Abstract
We have studied the molecular mechanism and signal transduction of pim-1, an oncogene encoding a serine-threonine kinase. This is a true oncogene which prolongs survival and inhibits apoptosis of hematopoietic cells. In order to determine whether the effects of Pim-1 occur by regulation of the mitogen-activated protein kinase pathway, we used a transcriptional reporter assay by transient co-transfection as a screening method. In this study, we found that Pim-1 inhibited the Elk-1 and NFkappaB transcriptional activities induced by activation of the mitogen-activated protein kinase cascade in reporter gene assays. However, Western blots showed that the induction of Elk-1-regulated expression of endogenous c-Fos was not affected by Pim-1. The phosphorylation and activation of neither Erk1/2 nor Elk-1 was influenced by Pim-1. Also, in the gel shift assay, the pattern of endogenous NFkappaB binding to its probe was not changed in any manner by Pim-1. These data indicate that Pim-1 does not regulate the activation of Erk1/2, Elk-1 or NFkappaB. These contrasting results suggest a pitfall of the transient co-transfection reporter assay in analyzing the regulation of transcription factors outside of the chromosome context. It ensures that results from reporter gene expression assay should be verified by study of endogenous gene expression.
Pim-1 kinase; Signal transduction; Mitogen-activated protein kinase pathway; Elk-1; Reporter assay
Braz J Med Biol Res, February 2006, Volume 39(2) 169-176
Pim-1 kinase inhibits the activation of reporter gene expression in Elk-1 and c-Fos reporting systems but not the endogenous gene expression: an artifact of the reporter gene assay by transient co-transfection
B. Yan1*
*
B. Yan and H. Wang contributed equally to this study. Received May 18, 2005. Accepted August 23, 2005.
, H. Wang2*
*
B. Yan and H. Wang contributed equally to this study. Received May 18, 2005. Accepted August 23, 2005.
, T. Kon1 and  Acknowledgments
Acknowledgments
 C.-Y. Li1
C.-Y. Li1
1Department of Radiation Oncology, 2Department of Medicine, Duke University Medical Center, Durham, NC, USA
 References
References
 Acknowledgments
Correspondence and Footnotes
Acknowledgments
Acknowledgments
Correspondence and Footnotes
Acknowledgments
Abstract

We have studied the molecular mechanism and signal transduction of pim-1, an oncogene encoding a serine-threonine kinase. This is a true oncogene which prolongs survival and inhibits apoptosis of hematopoietic cells. In order to determine whether the effects of Pim-1 occur by regulation of the mitogen-activated protein kinase pathway, we used a transcriptional reporter assay by transient co-transfection as a screening method. In this study, we found that Pim-1 inhibited the Elk-1 and NFkB transcriptional activities induced by activation of the mitogen-activated protein kinase cascade in reporter gene assays. However, Western blots showed that the induction of Elk-1-regulated expression of endogenous c-Fos was not affected by Pim-1. The phosphorylation and activation of neither Erk1/2 nor Elk-1 was influenced by Pim-1. Also, in the gel shift assay, the pattern of endogenous NFkB binding to its probe was not changed in any manner by Pim-1. These data indicate that Pim-1 does not regulate the activation of Erk1/2, Elk-1 or NFkB. These contrasting results suggest a pitfall of the transient co-transfection reporter assay in analyzing the regulation of transcription factors outside of the chromosome context. It ensures that results from reporter gene expression assay should be verified by study of endogenous gene expression.
Key words: Pim-1 kinase, Signal transduction, Mitogen-activated protein kinase pathway, Elk-1, Reporter assay
Introduction

Hematopoietic cells are absolutely dependent on peptide growth factors for survival and proliferation. Pim-1 is a serine-threonine kinase in hematopoietic cells whose expression is regulated by such growth factors as GM-CSF, IL-3, IL-4, IFN-g and so on (1-3). Pim-1 appears to be a true oncogene in that its enforced expression in transgenic mice leads to an increased incidence of tumors (4-6). Pim-1, as well as pim-2, prolongs survival and inhibits apoptosis (7-11). But the molecular mechanism of pim-1 has not been completely clarified although some clues have been reported (12-17). Some oncogenes encoding serine-threonine kinases such as raf (18,19), mos (20,21) and tpl-2 (22) are known to exert their survival effect by regulating the mitogen-activated protein kinase (MAPK) pathway. We hypothesized that Pim-1 might have a similar effect. Elk-1 and NFkB are transcription factors whose activities are regulated or partially regulated by MAPK (23,24). If Pim-1 affects the MAPK pathway at any level, this should be reflected on the transcriptional activity of Elk-1 or NFkB, the output of the MAPK pathway. We thus used the transcriptional reporter assay as a quick screening system for our study.
