Abstracts
Toxoplasma gondii strains displaying the Type I/III genotype are associated with acquired ocular toxoplasmosis in humans. Here, we used a mice model to characterize some immunological mechanisms involved in host resistance to infection with such strains. We have chosen the Type I/III strains D8, G2 and P-Br, which cause a chronic infection in mice that resembles human toxoplamosis. Mice deficient of molecules MyD88, IFN-gamma, and IL-12 were susceptible to all three parasite strains. This finding indicates the importance of innate mechanisms in controlling infection. On the other hand, MHC haplotype did not influenced resistance/susceptibility; since mice lineages displaying a same genetic background but different MHC haplotypes (H2b or H2d) developed similar mortality and cyst numbers after infection with those strains. In contrast, the C57BL/6 genetic background, and not MHC haplotype, was critical for development of intestinal inflammation caused by any of the studied strains. Finally, regarding effector mechanisms, weobserved that B and CD8+ T lymphocytes controlled survival,whereas the inducible nitric oxide synthase influenced cyst numbers in brains of mice infected with Type I/III strains. These findings are relevant to further understanding of the immunologic mechanisms involved in host protection and pathogenesis during infection with T. gondii.
Toxoplasma gondii strains; innate immunity; acquired immunity; TLR and MHC
Cepas de Toxoplasma gondii que apresentam o genótipo I/III são associadas a toxoplasmose ocular adquirida em humanos. No presente trabalho, nós utilizamos um modelo da doença em camundongos para caracterizar mecanismos imunológicos envolvidos na resistência do hospedeiro à infecção por aquelas cepas. Escolhemos as cepas D8, G2 e P-Br, que causam infecção crônica em camundongos, semelhante à toxoplasmose humana. Camundongos deficientes em MyD88, IFN-G e IL-12 foram susceptíveis a infecções com todas as três linhagens do parasita. Esses dados indicam a importância de mecanismos inatos no controle da infecção. Por outro lado, o haplótipo do MHC não influenciou na resistência/susceptibilidade, na medida em que linhagens de camundongos com um mesmo "background'' genético, mas diferentes haplótipos de MHC (H2b e H2d) apresentam o índice de mortalidade e número de cistos semelhantes após a infecção com aquelas cepas do parasita. Em contraste, o "background'' genético de C57BL/6, mas não o haplótipo de MHC, foi crítico para o desenvolvimento de inflamação intestinal causada pelas cepas estudadas. Finalmente, com relação aos mecanismos efetores, observamos que linfócitos B e T CD8+ controlam a sobrevivência após infecção. Por outro lado, a ativação da enzima óxido nítrico sintase induzida foi um fator importante para controle do número de cistos cerebrais em camundongos infectados com cepas do Tipo I/III. Esses achados são relevantes para o melhor entendimento dos mecanismos imunológicos envolvidos na proteção e patogênese durante infecção com T. gondii.
cepas de Toxoplasma gondii; imunidade inata; imunidade adquirida; TLR e MHC
BIOLOGICAL SCIENCES
The role of MHC haplotypes H2d/H2b in mouse resistance/susceptibility to cyst formation is influenced by the lineage of infective Toxoplasma gondii strain
Marianne G. ResendeI; Blima FuxI, IV ; Brália C. CaetanoI, II; Erica A. MendesII; Neide M. SilvaV; Adriana M. FerreiraIII; Maria Norma MeloIII; Ricardo W.A. VitorIII; Ricardo T. GazzinelliI, II, VI
ILaboratório de Imunopatologia, Instituto René Rachou, Av. Augusto de Lima, 1715, Centro 30190-002 Belo Horizonte, MG, Brasil
IIDepartamento de Bioquímica e Imunologia, Universidade Federal de Minas Gerais Av. Antônio Carlos, 6627, Pampulha, 31270-901 Belo Horizonte, MG, Brasil
IIIDepartamento de Parasitologia, Universidade Federal de Minas Gerais Av. Antônio Carlos, 6627, Pampulha, 31270-901 Belo Horizonte, MG, Brasil
IVDepartment of Molecular Microbiology, Washington University School of Medicine 660 S. Euclid Ave., 63110 Saint Louis, MO, U.S.A
VInstituto de Ciências Biomédicas, Universidade Federal de Uberlândia Av. João Pinheiro, 565, Centro, 38400-902 Uberlândia, MG, Brasil
VIDivision of Infectious Diseases and Immunology, University of Massachusetts Medical School 364 Plantation Street, 01605 Worcester, MA, U.S.A
Correspondence to Correspondence to: Ricardo T. Gazzinelli E-mail: ritoga@cpqrr.fiocruz.br
ABSTRACT
