory of convergence, can play a role in speciation, which requires divergence. However, rather than all converging on a single pattern, mimetic butterflies are characterized by a great diversity of color patterns. Closely related species commonly differ in pattern, while convergence commonly occurs between more distantly related species. The reasons for such a diversity of mimetic patterns remain unclear, but that diversity is most likely attributable, in part, to different patterns being adaptive in different microhabitats within the forest (Joron and Mallet 1998). Thus, there is potential for adaptive radiation in pattern through divergence to mimic different model species found in the local environment.
We have directly studied the role of color patterns in speciation among Heliconius butterflies. Heliconius are all distasteful and warningly colored, and commonly converge as Müllerian mimics on a shared warning signal. Differences in color pattern among species lead to reproductive isolation in at least two ways. First, patterns are used as cues in mate choice, such that populations and species differing in pattern tend to mate assortatively. Second, hybrids commonly have intermediate, rare, and nonmimetic patterns that are expected to be selected against. Although such selection has never been demonstrated directly for interspecies hybrids, mimicry selection can be extremely strong, as it is, for example, in interracial hybrid zones (Mallet and Barton 1989).
Experiments have shown that between closely related species males strongly prefer to court their own color patterns. In total, four different species have now been studied, H. melpomene, Heliconius pachinus, Heliconius heurippa, and H. cydno, and in all cases there was a strong preference for conspecific color patterns as compared with those of related species (Jiggins et al. 2001a, Kronforst et al. 2006, Mavárez et al. 2006). These results are repeatable using both printed wing pattern models and wings dissected from female butterflies (Jiggins et al. 2001a, Mavárez et al. 2006), or manipulated patterns on mounted butterflies (Kronforst et al. 2006), demonstrating that the butterflies are responding to color pattern and not to some other aspect of wing morphology or chemistry. Similar effects are seen intraspecifically. Subspecies within H. melpomene differ almost as dramatically in color pattern as distinct species such as H. melpomene and H. cydno. These subspecies also show strong preferences to court their own color patterns, although there were some interesting exceptions. A few forms actually preferred to court different color patterns rather than their own, implying some conflict between mimicry evolution and sexual selection (Jiggins et al. 2004b). Thus, the use of color pattern by males in identifying potential mates both contributes to reproductive isolation between species and causes incipient isolation between divergent populations.
Subspecies or races of H. melpomene show little differentiation at neutral molecular markers, and hybrid zones separate forms with no obvious ecological differences (Mallet et al. 1998). Thus, it appears that color pattern and color-based assortative mating are the first traits to diverge between adjacent populations. All mating experiments to date have been carried out with males, as they are known to search for potential mates using visual cues. It would be of considerable interest to know whether females also use color as a cue for mate choice, as is known in other butterflies (Costanzo and Monteiro 2007).
Until recently, we had documented a relatively straightforward story in which shifts in pattern between closely related species such as H. melpomene and H. cydno were driven by mimicry adaptation and led to reproductive isolation. In combination with ecological divergence and a degree of hybrid sterility, mimicry therefore led to speciation. Recent work has made this story more complex, however, and in some cases presents some significant challenges to our previous understanding.
Is mimicry necessary?
Selection for phenotypic convergence due to mimicry is undoubtedly an important force in the evolution of Heliconius patterns. Most species are convergent with at least one other butterfly, and this provides a strong selective force that can cause populations to evolve a novel pattern by converging on a different model species. In Panama, for example, the closely related H. melpomene and H. cydno mimic the more divergent Heliconius erato and Heliconius sapho, respectively. This clearly implies that H. cydno has diverged from an ancestral H. melpomene pattern (or vice versa) in order to mimic H. sapho. Thus, mimicry adaptation has caused the shift in color pattern.