In the present study, we found that Pim-1 inhibited the induction of Elk-1 and NFkB transcriptional activities by activation of MAPK in reporter gene assays but not under physiological conditions. The contrasting data revealed the potential pitfalls of luciferase reporter analysis outside of their natural chromosomal context.
Material and Methods

Cell culture and transient transfection
HeLa, COS7 and Jurkat cells were cultured in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum. The IL-3-dependent murine hematopoietic cell lines FDCP1 (obtained from Dr. Scott Boswell, Indiana University) were cultured in RPMI 1640 medium with 10% (v/v) iron-supplemented calf serum, and 10% (v/v) medium conditioned by the WEHI-3B cell line (a convenient source of IL-3). Transfection of HeLa, COS7 and Jurkat cells was done by SuperFect (QIAGEN, Valencia, CA, USA) and electroporation methods, respectively. Twenty-four hours after transfection, cells were starved for an additional 24 h by incubating with DMEM containing 0.1% fetal bovine serum prior to stimulation.
Plasmids and luciferase reporter assay
The c-Jun Trans-Reporting System, Elk-1 Trans-Reporting System and NFkB Cis-Reporting System which include pFR-Luc, pFA2-cJun, pFA2-Elk-1, pFC-MEK1, pFC-MEKK, pNFkB-Luc plasmids were products of Stratagene (La Jolla, CA, USA). pcFos-Luc containing a natural c-fos gene promoter upstream of the firefly luciferase gene was kindly provided by Dr. Andrew Kraft (University of Colorado Health Center).
Twenty-four to 36 h after transfection, cells were stimulated with phorbol myristate acetate (PMA) for 6 h. Cell lysates were prepared and luciferase activity was measured using the luciferase assay kit from Promega (Madison, WI, USA). A plasmid containing the renilla luciferase gene without promoter was co-transfected with the firefly luciferase reporter gene as an internal control. Firefly luciferase activity of individual transfection was normalized to renilla luciferase activity.
Western blotting
Cells were collected, washed in PBS and lysed in 1% Triton lysis buffer (20 mM Tris, pH 7.5, 100 mM NaCl, 5 mM EDTA, 1% Triton X-100, 10 mM NaF, 1 mM Na3VaO4, 20 µg/mL leupeptin, 10 µg/mL pepstatin, and 10 µg/mL aprotinin). Samples were denatured at 100ºC for 5 min. Equal amounts of total protein were loaded to each well for electrophoresis in 10% SDS polyacrylamide gels and then transferred to polyvinylidene fluoride microporous membranes (Millipore Corporation, Billerica, MD, USA). Membranes were then incubated with primary antibody followed by incubation with horseradish peroxidase-linked secondary antibodies. Antibody-antigen complexes were detected using chemiluminescence (Pierce). The primary antibodies are Pim-1 and c-Fos (Santa Cruz Biotechnology, Santa Cruz, CA, USA).
Northern blotting
Twenty micrograms total RNA was loaded to each lane of a formaldehyde gel. After electrophoresis, RNA was transferred to hybond-XL membrane (Amersham Pharmacia, Piscataway, NJ, USA) using a turboblotter rapid downward transfer system (Schleicher and Schuell, Keene, NH, USA). The membrane was then incubated with prehybridization buffer (Ambion, Austin, TX, USA) at 42ºC for 1 h before the 32P random primer-labeled probe (Stratagene) was added for an overnight incubation at 42ºC. Blots were washed three times with wash buffer (0.1X SSC, 0.5% SDS) and then subjected to autoradiography.