Toxoplasma gondii strains displaying the Type I/III genotype are associated with acquired ocular toxoplasmosis in humans. Here, we used a mice model to characterize some immunological mechanisms involved in host resistance to infection with such strains. We have chosen the Type I/III strains D8, G2 and P-Br, which cause a chronic infection in mice that resembles human toxoplamosis. Mice deficient of molecules MyD88, IFN-g, and IL-12 were susceptible to all three parasite strains. This finding indicates the importance of innate mechanisms in controlling infection. On the other hand, MHC haplotype did not influenced resistance/susceptibility; since mice lineages displaying a same genetic background but different MHC haplotypes (H2b or H2d) developed similar mortality and cyst numbers after infection with those strains. In contrast, the C57BL/6 genetic background, and not MHC haplotype, was critical for development of intestinal inflammation caused by any of the studied strains. Finally, regarding effector mechanisms, weobserved that B and CD8+ T lymphocytes controlled survival,whereas the inducible nitric oxide synthase influenced cyst numbers in brains of mice infected with Type I/III strains. These findings are relevant to further understanding of the immunologic mechanisms involved in host protection and pathogenesis during infection with T. gondii.
Key words:Toxoplasma gondii strains, innate immunity, acquired immunity, TLR and MHC.
RESUMO
Cepas de Toxoplasma gondii que apresentam o genótipo I/III são associadas a toxoplasmose ocular adquirida em humanos. No presente trabalho, nós utilizamos um modelo da doença em camundongos para caracterizar mecanismos imunológicos envolvidos na resistência do hospedeiro à infecção por aquelas cepas. Escolhemos as cepas D8, G2 e P-Br, que causam infecção crônica em camundongos, semelhante à toxoplasmose humana. Camundongos deficientes em MyD88, IFN-G e IL-12 foram susceptíveis a infecções com todas as três linhagens do parasita. Esses dados indicam a importância de mecanismos inatos no controle da infecção. Por outro lado, o haplótipo do MHC não influenciou na resistência/susceptibilidade, na medida em que linhagens de camundongos com um mesmo "background'' genético, mas diferentes haplótipos de MHC (H2b e H2d) apresentam o índice de mortalidade e número de cistos semelhantes após a infecção com aquelas cepas do parasita. Em contraste, o "background'' genético de C57BL/6, mas não o haplótipo de MHC, foi crítico para o desenvolvimento de inflamação intestinal causada pelas cepas estudadas. Finalmente, com relação aos mecanismos efetores, observamos que linfócitos B e T CD8+ controlam a sobrevivência após infecção. Por outro lado, a ativação da enzima óxido nítrico sintase induzida foi um fator importante para controle do número de cistos cerebrais em camundongos infectados com cepas do Tipo I/III. Esses achados são relevantes para o melhor entendimento dos mecanismos imunológicos envolvidos na proteção e patogênese durante infecção com T. gondii.
Palavras-chave: cepas de Toxoplasma gondii, imunidade inata, imunidade adquirida, TLR e MHC.
INTRODUCTION
Toxoplasma gondii is an obligate intracellular coccidian belonging to the phylum Apicomplexa. The parasite is globally distributed and has been described to infect more than 30 species of birds and 300 species of mammals, including humans (Dubey et al. 2002). Possibly one-third of the world's human population is infected with T. gondii (Dubey 1998, Tenter et al. 2000). Infection with the parasite occurs via three main routes: congenitally by transplacental transmission of tachyzoites; ingestion of food or water contaminated with oocysts shed in feces of infected cats (Bahia-Oliveira et al. 2003, de Moura et al. 2006); or by ingestion of raw or undercooked meat containing tissue cysts (Dubey 1996). Infection is asymptomatic in most individuals, whereas severe pathology and lethality due to toxoplasmosis is a common finding in congenitally infected or immunodeficient patients (Desmonts and Couvreur 1974). In addition, toxoplasmosis is one of the most common causes of infectious uveitis in both immunocompetent and immunocompromised persons (Holland 1999, Colombo et al. 2005). Importantly, some studies indicate that toxoplasmic retinocoroiditis and ocular disease is frequently found in cases of acquired toxoplasmosis (Glasner et al. 1992). Variation in the clinical presentation and severity of disease in susceptible persons has been attributed to several factors, including the genetic heterogeneity of the host and the genotype of the infective parasite (Sibley and Boothroyd 1992, Howe and Sibley 1995, Holland 1999).
