Abstracts
A total of 67 nests of Centris tarsata were obtained from wood trap-nests of different diameters, consisting of a linear series of brood cells built with sand mixed with oil. This species showed a preference for open habitats, since it occurred only in Swamp and Grassland areas and has never been found in the Araucaria forest. Nesting activity was bigger during the hot season, especially in December and January. The Sex ratio was of 1.48:1 (females/males), significantly different from 1:1. The females were larger than the males and these showed no dimorphism. Males were produced in the outermost cells and females in the innermost cells. C. tarsata presented a direct development without diapause in larval stage. They overwinter as adults. Development time was similar for males and females. Natural enemies are Bombyliidae Mesocheira bicolor, Coelioxys sp. and Meloidae.
nesting biology; bee; Apidae; Centris tarsata
Um total de 67 ninhos de Centris tarsata foi obtido utilizando-se ninhos armadilhas. Eles consistiram de uma série linear de células construídas com uma mistura de areia e óleo. Essa espécie apresentou preferência por ambientes abertos, ocorrendo apenas em áreas de Campo e de Várzea, e não ocorrendo em Florestas de Araucárias. Sua atividade de nidificação foi maior nos meses mais quentes, especialmente dezembro e janeiro. A razão sexual foi de 1,48:1 (fêmeas/machos), significativamente diferente de 1:1. Embora não haja dimorfismo sexual entre os sexos, as fêmeas foram maiores que os machos. Eles foram produzidos nas células mais externas e elas nas mais internas. C. tarsata apresentou desenvolvimento direto, sem diapausa na fase de larva, passando o inverno como adulto. O tempo de desenvolvimento foi similar para fêmeas e machos. Seus inimigos naturais foram: Bombyliidae, Mesocheira bicolor, Coelioxys sp. e Meloidae.
biologia de nidificação; abelha; Apidae; Centris tarsata
BIOLOGY
Nesting biology of Centris (Hemisiella) tarsata Smith in southern Brazil (Hymenoptera, Apidae, Centridini)
Biologia de nidificação de Centris (Hemisiella) tarsata Smith no sul do Brasil (Hymenoptera, Apidae, Centridini)
Buschini, M. L. T.; Wolff, L. L.
Departamento de Biologia, UNICENTRO, Rua Presidente Zacarias, 875, CEP 85010-990, Guarapuava, PR, Brazil
Correspondence to Correspondence to: Maria Luisa Tunes Buschini Depto de Biologia, UNICENTRO Rua Presidente Zacarias, 875 CEP 85010-990, Guarapuava, PR, Brazil e-mail: isatunes@yahoo.com.br
ABSTRACT
A total of 67 nests of Centris tarsata were obtained from wood trap-nests of different diameters, consisting of a linear series of brood cells built with sand mixed with oil. This species showed a preference for open habitats, since it occurred only in Swamp and Grassland areas and has never been found in the Araucaria forest. Nesting activity was bigger during the hot season, especially in December and January. The Sex ratio was of 1.48:1 (females/males), significantly different from 1:1. The females were larger than the males and these showed no dimorphism. Males were produced in the outermost cells and females in the innermost cells. C. tarsata presented a direct development without diapause in larval stage. They overwinter as adults. Development time was similar for males and females. Natural enemies are Bombyliidae Mesocheira bicolor, Coelioxys sp. and Meloidae.
Keywords: nesting biology, bee, Apidae, Centris tarsata.