Electrophoresis mobility shift assay
The oligonucleotide probes used in the electrophoretic mobility shift assay are 5'-TGA CCT GGG GAC ATC CCC TTC CCT-3' and 5'-ACA CCT GGG GAA TTC CCA CAC G-3'. DNA probes were end-labeled with [a-32P]dCTP using the Klenow fragment of DNA polymerase I. Nuclear extracts were prepared as described by Hussain et al. (25). The binding reactions were carried out using the gel shift assay core system from Promega. Binding reactions contained 2-5 µg nuclear extract and 10-50,000 cpm radiolabeled oligonucleotide in 10 mM Tris-HCl, pH 7.5, 1 mM MgCl2, 0.5 mM EDTA, 0.5 mM DTT, 50 mM NaCl, 1 µg polydeoxyinosylate-polydeoxycytidylate, and 4% glycerol. Binding reactions were electrophoresed at 4ºC in 5-6% native polyacrylamide gels using 0.5X Tris-borate buffer followed by autoradiography.
Results

Pim-1 blocks transcriptional activation of Elk-1
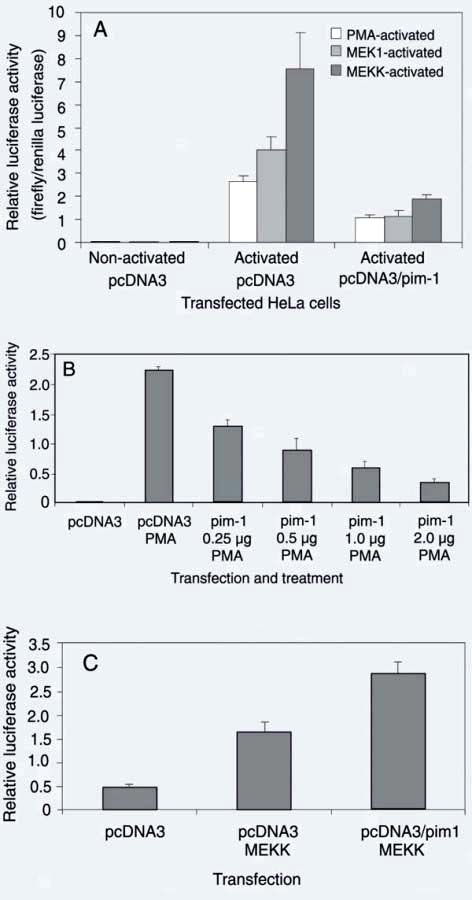
Activated MAPK is able to phosphorylate numerous cellular proteins and thus directly regulate their functions. One of the best characterized ERK substrates is Elk-1, which cooperates with the serum response factor to regulate transcription of promoters containing the serum response element (24, 26-28). To test the effect of Pim-1 on Elk-1 transcriptional activity, the activity of a Gal4-Elk-1 reporter, which contains the c-terminal ERK responsive domain of Elk-1 fused to the DNA-binding domain of Gal4, was tested. The transcription activity of Gal4-Elk-1 is dependent on phosphorylation of the Elk-1 transactivation domain by ERK. We observed that pim-1 co-transfection effectively inhibited PMA-stimulated and constitutively active MEK1- and MEKK-induced expression of the reporter (Figure 1A). The inhibition occurs in a dose-dependent manner (Figure 1B).
To test whether the inhibition of Gal4-Elk-1 by Pim-1 is specific to Elk-1 rather than a global inhibition of transcription, we performed similar experiments with the Gal4-Jun reporter (Stratagene). The constitutively active MEKK is used as a positive control in the Gal4-Jun reporter system which can activate the Gal4-Jun reporter. Pim-1 did not inhibit the activation of the Gal4-Jun reporter induced by constitutively active MEKK (Figure 1C). It should be emphasized that the Gal4-Jun reporter and the Gal4-Elk reporter are identical except that the Gal4 DNA-binding domain is fused to a different transactivation domain of either the C-terminal ERK-responsive domain of Elk-1 or the N-terminal JNK-responsive domain of c-Jun.
Pim-1 inhibits Elk-1-dependent, but not Jun-dependent transcription activity. A, Pim-1 inhibits Gal4-Elk-1 reporter expression induced by phorbol myristate acetate (PMA), constitutively active MEK1 or MEKK. HeLa cells were transfected with Gal4-Luc, Gal4-Elk-1 together with pcDNA3/pim-1 or pcDNA as control. A renilla luciferase plasmid was co-transfected as an internal control. Gal4-Elk-1 was activated either by treatment with PMA for 6 h or co-transfection and expression of constitutively active MEKK or MEK1. Luciferase activity was measured and normalized against the co-transfected renilla luciferase activity. B, Pim-1 inhibits Elk-1 transcription activity in a dose-dependent manner. HeLa cells were transfected with Gal4-Luc, Gal4-Elk-1 together with pcDNA3 or increasing amounts of pcDNA3/pim-1. The transfected cells were left untreated or treated with PMA for 6 h before measuring luciferase activity. C, Pim-1 does not inhibit Gal4-Jun activity. Cells were transfected with Gal4-Luc, Gal4-Jun and either pcDNA3, pcDNA3/pim-1 or a contitutively active mutant of MEKK as indicated in the figure. Twenty-four to 48 h after transfection, luciferase activity was determined as above.