Studies performed with strains of T. gondii isolated in North America and Europe showed that they were morphologically similar yet could be grouped into three distinct clonal lineages by isoenzymes or DNA restriction fragment length polymorphisms (Darde et al. 1992, Sibley and Boothroyd 1992, Sibley et al. 1992, Howe et al. 1997). The structure of T. gondii population in those geographic areas is clonal, and most strains fall into one of the three categories denominated Type I, Type II and Type III lineages (Sibley and Boothroyd 1992, Howeand Sibley 1995). The three clonal types are apparently not minor or random polymorphic states of any phenotypic consequence: Type I lineage strains are highly virulent in outbred mice and perhaps humans (Grigg et al. 2001a), whereas type II and III lineage strains are relatively less virulent (Sibley et al. 1992, Howe et al. 1996, 1997). A small percentage of strains are recombinant between two of three parasite lineages, and vary in terms of their virulence phenotype in mice (Grigg et al. 2001a). In North America and Europe, Type II strains are most common in human toxoplasmosis and chronic infections in food animals (Howe and Sibley 1995). Importantly, while type I strains are relatively rare in animals, they occur with increasing frequency in human congenital toxoplasmosis (Fuentes et al. 2001)and ocular disease (Grigg et al. 2001a), whereas recombinant Type I/III strain are more often found in patients with ocular toxoplasmosis (Grigg et al. 2001b). In Brazil, T. gondii isolates present high genetic variability, as demonstrated by recent studies on multilocus PCR RFLP, and the parasite population has an epidemic structure with a few expanded clonal lineages (Ferreira Ade et al. 2006, Pena et al., in press).
Immunogenetic studies provide powerful tools for understanding protective and pathogenic mechanismsin such infectious disease (McLeod et al. 1995, 1996, Mack et al. 1999). Specifically, in the case of T. gondii infection, genes located in different regions of the host genome have been implicated in resistance to infection. Particularly, major histocompatibility (MHC) alleles are important determinants of resistance and susceptibility to early infection, as well as controllers of cyst numbers and encephalitis at later stages of infection with T. gondii, both in mice and humans (McLeod et al. 1989a, Brown and McLeod 1990, Brown et al. 1994, Blackwell et al. 1993, Johnson et al. 2002a, b). These studiesare confirmed by the critical role of CD8+ T as well as CD4+ T cells in host resistance to toxoplasmosis (Brown and McLeod 1990, Suzuki et al. 1991, Gazzinelli et al. 1992). In addition, various cytokines have shown to be critical in host resistance to T. gondii infection (Denkers and Gazzinelli 1998). In the acute phase of toxoplasmosis, T. gondii tachyzoites trigger the synthesis of IL-12 and other co-stimulatory cytokines (e.g. TNF-a), which initiate the synthesis of IFN-g by "natural killer'' (NK) cells and CD4+ ab T lymphocytes (Gazzinelli et al. 1993b, 1994b, Hunter et al. 1994, Cai et al. 2000). IFN-g and TNF-a remain critical to host resistance to chronic toxoplasmosis, probably by their ability to activate the inducible nitric oxide synthase (iNOS) and production of reactive nitrogen intermediates (RNI) thatexert both microbicidal and microbiostatic effects.