RESUMO
Um total de 67 ninhos de Centris tarsata foi obtido utilizando-se ninhos armadilhas. Eles consistiram de uma série linear de células construídas com uma mistura de areia e óleo. Essa espécie apresentou preferência por ambientes abertos, ocorrendo apenas em áreas de Campo e de Várzea, e não ocorrendo em Florestas de Araucárias. Sua atividade de nidificação foi maior nos meses mais quentes, especialmente dezembro e janeiro. A razão sexual foi de 1,48:1 (fêmeas/machos), significativamente diferente de 1:1. Embora não haja dimorfismo sexual entre os sexos, as fêmeas foram maiores que os machos. Eles foram produzidos nas células mais externas e elas nas mais internas. C. tarsata apresentou desenvolvimento direto, sem diapausa na fase de larva, passando o inverno como adulto. O tempo de desenvolvimento foi similar para fêmeas e machos. Seus inimigos naturais foram: Bombyliidae, Mesocheira bicolor, Coelioxys sp. e Meloidae.
Palavras-chave: biologia de nidificação, abelha, Apidae, Centris tarsata.
INTRODUCTION
Important pollinator bee families are Anthophoridae, Apidae, Halictidae and Megachilidae (Roubik, 1989). Within the Apidae family, members of the genus Centris are particularly important because of their numerical abundance and their effectiveness as outcrossers (Frankie et al., 1976).
Centris is a primarily tropical genus, whose species are separated into twelve sub-genera (Snelling, 1984). Species of the sub-genera Hemisiella, Heterocentris and Xanthemisia build nests in existing holes.
According to Pereira et al. (1999) information on the nesting biology of species that nest in preexisting cavities remains limited. Jesus & Garófalo (2000) also stated that, although data on nesting biology is available for several nest-excavating species, detailed studies on this aspect for species that nest in preexisting cavities are scarce.
Frankie et al. (1993) carried out a Centris monitoring study in a Costa Rican dry forest. They emphasized that in order to examine the biological basis for changes in Centris bee frequencies throughout time, more information is needed on seasonal behavior and on the basic elements of the reproductive capacity of each species under a variety of environmental conditions. According to the authors, sex ratios vary from species to species and from year to year and information on mortality is necessary for an understanding of the natural enemies and their relation to the changes in Centris bees' frequencies.
In this paper, we present information on nesting biology of Centris (Hemisiella) tarsata, an indigenous solitary bee, widespread in Brazil.
MATERIAL AND METHODS
Study areas
This study was carried out at the Parque Municipal das Araucárias, in the municipality of Guarapuava, state of Paraná, southern Brazil (25° 23' 36'' S and 51° 27' 19'' W, 1.120 m of altitude). This area is characterized by a wet, cool season, and during the warmest months the average temperature is less than 22 °C. Hard frosts are common and severe in this region.
Sampling program
Nests of Centris tarsata were obtained using trap-nests of 0.7, 1.0, and 1.3 cm diameter and 8.0 cm depth. The traps were set up in the field from December 2001 to July 2004 and were concentrated in a very heterogeneous site which included Araucaria forests, swamps and grasslands. Two areas were studied in each habitat, with 2 transects per area and 4 sampling stations per transect. Twelve trap nests were placed in each sampling station, four of each had an opening diameter (0.7, 1.0 and 1.3 cm, respectively), making a total of 576 traps. They were placed 1.5 m above the ground and were inspected every two weeks.
Field-collected traps were brought to the laboratory in order to investigate their contents. If eggs and/or larvae were present, the nest was closed to allow the completion of the life-cycle and the emergence of the adults. Recently emerged adults were carefully removed and weighed. Head widths were also measured.
Humidity and temperature in each habitat were measured throughout the sampling period.
Data analysis
Mann-Whitney's U and Kruskal-Wallis´s H tests were used to test the null hypothesis related to females and males' length and width of cells, premature development time and adults´ head width. Development time was determined by the interval between the date of the collection of the nest and the emergence of the adults. Thus, it was an estimate of that period.
The ANOVA parametric test was used to test the null hypothesis related to the total nest length and nest cell number. Since the variances related to adults' body mass were not homogeneus, all the data was transformed to logarithms and then the ANOVA was run. In all analyses in which this hypothesis was rejected and more than one mean had been compared, the TUKEY test was used to compare ranges of pairs of means.