Nonetheless, the great diversity of patterns demonstrates that novel patterns must occasionally arise, and these cannot be explained by straightforward mimicry theory (after all, mimicry theory predicts convergence, not divergence). Indeed, two of the well-studied species with respect to speciation, Heliconius himera and H. heurippa, are both nonmimetic (Jiggins et al. 1996, Mavárez et al. 2006). In both cases, these species can be very abundant in their respective habitats, and it seems probable that they have effectively established their own aposematic pattern with no particular need for mimicry. As demonstrated by both theoretical and empirical studies, warning coloration and Müllerian mimicry is in essence a density-dependent phenomenon, with the benefit proportional to the number of individuals found locally with a particular pattern (Mallet and Joron 1999, Rowland et al. 2007). If 10 individuals need to be sampled in order for the local predator community to learn to avoid a particular pattern, then the per capita cost to the butterflies is greater if the local population numbers 100 individuals than it is if there are 1000. However, a similar level of protection is provided whether those 1000 individuals are composed of a single abundant species or several mimetic species (assuming a similar degree of distastefulness). Thus, provided the species is sufficiently abundant, a novel aposematic pattern can be evolutionarily stable once established, even in the absence of mimicry. The initial shift to an entirely novel pattern must occur through genetic drift or sexual selection, rather than through mimicry adaptation. This hypothesis is supported by the observation that both the nonmimetic species H. heurippa and H. himera can be very abundant locally in their respective habitats.
Origin of premating isolation
Although color pattern has been clearly shown to play an important role in mate choice, a shift in pattern is not enough in itself. Divergence in mate preference is also required, such that individuals both recognize and prefer mates with patterns similar to their own. One possibility might be that butterflies determine their preference by learning the pattern associated with local conspecific individuals, or even somehow by sensing their own pattern. However, there is no experimental evidence to support such learning of preferences. In one experiment, freshly emerged males of Heliconius malpomene malleti were separated into two groups and exposed to females of either H. m. malleti or Heliconius malpomene plesseni, which have very different patterns (figure 1a; Jiggins et al. 2004b). Subsequent experiments showed no significant difference in preference between the two groups. In an earlier experiment, males were allowed to emerge in darkness, with their pattern blacked out before exposing them to natural light, thus preventing males from learning their own phenotype (Crane 1955). Similarly, this had no effect on subsequent behavior. Thus, there is no evidence for learning of pattern preferences. Furthermore, in at least one case, preferences have been clearly shown to have a genetic basis (Kronforst et al. 2006).
We have previously speculated that patterns and preferences evolve by a two-step process whereby novel patterns first establish in geographically adjacent (parapatric) populations. This would then be followed by divergence in mate-finding preferences in already partly isolated populations (Jiggins et al. 2004b). Under this hypothesis, there would be no reason to expect genes controlling color pattern and mate choice to be close to one another in the genome, as the two evolved independently. Therefore, the recent discovery of a genetic association between color pattern and mate preference is surprising and casts doubt on this model. The parapatric species H. cydno and H. pachinus differ in several aspects of color and pattern (figure 1b). Most notably, the H. pachinus pattern has yellow pattern elements, whereas the adjacent population of H. cydno is white, with the change in forewing color controlled by a single locus, K. It has recently been shown that a large proportion of the variance in preference for white versus yellow in these two species is controlled by a locus within a 20-centimorgan window around the K locus (Kronforst et al. 2006). Although the resolution of this study was crude, for trait and preference to be so closely associated by chance would nonetheless be unexpected.
The molecular basis for this association remains unclear. It seems improbable, although perhaps not entirely impossible, that the same mutation has caused a change in both color and preference, so we need to explain how independent mutations causing the two traits have arisen in the same region of the genome. This could be due to a functional association—it has been speculated that the same gene might regulate both wing and eye ommochrome pigment placement, perhaps through tissue-specific cisregulatory regions (Kronforst et al. 2006). Alternatively, or perhaps additionally, there may be an evolutionary reason for such an association. Genetic associations between traits and preferences necessary for runaway divergence in sexually selected traits might be facilitated by such genetic linkage (Fisher 1958). It is not immediately obvious that sexual selection would promote linkage, but perhaps alleles that happened to arise in close linkage with color pattern genes would be more likely to trigger sexual selection. Occasional bouts of rapid divergent sexual selection could contribute to the observed diversity of mimicry patterns, which is difficult to explain under mimicry theory alone (Mallet and Joron 1999).
Speciation theory also predicts genetic associations between traits and preferences for related reasons. If genes causing reproductive isolation are genetically associated, this reduces recombination between loci and facilitates divergence in sympatry or parapatry (Kirkpatrick and Barton 2006). Essentially, coadapted genes, such as genes for a wing pattern and those for preference of that pattern, or different genes for adaptation to a novel habitat, are less likely to become dissociated through hybridization if they are tightly linked. This facilitates adaptation to divergent environments under ongoing gene flow. Thus, the observation of genetic associations between traits and preferences in Heliconius may provide an additional line of support for divergence having occurred in the face of gene flow.