Pim-1 expression blocks induction of a c-fos reporter gene
The inhibition of Gal4-Elk-1 by Pim-1 observed here might simply be an artifact of an artificial chimera transcription factor. To test the effect of Pim-1 on a physiological promoter activated by endogenous transcription factors, we examined the c-fos reporter for gene expression. The natural c-fos promoter contains DNA elements for multiple transcription factors, including a functional serum response element (29). Ternary complex factors together with serum response factors are responsible for the transcription activation of the serum response elements (29). Elk-1 is a member of the ternary complex factor family. Pim-1 expression effectively blocked PMA-induced activation of the reporter (Figure 2) as well as constitutively active MEK1- and MEKK-induced c-fos reporter activity.
Pim-1 inhibits activation of c-fos reporter. A, In HeLa cells: a luciferase reporter controlled by the c-fos promoter was co-transfected with either pcDNA3 or pcDNA3/pim-1. The reporter was left untreated or activated by either treatment with phorbol myristate acetate (PMA) or co-transfection with constitutively active mutant MEK1 or MEKK as indicated in the figure. B, In Jurkat cells: c-fos reporter was co-transfected by electroporation with either pcDNA3, pcDNA3/pim-1 or constitutively active MEK1 as indicated. Renilla luciferase plasmid was co-transfected as an internal control and luciferase activity was measured as described in Figure 1.
Neither MAPK nor Elk-1 activation was affected by Pim-1
Phosphorylation of Ser383 has been shown to be critical for the transcription activity of Elk-1 (24,26-28). Pim-1 could inhibit Elk-1 activation by either directly blocking Elk-1 phosphorylation or indirectly repressing Elk-1 activity. To examine the effect of Pim-1 on Elk-1 phosphorylation directly, the PMA-stimulated time course of Elk-1 phosphorylation was determined using a phosphorylation state-specific Elk-1 antibody, which recognizes only the Ser383 phosphorylated form of Elk-1. Data in Figure 3A show that Elk-1 phosphorylation was acutely stimulated by PMA. Co-transfection of Pim-1 did not affect PMA-stimulated Elk-1 phosphorylation. Western blots using anti-Elk- 1 showed that similar amounts of Elk-1 were expressed in all samples. ERK1/2 phosphorylates and activates Elk-1. A parallel experiment examined the effect of Pim-1 on ERK phosphorylation and activation using an antibody specifically recognizing phosphorylated ERK. Figures 3B and 3C show that Pim-1 did not affect PMA-stimulated ERK1/2 phosphorylation. These data cast doubts on the data from the reporter luciferase assays.
Pim-1 does not affect the phosphorylation and activation of ERK and Elk-1. A, Pim-1 does not inhibit the phosphorylation of Elk-1 at Ser383. HeLa cells were transfected with pcDNA3/Elk-1 and either pcDNA3 or pcDNA3/pim-1. Cells were treated with phorbol myristate acetate (PMA) for the indicated times. Cell lysates were subjected to immunoblot analysis with anti-p-Elk-1 or anti-Elk-1 as indicated on the left side of each panel. Anti-Elk-1 recognizes both the phosphorylated and unphosphorylated Elk-1 while anti-p-Elk-1 specifically recognizes Elk-1 phosphorylated at Ser383. B,C, Pim-1 does not affect the phosphorylation of ERK. HeLa cells were transfected with either pcDNA3 or pcDNA3/pim-1. Cells were treated with PMA (B) or TNF-a (C) for the indicated times. Cell lysates were subjected to immunoblot analysis with anti-p-ERK (phosphoMAPK) or anti-ERK (MAPK) as indicated on the lower side of each panel. Anti-ERK recognizes both the phosphorylated and unphosphorylated ERK while anti-p-ERK specifically recognizes phosphorylated ERK. p-Elk-1, p-ERK = phosphorylated Elk-1 or ERK; MAPK = mitogen-activated protein kinase; mpim44 = 44-kD form of mouse Pim-1 protein.