The sex ratio was calculated by the ratio between the number of females and the number of males, and the chi-square test was used to check the extent by which the observed sex ratio deviated from the expected frequency (1 female:1 male).
RESULTS
Seasonality and nesting activity in different habitats
A total of 67 nests were collected during this study; 19 in the swamp and 48 in the grassland. No nests were built in the Araucaria forest (Fig. 1).
Nests were collected during the hot season (November-March) and nesting activity was more active in December and January (Fig. 2).
Analyzing C. tarsata´s development time, we noticed that this species probably has two generations throughout the year. One raised from nests built by the adults that overwintered, from the middle of November until the middle of January, and the other raised from the middle of January until the end of March and the beginning of April.
Nest Architecture
Nests were obtained in trap-nests of 0.7 cm (n = 18), 1.0 cm (n = 39) and 1.3 cm of diameter (n = 10).
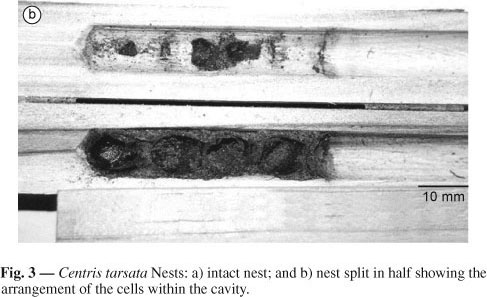
C. tarsata built its nests with sand mixed with an oily substance and probably with wax (Fig. 3a and Fig 3b). The nests consisted of 1 to 4 cells in trap-nests of 0.7 cm and 1 to 6 in those of 1.0 and 1.3 cm diameter, forming a linear series. No significant difference was found in the nest length (F = 0.337; P = 0.715). Regarding the cell number, we noticed that the only significant difference occurred between the average number of trap-nests of 0.7 and 1.3 cm diameter (F = 4.068; P = 0.022).
The cells of trap-nests of 0.7 cm diameter always had a cylindrical shape, but those of 1.0 and 1.3 cm were usually urn-shaped. Sometimes they were elongated ovals and in rare cases ovoid. Regardless of their shape, all cells were rounded at the bottom and truncated at the cell closure. The inner cell wall had a shiny and smooth appearance. Only one 1.0 cm nest had cells which were obliquely disposed. Both the cell partition and nest plug were made of the same material that was used for building the actual cell. The only difference between them is that only the outer side of the plug is covered with an abundant amount of yellowish oily liquid. The cell partitions and nest plugs were a continuation of the cells. Thus, they did not have a cap shape. The average thickness of cell partitions was 0.184, 0.166 and 0.171 cm, and for the nest plug 0.210, 0.179 and 0.224 cm for the nests with 0.7, 1.0 and 1.3 cm diameter, respectively. Vestibular cells occurred only in two nests of 1.0 cm diameter and their number ranged from 1 to 2. Their average length was 0.888 cm (Table 1).