A further line of evidence for divergence in sympatry comes from reproductive character displacement. Studies in Panama and Costa Rica have shown that H. melpomene collected from populations sympatric with H. cydno are less likely to court H. cydno than those from allopatric populations (Jiggins et al. 2001b, Kronforst et al. 2007). Similarly, H. pachinus and H. cydno show decreasing levels of isolation with increasing distance from a zone of contact in central Costa Rica (Kronforst et al. 2007). This implies that the latter stages of speciation involve divergence resulting from sympatric interactions between the species, which has been interpreted as evidence for reinforcement (Kronforst et al. 2007).
Studies of genes for mating preference that are currently under way—in particular, quantitative trait locus (QTL) mapping and molecular analysis of reproductive isolation between species—promise new insights into this system. First, sequencing of these regions in combination with functional analysis of divergent alleles will enable dissection of the regions controlling mate preference and color to determine whether they are one and the same. Second, further QTL mapping will determine whether there are other genes in this region contributing to reproductive isolation. Sympatric speciation theory would predict that genes for divergent traits unrelated to sexual selection, such as hybrid incompatibility and ecological preferences, should also be found in the same genomic region.
How important is color pattern?
How important are changes in color pattern for butterfly speciation? This question can be broken down into two distinct parts. First, in specific cases where divergence does involve a change in color pattern, what contribution does the pattern shift make to speciation? In other words, what proportion of the reproductive isolation observed can be attributed to color pattern? Second, at a species level, what proportion of speciation events involves changes in color pattern? I will discuss these two questions in turn.
The relative contribution of mimicry to reproductive isolation.
In closely related species that still hybridize, it should be possible to estimate the relative contributions of different factors to the total degree of reproductive isolation (Coyne and Orr 2004), giving a snapshot view of the importance of different factors at a particular stage of divergence. The only pair of butterfly species that has been studied in sufficient detail to do this is H. melpomene and H. cydno. Naisbit and colleagues (2002) made the first attempt, showing that hybrid incompatibility represents a barrier of around 70%, while the barrier due to selection against hybrids by predation could be as high as 65%, assuming selection is similar to that estimated from H. erato hybrid zones. Premating isolation is much stronger. Previous attempts to quantify reproductive isolation in this system used a frequency of hybrids in wild populations of 0.001 as an estimate of premating isolation, although this frequency has never been accurately quantified (Mallet 2006). However, even if it were accurate, this figure would not be a good estimator of premating isolation, as the observed frequency of wild hybrids must also include a degree of post-mating isolation because of selection against hybrids.
What we now have are mate preference and field experiments that directly estimate premating isolation. In choice experiments, published data show zero matings between heterospecifics in 41 replicate experiments (combining data from Jiggins et al. [2001b] and Mavárez et al. [2006]), giving at least a 97% barrier. Similarly, in no-choice experiments, Naisbit obtained zero hybrid matings in 30 trials (one male for each female; Naisbit et al. 2001). Although Mavárez and colleagues observed a higher rate of interspecific mating in experiments with a ratio of 10 males for each female (8 out of 45 matings for male melpomene X female cydno; 6 out of 50 matings for the reciprocal direction [Mavárez et al. 2006]), a 10 to 1 ratio of males to females is unlikely to occur in the wild, so the former experiments more likely represent a natural situation where males encounter females singly. Conservatively, therefore, assortative mating must account for isolation of at least 97%.
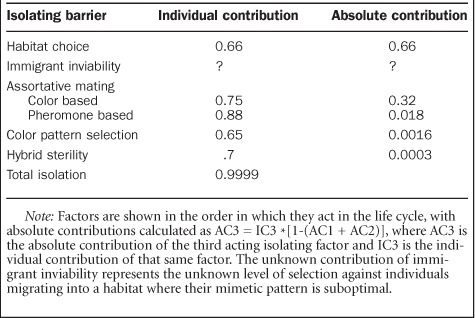
Assortative mating is caused by a number of factors, most notably color pattern and pheromone-mediated mate choice, although the latter has not been studied in detail. Published results suggest that males show approximately a 75% reduction in the probability of courting heterospecific female color patterns, compared with conspecifics (Jiggins et al. 2001b). An additional barrier of 88% (0.25 × 0.12 = 0.03) is therefore required to explain the overall strength of assortative mating, most of which is probably due to pheromonal cues. Color pattern therefore accounts for slightly less than half of the assortative mating between these species. However, the fact that males first use color pattern to find mates, such that it acts before other sexual signals, means that color pattern actually plays a much greater absolute role in isolation (table 1).