Pim-1 does not inhibit the induction of endogenous c-fos expression
In order to determine whether Pim-1 regulates the endogenous Elk-1 activity and c-fos expression, we examined the induction of c-fos mRNA and protein by PMA in HeLa cells. In both Northern blot and Western blot, induction of c-fos was acutely induced by PMA. But the overexpression of pim-1 did not inhibit the induction of c-fos (Figure 4A and B). These data suggest that Pim-1 does not regulate c-fos expression under physiological conditions.
Pim-1 does not inhibit the induction of c-fos expression by phorbol myristate acetate (PMA). HeLa cells were transfected with either pcDNA3 or pcDNA3/pim-1 and grown for at least 24 h. Then cells were left untreated or treated with PMA for 30 or 60 min. A, Total RNA was extracted for a Northern blotting - 20 µg RNA was loaded to each lane. Probe is a 1-kb c-fos cDNA amplified by PCR. B, Cell lysates were used for Western blotting with anti-Fos antibody. mpim-1 = mouse pim-1.
Pim-1 expression blocks induction of the NF k B reporter gene but does not affect endogenous NF k B activation in an electrophoresis mobility shift assay
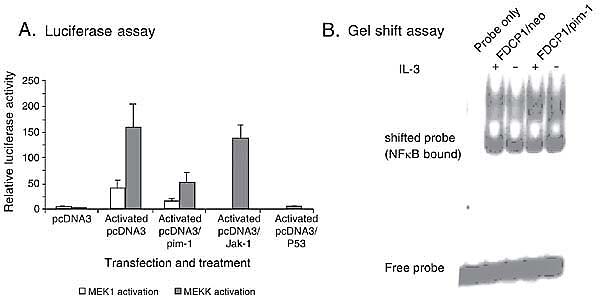
Similarly, we obtained contrasting data for the NF kB cis-reporting system. Figure 5A shows that induction of luciferase activity by constitutively active MEK1 or MEKK was inhibited by overexpression of Pim-1. P53, which is known to inhibit NF kB activity, was used as a positive control while the mutant JAK (encoding a tyrosine kinase), which does not affect NF kB activity, was used as a negative control in these experiments. We then tried to determine whether this is an artifact by a gel shift assay using stable FDCP1 cells expressing Pim-1. The result showed that the pattern of NF kB binding to its probe was not altered by Pim-1 at all (Figure 5B).
Pim-1 inhibits NFkB activity in a reporter assay but not in electrophoretic mobility shift assay. A, Pim-1 blocks NFkB activity in reporter assay. An NFkB luciferase reporter was co-transfected with either pcDNA3, pcDNA3/pim-1, pcDNA3/Jak-1 (negative control) and pcDNA3/P53 (positive control). The cells were left untreated or activated by co-transfection with the constitutively active mutant MEK1 or MEKK as indicated in the figure. At least 24 h after transfection, luciferase activities were assayed as described in Figure 1. B, Pim-1 does not inhibit NFkB binding to its probe. FDCP1 cells were stably transfected with pcDNA3 (FDCP1/neo) or pcDNA3/Pim-1 (FDCP1/pim-1). Cells were grown in the presence or absence of interleukin 3 (IL-3) for 8 h before the nuclear extracts were obtained for the gel shift assay. An oligonucleotide that contains the NFkB cis-acting element sequence was radioactively labeled as a probe.
Discussion

Bioluminescent reporters of genetic transcription have many applications in cell biology due to the speed, simplicity and precision of their assays. In general, applications have focused primarily on functional analyses of genetic elements, such as promoters and enhancers. However, the range of current uses is much broader, including areas such as receptor function, signal transduction and protein-protein interactions. Because the assays are simple and widely applicable, bioluminescent reporters have become increasingly popular as analytical methods for biomedical research. Transient co-transfection assays have often been used to study protein-protein interactions involved in promoter targeting and transcriptional synergy and antagonism.