The medians of the males´ cell lengths were significantly different between nests of 0.7 and those of 1.0 and 1.3 cm diameter, but no difference was observed between nests of 1.0 and 1.3 cm (Kruskal-Wallis = 22.820, P = 0.000; P(0.7 cm x 1.0 cm)= 0.000; P(0.7 cm x 1.3 cm) = 0.003; P(1.0 cm x 1.3 cm) = 0.687). For the females´ cells, significant differences were found between the nests of 0.7 and 1.3 cm and those of 1.0 and 1.3 cm (Kruskal-Wallis = 13.086, P = 0.001; P(0.7 cm x 1.0 cm)= 0.051; P(0.7 cm x 1.3 cm) = 0.000; P(1.0 cm x 1.3 cm) = 0.021). When the comparison was made between males and females from the same type of trap-nest, the only situation that did not show a statistical difference was that of males and females from trap-nests of 1.3 cm diameter (Mann-Whitney ( 1.0 cm X
1.0 cm X  1.0 cm) = 69.00; P = 0.001; Mann-Whitney (
1.0 cm) = 69.00; P = 0.001; Mann-Whitney ( 0.7 cm X
0.7 cm X  0.7 cm) = 20.50 P = 0.003; Mann-Whitney (
0.7 cm) = 20.50 P = 0.003; Mann-Whitney ( 1.3 cm X
1.3 cm X  1.3 cm) = 40.50 P = 0.902). The males' cells from trap-nests of 1.0 and 1.3 cm diameter were significantly wider than those from trap-nests of 0.7 cm, but they were similar between themselves (Kruskal-Wallis = 22.613, P = 0.000; P(0.7 cm x 1.0 cm)= 0.000; P(0.7 cm x 1.3 cm) = 0.001; P(1.0 cm x 1.3 cm) = 0.895). The same results was found between the females´ cells (Kruskal-Wallis = 23.229, P = 0.000; P(0.7 cm x 1.0 cm)= 0.000; P(0.7 cm x 1.3 cm) = 0.000; P(1.0 cm x 1.3 cm) = 0.156) (Table 1).
1.3 cm) = 40.50 P = 0.902). The males' cells from trap-nests of 1.0 and 1.3 cm diameter were significantly wider than those from trap-nests of 0.7 cm, but they were similar between themselves (Kruskal-Wallis = 22.613, P = 0.000; P(0.7 cm x 1.0 cm)= 0.000; P(0.7 cm x 1.3 cm) = 0.001; P(1.0 cm x 1.3 cm) = 0.895). The same results was found between the females´ cells (Kruskal-Wallis = 23.229, P = 0.000; P(0.7 cm x 1.0 cm)= 0.000; P(0.7 cm x 1.3 cm) = 0.000; P(1.0 cm x 1.3 cm) = 0.156) (Table 1).
Males were reared in the outer cells and females in the inner cells (Fig. 4). Table 2 shows that the more inner the cell, the longer it was. Their length medians were significantly larger than that of the outer cells.
Cocoon structure and adult size
Cocoons were thin, whitish and translucent. They were compound with only one layer. Thus, the faeces were deposited in some compressed layers at the bottom of the cell. The cocoons were fused along the entire cell and with the faeces layers at the bottom so that when they were removed, the faeces layer was removed with it.
The head width of females from different types of trap-nests were not statistically different (H = 2.859, P = 0.239). The same results were found for males (H = 2.619 P = 0.270). Comparing males and females from the same type of trap-nest, the females were always larger than the males (U ( 0.7 cm X
0.7 cm X  0.7 cm) = 2.00, P = 0.004; U (
0.7 cm) = 2.00, P = 0.004; U ( 1.0 cm X
1.0 cm X  1.0 cm) = 62.50, P = 0.000; U (
1.0 cm) = 62.50, P = 0.000; U ( 1.3 cm X
1.3 cm X  1.3 cm) = 0.00 P = 0.001). Only for the adults of the 1.3cm trap-nests was there no overlay in the head width measures (Table 3).
1.3 cm) = 0.00 P = 0.001). Only for the adults of the 1.3cm trap-nests was there no overlay in the head width measures (Table 3).
Life-History and Sex ratios
The Sex ratio was of 1.48:1 (females/males) since 62 females and 42 males emerged (significantly different from 1:1, c2 = 3.846, df = 1, 0.05 < P < 0.025).
Of the 67 collected nests, 19 produced both males and females. Only females or males were produced in 2 nests. At least 2 nests were needed so that 1 would produce only females and the other only males. From 2 nests, only parasites and males emerged and from 6 nests only parasites and females emerged. Dead juveniles were also found in nests that produced only males (n = 1) and only females (n = 16).