Finally, ecological isolation attributable to habitat preference must act in addition to assortative mating, as the latter is estimated by forcing individuals into close proximity in a small insectary. Data from a habitat transect in Panama (figure 2) showed that 21% of H. cydno and 44% of H. melpomene individuals occurred in regions of local sympatry, suggesting an averaged ecological isolation of around 66% (Estrada and Jiggins 2002). This habitat divergence is also associated with mimicry, as H. melpomene and H. cydno overlap ecologically with their comimics. The degree of isolation due to habitat choice may be underestimated—there is also likely to be some degree of “immigrant inviability” (sensuNosil et al. 2006). Individuals found in the “wrong” habitat will be selected against as having a locally less abundant color pattern, even before they have a chance to mate. This selection has not been quantified, and given the considerable degree of overlap between the mimicry rings, may not be very strong. Overall, the degree of ecological isolation is likely to be highly dependent on local environments, seasonality, and other variables, but nonetheless these results suggest that ecological separation is of a similar magnitude to that caused by other factors.
These estimates do not take into account, however, the order in which isolating barriers act. As has been highlighted previously, barriers that act earlier in the life cycle necessarily make a greater contribution to isolation in absolute terms. For example, if habitat isolation is 0.66, then assortative mating of 0.95 represents an absolute barrier of only 0.32 (0.95 × [1 − 0.66]) (table 1; Coyne and Orr 2004, Nosil et al. 2006). Although there are similar individual contributions to reproductive isolation from at least five factors—ecological habitat isolation (66%); color pattern preference (75%); assortative mating due to other factors, including pheromones (80%); selection against color pattern hybrids (65%); and intrinsic incompatibility (70%)—when absolute contributions are calculated, habitat choice and color-based mate selection are by far the most important because they act before other factors (table 1).
Nonetheless, the current importance of isolating barriers may not reflect barriers' historical importance (Coyne and Orr 2004). The later-acting barriers may have evolved first, and therefore may have played a more important causative role in speciation. However, this seems unlikely in Heliconius, as there are many examples of within-species divergence in color pattern and mate preference (Jiggins et al. 2004b), but only one known case of intraspecific hybrid inviability (Jiggins et al. 2001a). This suggests that the color pattern (and the associated mate preference) is commonly the first trait to diverge between populations.
How often does speciation involve pattern shifts?
Obviously, not all species pairs can be studied in as much detail, but to estimate the generality of the phenomena we can at least count the proportion of speciation events that involve a change in color pattern. Of course, associations between cladogenesis and pattern change cannot be taken as proof of causation, but they at least put an upper bound on the number of speciation events that have been caused in part by a pattern shift. In Heliconius, analysis of the published taxonomy suggests that a very high proportion of sister species differs in color pattern (Beltrán 2004). However, recent results from molecular bar-coding studies have identified a number of cryptic Heliconius sister species with very similar color patterns that had not previously been recognized. The first of these, Heliconius tristero, was described on the basis of just two individuals collected in Colombia (Brower 1996, 2000). This was initially met with skepticism by Heliconius biologists, given the possibility that these specimens might represent occasional interspecific hybrids. However, subsequent analysis of other eastern Andean populations of H. melpomene has identified other examples of cryptic species more closely related to H. cydno, the species complex sister to H. melpomene. One of these species has an orange-rayed pattern and occurs around the town of Florencia in Colombia (Nathalia Giraldo Herrèra and Mauricio Linares Porto, Universidad de los Andes, Bogota, Colombia, personal communication, December 2007). The second has a postman pattern (red and yellow bands on a black background) and occurs in the mountains above Tarapoto in northern Peru (Mallet 2008). Detailed ecological and genetic studies of larger samples support these new examples. It seems likely that a complex of forms found in the eastern Andes are related to H. cydno and mimic H. melpo mene. These may in fact be races of the already recognized species Heliconius timareta. These cryptic species therefore clearly demonstrate that closely related species in the H. melpomene/cydno group can occur sympatrically without differing in color pattern, and that color pattern shifts are perhaps not as critical to speciation in this group as had previously been supposed.