There are three possible reasons for our contradictory results. The first is that Pim-1 inhibited the luciferase activity directly but this is unlikely since we had the negative control: Pim-1 did not inhibit the induction of the c-Jun reporter (Figure 1C). The second is that the Elk-1 trans-reporting system used the artificial Gal4-binding element and Gal4-Elk-1 fusion expression plasmid rather than the natural Elk-1-binding element and Elk-1. For this concern, we tested it in the natural c-fos promoter reporter construct. The data from a series of reporter assays seem to be highly consistent but do not correspond to the data of endogenous c-Fos induction. This casts doubts on the accuracy of the reporter gene assay using a plasmid reporter that is out of the natural chromosomal context. It is likely that the transient co-transfection assay produced artifacts.
This drawback of the transient co-transfection assay has already been noticed by researchers (30). A solution to this problem is to prepare stable cells with a reporter plasmid integrated into the chromosome. This places the promoter and reporter gene in a chromosomal context although the context is not at the same site of the chromosome as the natural promoter. However, it is always necessary to double-check the data from reporter assays in the endogenous system at the mRNA or protein level.
We thank Dr. Michael Lilly and Dr. Marina Zemskova of Center for Molecular Biology and Gene Therapy in Loma Linda University, Loma Linda, CA, USA, for discussion and insightful input to our manuscript.
Correspondence and Footnotes

 Address for correspondence: C.-Y. Li, Department of Radiation Oncology, Duke University Medical Center, Box 3455, Durham, NC 27710, USA. Fax: +1-919-684-8718. E-mail: cyli@radonc.duke.edu
Address for correspondence: C.-Y. Li, Department of Radiation Oncology, Duke University Medical Center, Box 3455, Durham, NC 27710, USA. Fax: +1-919-684-8718. E-mail: cyli@radonc.duke.edu
-
1Saris CJ, Domen J & Berns A (1991). The pim-1 oncogene encodes two related protein-serine/threonine kinases by alternative initiation at AUG and CUG. EMBO Journal, 10: 655-664.
- 2. Hoover D, Friedmann M, Reeves R et al. (1991). Recombinant human pim-1 protein exhibits serine/threonine kinase activity. Journal of Biological Chemistry, 266: 14018-14023.
- 3. Padma R & Nagarajan L (1991). The human PIM-1 gene product is a protein serine kinase. Cancer Research, 51: 2486-2489.
- 4. Breuer M, Wientjens E, Verbeek S et al. (1991). Carcinogen-induced lymphomagenesis in pim-1 transgenic mice: dose dependence and involvement of myc and ras. Cancer Research, 51: 958-963.
- 5. van Lohuizen M, Verbeek S, Krimpenfort P et al. (1989). Predisposition to lymphomagenesis in pim-1 transgenic mice: cooperation with c-myc and N-myc in murine leukemia virus-induced tumors. Cell, 56: 673-682.
- 6. Allen JD & Berns A (1996). Complementation tagging of cooperating oncogenes in knockout mice. Seminars in Cancer Biology, 7: 299-306.
- 7. Yan B, Zemskova M, Holder S et al. (2003). The PIM-2 kinase phosphorylates BAD on serine 112 and reverses BAD-induced cell death. Journal of Biological Chemistry, 278: 45358-45367.
- 8. Moroy T, Grzeschiczek A, Petzold S et al. (1993). Expression of a Pim-1 transgene accelerates lymphoproliferation and inhibits apoptosis in lpr/lpr mice. Proceedings of the National Academy of Sciences, USA, 90: 10734-10738.
- 9. Lilly M & Kraft A (1997). Enforced expression of the Mr 33,000 Pim-1 kinase enhances factor-independent survival and inhibits apoptosis in murine myeloid cells. Cancer Research, 57: 5348-5355.
- 10. Pircher TJ, Zhao S, Geiger JN et al. (2000). Pim-1 kinase protects hematopoietic FDC cells from genotoxin-induced death. Oncogene, 19: 3684-3692.
- 11. Wang Z, Bhattacharya N, Weaver M et al. (2001). Pim-1: a serine/threonine kinase with a role in cell survival, proliferation, differentiation and tumorigenesis. Journal of Veterinarian Science, 2: 167-179.
- 12. Maita H, Harada Y, Nagakubo D et al. (2000). PAP-1, a novel target protein of phosphorylation by pim-1 kinase. European Journal of Biochemistry, 267: 5168-5178.