Centris tarsata showed a direct development without diapause in the larval stage (Table 3). They overwintered as adults. Development time was similar for males (H = 3.821, P = 0.148) and females (H = 1.363, P = 0.506) from different types of trap-nests, and for males and females from the same trap-nest (U ( 0.7 cm X
0.7 cm X  0.7 cm) = 18.50, P = 0.636; U (
0.7 cm) = 18.50, P = 0.636; U ( 1.0 cm X
1.0 cm X  1.0 cm) = 50.50, P= 0.224; U (
1.0 cm) = 50.50, P= 0.224; U ( 1.3 cm X
1.3 cm X  1.3 cm) = 14.00 P = 0.570).
1.3 cm) = 14.00 P = 0.570).
Detailed observations made it possible to measure the duration of the different stages of development. Measurements included the average number of days for the newlyt-laid hatched ( = 2.7 ± 1.60; n = 13), completion of feeding (
= 2.7 ± 1.60; n = 13), completion of feeding ( = 11.4 ± 2.62 n = 8), spinning the cocoon (
= 11.4 ± 2.62 n = 8), spinning the cocoon ( = 2.6 ± 1.14; n = 35) and pupa stage (
= 2.6 ± 1.14; n = 35) and pupa stage ( = 49.8 ± 5.65; n = 4).
= 49.8 ± 5.65; n = 4).
Mortality and Natural Enemies
Out of 40 provisioned cells in 0.7 cm trap-nests, 28 (70%) resulted in death. In 1.0 and 1.3 cm trap-nests, 94 out of 140 (67.14%) and 24 out of 41 (58.54%) provisioned cells, respectively, resulted in mortality.
In 0.7 cm trap-nests, the most important mortality factor was dead juveniles. They were observed in 20 cells (50%), probably due to development failure that occurred in the egg (20%) or in pre-emergent adult stages (22.5%). In three cells no egg or larvae was found, only pollen (7.5%). Parasitoids occurred in 8 cells (20%). Bombyliidae accounted for 12.5% of the attacks, followed by Mesocheira bicolor (Hymenoptera, Apidae) 7.5%.
In 1.0 cm trap nests, dead juveniles were observed in 70 cells (50%), probably due to development failure that occurred in the egg (2.14%), larvae (18.57%), pupa (4.29%) or pre-emergent adult stages (17.86%). In 10 cells (7.14%) no egg or larvae was found, only pollen. Twenty four cells (17.14%) were attacked by parasitoids. Bombyliidae accounted for 7.86% of the cases followed by Mesocheira bicolor (5%), Coelioxys sp. (Hymenoptera, Megachilidae) (3.57%) and Meloidae (Coleoptera) (0.71%).
In nests of 1.3 cm diameter, dead juveniles were observed in 22 cells (53.66%), probably due to development failure that occurred in larvae (7.32%) or pre-emergent adult stages (26.83%). In 8 cells (19.51%) no egg or larvae was found, only pollen. Two cells (4.88%) were attacked by parasitoids, one of them by Bombyliidae (2.44%) and the other by Meloidae (Coleoptera) (2.44%).
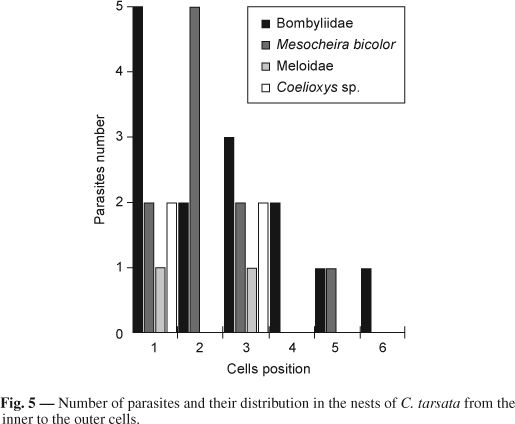
The parasitoid percentage was higher in the first three cells and the inner cells were the most attacked. Bombyliidae was the most frequent parasitoid in these cells. On the other hand, the outer cells were less attacked (Fig. 5).