These new discoveries paint a complicated picture in which two sister clades, the widespread H. melpomene and H. cydno species complex, overlap in distribution through the Andes and Central America. In most areas, they differ in color pattern, and this pattern contributes to reproductive isolation. However, in a few areas, the two clades coexist without major differences in pattern. How can this be? Speciation may have initially occurred through divergence in factors unrelated to pattern, leading to phenotypically similar proto-H. cydno and proto-H. melpomene. Some populations subsequently diverged in pattern, which enhanced reproductive isolation, leading to the present situation. Alternatively, divergence may have occurred initially in color pattern, followed by changes in ecology and hybrid inviability. Subsequently, mimetic selection led to convergence in pattern between some sympatric populations of these species, perhaps facilitated by hybrid introgression of pattern alleles. Neither of these hypotheses negates the current role of color pattern in reproductive isolation between the sympatric populations that have been studied, but the observation of cryptic sister species does stir uncertainty about the initial cause of speciation. A resolution of the branching order between these different populations might resolve the issue, but given the large amount of shared genetic variation between these species, such resolution may be difficult to achieve.
Perhaps more strikingly, in another group, the Ithomia butterflies, phylogenetic analysis has shown that there are many sister species with shared color patterns (Mallarino et al. 2005, Jiggins et al. 2006). Other ithomiine genera, notably Oleria, also contain large numbers of species with very similar color patterns. Unlike in Heliconius, speciation in Ithomia commonly occurs without a change in color pattern. Nonetheless, a number of pattern shifts did occur in the Ithomia tree, which raises the question of whether color pattern change earlier in the history of the group might have been associated with speciation events.
Using a likelihood reconstruction of color-pattern evolution, we tested whether a punctuationist model, in which pattern changes occur at nodes, was a better fit to the data than a gradualist model, in which color-pattern change is proportional to evolutionary time (i.e., branch length). This analysis demonstrated that where color pattern shifts do occur, they are significantly correlated with speciation events (Jiggins et al. 2006). Although such correlations cannot prove a causal association, this analysis certainly suggests that pattern shifts do contribute to speciation where they occur, even where a majority of speciation events might not involve pattern shifts.
Wider implications.
Despite the continued fascination with sympatric speciation, sympatry and allopatry are in fact the extremes along a continuum of geographic separation from complete panmixia to complete isolation (Gavrilets 2004). Indeed, several putative examples of sympatric speciation have been shown to involve an element of geographic separation (Schluter 1998, Feder et al. 2003). The genetic variation that facilitated sympatric divergence of Rhagoletis fruit flies initially arose in a geographically distant population in the southern United States (Feder et al. 2003). Similarly, some well- studied sympatric stickleback ecomorphs found in postglacial lakes very likely arose through multiple invasions from marine habitats (Schluter 1998). These observations do not negate the crucial role of ecological displacement in sympatry for speciation in both cases, but they do highlight the complicated nature of the real world. In diverse and continuous continental ecosystems such as the Amazonian rainforest, populations are unlikely to become completely isolated, but similarly have ample opportunity for some degree of spatial segregation, whether through isolation by distance or by choosing to fly in distinct and spatially segregated niches.
Two aspects of speciation in Heliconius are therefore likely to have general importance. First, parapatric divergence between abutting populations, as observed in Heliconius color-pattern races, is likely to be far more common than the emphasis in the literature on sympatry versus allopatry might imply (see Schneider et al. [1999]). Second, habitat choice on a finer spatial scale, as observed in H. cydno and H. melpomene, offers a powerful mechanism for spatial segregation and is most likely a predominant force in ecological speciation. Importantly, in the latter case, spatial isolation is determined by the genotype of an individual determining choice of a particular habitat, and leads to strong premating isolation.