- 13. Ishibashi Y, Maita H, Yano M et al. (2001). Pim-1 translocates sorting nexin 6/TRAF4-associated factor 2 from cytoplasm to nucleus. FEBS Letters, 506: 33-38.
- 14. Leduc I, Karsunky H, Mathieu N et al. (2000). The Pim-1 kinase stimulates maturation of TCRbeta-deficient T cell progenitors: implications for the mechanism of Pim-1 action. International Immunology, 12: 1389-1396.
- 15. Leverson JD, Koskinen PJ, Orrico FC et al. (1998). Pim-1 kinase and p100 cooperate to enhance c-Myb activity. Molecular Cell, 2: 417-425.
- 16. Lilly M, Sandholm J, Cooper JJ et al. (1999). The PIM-1 serine kinase prolongs survival and inhibits apoptosis-related mitochondrial dysfunction in part through a bcl-2-dependent pathway. Oncogene, 18: 4022-4031.
- 17. Rainio EM, Sandholm J & Koskinen PJ (2002). Cutting edge: Transcriptional activity of NFATc1 is enhanced by the Pim-1 kinase. Journal of Immunology, 168: 1524-1527.
- 18. Kyriakis JM, App H, Zhang XF et al. (1992). Raf-1 activates MAP kinase-kinase. Nature, 358: 417-421.
- 19. Dent P, Haser W, Haystead TA et al. (1992). Activation of mitogen-activated protein kinase by v-Raf in NIH 3T3 cells and in vitro Science, 257: 1404-1407.
- 20. Haccard O, Sarcevic B, Lewellyn A et al. (1993). Induction of metaphase arrest in cleaving Xenopus embryos by MAP kinase. Science, 262: 1262-1265.
- 21. Posada J, Yew N, Ahn NG et al. (1993). Mos stimulates MAP kinase in Xenopus oocytes and activates a MAP kinase kinase in vitro Molecular and Cellular Biology, 13: 2546-2553.
- 22. Salmeron A, Ahmad TB, Carlile GW et al. (1996). Activation of MEK-1 and SEK-1 by Tpl-2 proto-oncoprotein, a novel MAP kinase. EMBO Journal, 15: 817-826.
- 23. Ghoda L, Lin X & Greene WC (1997). The 90-kDa ribosomal S6 kinase (pp90rsk) phosphorylates the N-terminal regulatory domain of IkappaBalpha and stimulates its degradation in vitro Journal of Biological Chemistry, 272: 21281-21288.
- 24. Janknecht R, Ernst WH, Pingoud V et al. (1993). Activation of ternary complex factor Elk-1 by MAP kinases. EMBO Journal, 12: 5097-5104.
- 25. Hussain S, Zwilling BS & Lafuse WP (1999). Mycobacterium avium infection of mouse macrophages inhibits IFN-gamma Janus kinase-STAT signaling and gene induction by down-regulation of the IFN-gamma receptor. Journal of Immunology, 163: 2041-2048.
- 26. Kortenjann M, Thomae O & Shaw PE (1994). Inhibition of v-raf-dependent c-fos expression and transformation by a kinase-defective mutant of the mitogen-activated protein kinase Erk2. Molecular and Cellular Biology, 14: 4815-4824.
- 27. Marais R, Wynne J & Treisman R (1993). The SRF accessory protein Elk-1 contains a growth factor-regulated transcriptional activation domain. Cell, 73: 381-393.
- 28. Gille H, Sharrocks AD & Shaw PE (1992). Phosphorylation of transcription factor p62TCF by MAP kinase stimulates ternary complex formation at c-fos promoter. Nature, 358: 414-417.
- 29. Hill CS & Treisman R (1995). Differential activation of c-fos promoter elements by serum, lysophosphatidic acid, G proteins and polypeptide growth factors. EMBO Journal, 14: 5037-5047.
- 30. Chattopadhyay S, Whitehurst CE & Chen J (1998). A nuclear matrix attachment region upstream of the T cell receptor beta gene enhancer binds Cux/CDP and SATB1 and modulates enhancer-dependent reporter gene expression but not endogenous gene expression. Journal of Biological Chemistry, 273: 29838-29846.
Acknowledgments

Publication Dates
-
Publication in this collection
04 May 2006 -
Date of issue
Feb 2006
History
-
Accepted
23 Aug 2005 -
Received
18 May 2005