DISCUSSION
Results in this paper show that C. tarsata preferred open habitats, since it occurred only in Swamp and Grassland areas and never in the Araucaria forest, possibly reflecting the high humidity and lower temperatures found in this environment (Figs. 6 and 7). On the other hand, temperature and humidity were practically similar in the swamp and in the grassland.
Frankie et al. (1988), in a Centris' monitoring study, observed an overall preference of tree hole nesting Centris for more shaded habitats. These authors stated that this type of habitat presented lower temperatures that were more optimal for Centris development since higher temperatures (38 °C to 40 °C) seemed responsible for the mortality of early larval instars. Forysth (1980) demonstrated that certain wasp species could not survive in the general dry forest without these cooler environments.
From 1987 to 1991 Frankie et al. (1993) did another Centris monitoring study in the same areas and noted that C. nitida and C. lutea can apparently tolerate more open areas. In one forest remnant in Minas Gerais (Brazil), C. tarsata was mostly observed nesting in open areas with secondary vegetation than deep inside the forest (Perez-Maluf, 1993). According to Aguiar & Garófalo (2004), C. tarsata is well adapted to nesting in hot, sunny habitats with open vegetation, such as sand dunes and caatingas, but also in forested habitats in northeastern Brazil.
Nesting activites of the C. tarsata occurred in the spring and summer, corresponding to the hot period. This activity pattern was also observed for some Centris species (Heithaus, 1979; Camilo et al., 1993; Pereira et al., 1999; Morato et al., 1999; Aguiar & Garófalo, 2004).
An interesting point that Frankie et al. (1993) discussed is related to bivoltinism in C. analis, C. bicornuta and in C. nitida. According to the authors, bivoltinism is part of the seasonal history of these species and overwintering habitats are known for some species, however they do not know if the tree hole nesters have the capacity to hold over (in diapause) for more than one year. In the present study C. tarsata has never overwintered in diapause during the larval stage. The adults raised from nests built in March and April emerged during the Autumn and spent the cold period taking shelter in unknown places, as observed by Pereira et al. (1999) for C. vittata. As such, we do not believe that C. tarsata stays in diapause as an adult for more than eight months. Pereira et al. (1999) suggested that C. vittata had two generations per year. By C. tarsata´s development time, we also noticed the presence of two generations during the hot season. Aguiar & Garófalo (2004) found that C. tarsata had four generations per year in one of their study area. According to them, while the role of diapause is similar for all species, the survival strategies differ among species and C. tarsata uses two different survival strategies in the presence of adverse conditions, i.e., spending stressful times either as adults or as prepupae in diapause.
Similar to the results presented by Perez-Maluf (1993), Silva et al. (2001) and Aguiar & Garófalo (2004), C. tarsata nested in all trap-nest types, however it preferred those of 1.0 cm diameter. Pereira et al. (1999) reported that C. vittata used trap-nests of various diameters.
As observed by Silva et al. (2001) and by Aguiar & Garófalo (2004), C. tarsata used sand mixed with oil for building nest cells. According to Pereira et al. (1999), it might be a characteristic of the Hemisiella species, and the oily covering of the nest plug provides greater protection to the nest, since the plug becomes harder after being covered. Although Jesus & Garófalo (2000) stated that the cell arrangement and number in Heterocentris, Hemisiella and Xanthemisia species nests depend on the size of the cavity used, as Silva et al. (2001), we observed that the cell number per nest, for C. tarsata, seems to be not influenced by the nest length, since this species did not use all the trap-nest space. However the cell number and disposition observed in our study were different from those observed by Silva et al. (2001).
The C. tarsata cocoons were similar to those of C. vittata (Pereira et al., 1999) since they were thin, whitish and translucent and were fused along the entire cell. On the other hand, they were compound with only one layer and the faeces were deposited in some compressed layers at the bottom of the cell. Our results were similar to those found by Aguiar & Garófalo (2004).