Despite the continued fascination with sympatric speciation, there has been a shift in the speciation literature away from an emphasis on geography. Instead, recent theoretical and empirical work on speciation has tended to emphasize the kinds of traits that diverge in the early stages of speciation, and the ways in which selection can promote divergence (Via 2002, Gavrilets 2004, Nosil 2004). In this context, mimicry represents an excellent example of a trait in which ecological selection driven by predation can lead to divergence, with reproductive isolation and speciation as a side effect. Other examples include the color patterns of sticklebacks and reef fish (Boughman 2001, Puebla et al. 2007), host choice of phytophagous insects (Bush 1994, Feder 1998, Emelianov et al. 2001), and beak size of Darwin's finches (Podos 2001). Such traits—recently termed “magic traits” (Gavrilets 2004)—are likely to be common in nature, and they may represent the most likely cause of speciation (Jiggins et al. 2004a).
Acknowledgements
I would like to thank Jim Mallet for sparking my interest in the role of color patterns in speciation, and the Royal Society, the Biotechnology and Biological Sciences Research Council, the Natural Environment Research Council, and the Leverhulme Trust for funding that contributed to this research. Many thanks to Lawrence E. Gilbert for photos of H. cydno and H. pachinus specimens. I would also like to thank the numerous collaborators who have been involved in the work described here.
References cited
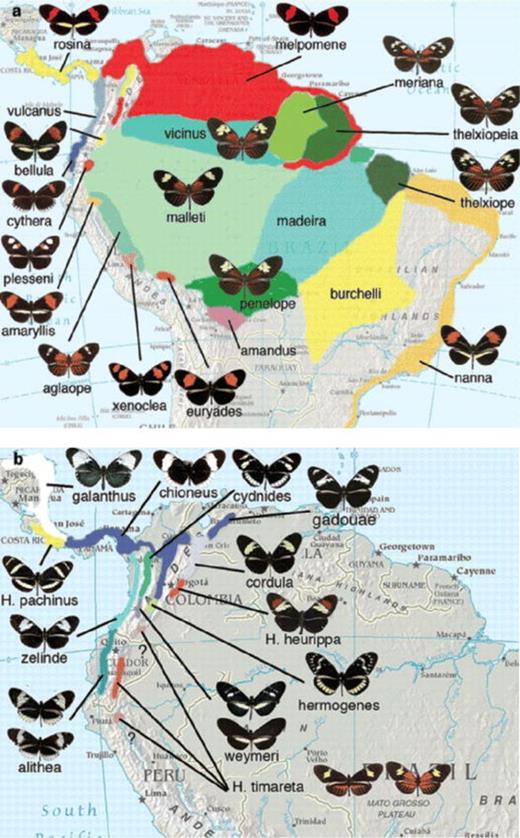
(a) Distribution of color pattern races of Heliconius melpomene. Note that not all named forms are shown. The form Heliconius melpomene melpomene in particular is divided into a number of named forms that differ in the shape and width of the forewing band, although this variation is subtle. Note the disjunct distribution of the red forewing band that is separated by the orange-rayed Amazonian forms shown in green. (b) Distribution of the Heliconius cydno species complex. Note the polymorphic populations, Heliconius cydno weymeri, Heliconius cydo alithea, and Heliconius timareta. There are three parapatric forms considered distinct species, Heliconius pachinus, Heliconius heurippa, and H. timareta. The northern and southern populations of H. timareta were recently discovered and remain undescribed, but resemble Heliconius melpomene aglaope and Heliconius melpomene amaryllis, respectively. Polymorphism is unusual in Müllerian mimicry, but seems to be maintained by spatial heterogeneity in the mimicry environment in the H. cydno group (Kapan 2001). Not all named subspecies of either species are shown. Source: Background map courtesy of the University of Texas Libraries, University of Texas at Austin.
Individual and absolute contributions to reproductive isolation by different factors between Heliconius melpomene and Heliconius cydno in Panama.
Soberania National Park and the Chagres River, Panama (a), and the Pipeline Road field site (b), where Heliconius melpomene and Heliconius cydno populations have been extensively studied. A mating pair consisting of a Heliconius melpomene melpomene male (c, right) and a H. cydno female (c, left). Hybridizing species such as these offer the opportunity to study speciation in progress. Photographs: Chris D. Jiggins.
Author notes
Chris D. Jiggins (e-mail: c.jiggins@zoo.cam.ac.uk) is with the Department of Zoology at the University of Cambridge in the United Kingdom.