No dimorphism was found in males, and the females were significantly larger than the males as reported for other Hemisiella species (Pereira et al., 1999; Jesus & Garófalo, 2000; Silva et al., 2001).
As reported by Silva et al. (2001) and as observed in the present study, the innermost cells of the nest produced females and the outermost cells males. Similar results were found for C. vittata (Pereira et al., 1999) and C. analis (Jesus & Garófalo, 2000). Pereira et al. (1999) emphasized that since males emerge before females, this spatial arrangement facilitated their emergence. Since we found no statistical difference between their development time, it can be presumed that more observations need to be made in order to elucidate the main factors associated to their arrangement, since this is a pattern which occurs by many solitary bees and wasp species (Krombein, 1967).
One factor that has been considered a possible explanation for sex arrangement in the nests is parasitism. According to Jayasingh & Taffe (1982), the cells closer to the nest entrance should have higher mortality rates due to natural enemies because of their greater exposure. PerezMaluf (1993) reported that the female strategy of laying male eggs in cells that are closest to the nest entrance and female eggs in the innermost cells could be a response to the behavior of parasitoid species that enter the nests by their entrance, causing outer cells to be more heavily parasitized. Given that sons are less costly to produce than the daughters, their position in the outer cells should decrease the investment lost by the female founder. It can be presumed that even this fact needs to be investigated more closely because in our study we observed that the parasitism was more frequent in the inner cells, mainly the first one. For us, this is evidence that female cells have higher parasitism rates.
The percentage of dead C. tarsata juveniles was high, as observed by Perez-Maluf (1993) and by Aguiar & Garófalo (2004) for this species and by Jesus & Garófalo (2000) for C. analis. Frankie et al. (1988) found the same results for some Centris species in Costa Rica. These authors emphasized that high environmental temperatures could be the cause of mortality and would affect mainly early instar larvae. This is probably an important factor related to mortality but we cannot forget the fact that their nests are removed from the natural environment and maintained in the laboratory. We suggest that the main cause of mortality is humidity, because Guarapuava is a subtropical region and even during the hot season the temperature is not as high as in Costa Rica or southeastern Brazil. Since this region does not have a dry season, under natural conditions the nest walls are able to maintain their humidity, but in the laboratory they turn very dry. The percentage of dead pre-emergent adults was always higher than that in the egg or early larval stages.
Regarding the parasites, the percentage of parasitized cells was low. Bombyliidae, Mesocheira bicolor, Coelioxys and meloid beetles were the parasites associated with C. tarsata´s nests both in Guarapuava and in Viçosa (Minas Gerais, Brazil) (Perez-Maluf, 1993). These parasites were also found in C. vittata nests (Pereira et al., 1999), C. analis (Jesus & Garófalo, 2000) and C. dichrootricha (Morato, 1999).
In spite of the fact that female cells were more parasitized, the total sex ratios were female biased. Frankie et al. (1993) pointed out that information on the basic elements of the reproductive capacity of each Centris species, such as sex ratios, must be developed under a variety of environment conditions since they may vary from species to species, and from year to year. Considering their point of view, we calculated the C. tarsata´s sex ratios for each year of our study and it was never significantly different from 1:1. This leads us to think that the founder invested more in female production.
Acknowledgments Partial financial support was provided by the Fundação Araucária (The State of Paraná Research Foundation) and UNICENTRO (Guarapuava PR, Brazil) .We thank Prof. Dr. Miguel Petrere Jr. from UNESP (Rio Claro SP, Brazil) for statistical discussion. We also thank Prof. Dr. Gabriel Augusto R. Melo and Profa Dra. Danuncia Urban from UFPR (PR, Brazil) for identifying the bees and Prof. Ms Sérgio Bazílio from the UNICENTRO (PR, Brazil) for some help.
Received February 11, 2005
Accepted April 12, 2005
Distributed November 1, 2006
- AGUIAR, C. M. L. & GARÓFALO, A. G., 2004, Nesting biology of Centris (Hemisiella) tarsata Smith (Hymenoptera, Apidae, Centridini). Rev. Bras. Zool., 21(3): 477-486.
- CAMILLO, E., GARÓFALO. C. A. & SERRANO J. C., 1993, Hábitos de nidificação de Melitoma segmentaria, Centris collaris, Centris fuscata e Paratetrapedia gigantea (Hymenoptera: Anthophoridae). Rev. Bras. Entomol., 37: 145-156.
- FORSYTH, A., 1980, Nest side and habitat selection by the social wasps, Metapolybia azteca (Hymenoptera, Vespidae). Brenesia. 17: 157-162.
- FRANKIE, G. W., OPLER, P. A. & BAWA, K. S., 1976, Foraging behavior of solitary bees: implications for outcrossing of a neotropical forest tree species. J. Ecol., 64: 1049-1057.
- FRANKIE, G. W., VINSON, S. B., NEWSTRON, L. E. & BARTHELL, J. F., 1988, Nest side and habitats preferences of Centris bees in the Costa Rican dry forest. Biotropica, 20(4): 301-310.
- FRANKIE, G. W., NEWSTRON, L. E., VINSON, S. B. & BARTHELL J. F., 1993, Nesting-habitat preferences of selected Centris bee species in Costa Rican Dry forest. Biotropica, 25(3): 322-333.
- HEITHAUS, E. R., 1979, Community structure of neotropical flower visiting bees and wasps: diversity and phenology. Ecology, 60: 190-202.
- JAYASINGH, D. B. & TAFFÉ, C. A., 1982, The biology of the eumenid mud-wasp Pachodynerus nasidens in trapnests. Ecological Entomology, 7: 283-289.
- JESUS, B. M. & GARÓFALO, C. A., 2000, Nesting behaviour of Centris (Heterocentris) analis (Fabricius) in southeastern Brazil (Hymenoptera, Apidae, Centridini). Apidologie, 31(4): 503-515.
- KROMBEIN, K. V., 1967, Trap-nesting Wasps and Bees Life Histories. Nests and Associates, Smithsonian Press, Washington.
- MORATO, E. F., GARCIA, M. V. B. & CAMPOS, L. A. O., 1999, Biologia de Centris Fabricius (Hymenoptera, Anthoporidae, Centridini) em matas continuas e fragmentos na Amazônia Central. Rev. Bras. Zool., 16: 1213-1222.
- PÉRES-MALUF, R., 1993, Biologia de vespas e abelhas solitárias, em ninhos-armadilhas, em Viçosa- MG. M. S. thesis, Universidade Federal de Viçosa, Viçosa, Minas Gerais, Brasil.
- PEREIRA, M., GARÓFALO, C. A., CAMILLO, E. & SERRANO, J. C., 1999, Nesting biology of Centris (Hemisiella) vittata Lepeletier in southeastern Brazil (Hymenoptera, Apidae, Centridini). Apidologie, 30: 327338.
- SILVIA, F. O., VIANA, B. F. & NEVES, E. L., 2001, Biologia e Arquirtetura de Ninhos de Centris (Hemisiella) tarsata Smith (Hymenoptera: Apidae: Centridini). Neotropical Entomology, 30(4): 541545.
- SNELLING, R. R., 1984, Studies on the taxonomy and distribution of American Centridine bees (Hymenoptera, Anthophoridae). Contrib. Science, Los Angeles, California Museum Nat. Hist., 347: 169.
- ROUBIK, D. W., 1989, Ecology and natural history of tropical bees, Cambridge University Press, Cambridge.
Publication Dates
-
Publication in this collection
13 Apr 2007 -
Date of issue
Nov 2006
History
-
Accepted
12 Apr 2005 -
Received
11 Feb 2005