We have recently reported a non-clonal distribution of natural recombinant Type I/III strains, in different geographic areas of Brazil, where acquired ocular toxoplasmosis (up to 20% of the infected population) is commonly found among patients with chronic toxoplasmosis (Glasner et al. 1992, Dubey et al. 2002, Fux et al. 2003, Ferreira Ade et al. 2004, 2006, Portela et al. 2004). Importantly, we and others were unable to isolate the avirulent Type II clonotype T. gondii strains in Brazilian territory, where approximately 80% of all isolated strains were virulent (Dubey et al. 2002, Ferreira Ade et al. 2004, 2006). However, most of the immunological studies concerning T. gondii infections have been performed with the ME49, a Type II strain. In the present study, we evaluated the role of different immunological compartments on host resistance to three different recombinant Type I/III strains of T. gondii, which presents low virulence during acute infection and are cystogenic during chronic phase, thus resembling human toxoplasmosis. Our results indicate that components of innate immunity are critical in a similar manner in infections with Type II or the Type I/III strains of T. gondii studied here. In contrast, MHC haplotypes H2b and H2d were not determinants of mice susceptibility and resistance to Type I/III strains, as previously shown for Type II strains of T. gondii (Brown and McLeod 1990).
MATERIALS AND METHODS
ANIMALS
BALB/c, C57BL/6, MyD88-/-, TLR2-/-, TLR4-/-, TLR9-/-, IL-12-/-, IFN-g-/-, iNOS-/-, CD8-/-, Igµ-chain-/-, congenic CB10H2 and C57BL/KsJ and outbreed Swiss Webster mice were housed at the animal facilities of Institute of Biological Sciences (ICB), of the Federal University of Minas Gerais (UFMG), Brazil. Congenic and knockout mice were bred as homozygotes, and knockout mice were backcrossed to at least 8 generations into the genetic background of C57BL/6. All animals were 8 weeks old, and were managed according to institutional standard guidelines.
PARASITE STRAINS
Strains BV, EGS, EFP (virulent), D8, G2 and P-Br (avirulent) were used as representative of Type I/III isolates, whereas strain ME49 was used as a representative of a Type II strain. Strains P-Br and D8 were isolated from dogs, BV and G2 were isolated from goats, and EGS and EFP were isolated from congenitally infected humans (Jamra and Vieira 1991, Fux et al. 2003, Ferreira Ade et al. 2004). ME49 was isolated from a sheep (Lunde and Jacobs 1983). Each strain was maintained by successive inoculation of free tachyzoite and/or tissue cysts in female Swiss Webster mice. Cyst and/or tachyzoites obtained from reservoir mice were used to infect experimental animals.
EXPERIMENTAL INFECTIONS
Experimental infections with tachyzoites from BV, EGS, EFP, D8, G2 and P-Br were performed as described: free tachyzoites were harvested from Swiss Webster reservoirs, 5 to 7 days after infection, by peritoneal washing with PBS. Tachyzoite inoculums were adjusted to 1, 10, 100 and 1,000 cells per 100µL PBS and administered to mice by i.p. injection. Survival was followed thereafter in groups submitted to each tachyzoite dose. Experimental infections with cysts of strains D8, G2, P-Br and ME49 were performed as follows: brains were collected from Swiss Webster reservoirs, 60 days after infection, and homogenized in 1mL PBS. Cysts numbers were determined by microscopic analysis of 10µL aliquots of brain homogenates, in duplicates. Inoculums were adjusted to 4, 20 or 100 cysts per 200µL and administered orally. Survivors were killed 60 days after infection for determination of brain cyst loads, as described above.
HISTOPATHOLOGY
Histological examination was carried out in small intestine samples obtained 7 days after infection with different T. gondii strains. Small intestine samples were embedded in paraffin and cut in 4µm-width sections, which were stained with Hematoxylin-Eosin dye. Slides containing 2 sections of each intestinal sample were analyzed under light microscope for presence of inflammatory infiltrates, and scored from 1 (+) to 4 (+ + + +) based on the intensity of pathological changes.
FLOW CYTOMETRY
Spleen homogenates were obtained individually frominfected or non-infected mice (3 animals per group) andred blood cells were disrupted with ACK lysis buffer. A total of 2 ×106 purified splenocytes were stained with 15µl of optimal concentrations of FITC-conjugated anti-Vb 8, 6 or 5 specific monoclonal antibodies (BDPharmingen, San Diego, CA) in combination with PE-conjugated anti-CD4+ or CD8+ antibodies (BD Pharmingen). Analysis of stained cells was performed inFACScan® (BD Biosciences, San Jose, CA) with Cell Quest® software (BD Biosciences).
STATISTICAL ANALYSIS
To compare brain cyst numbers obtained in different mice lineages infected with different T. gondii strains we used ANOVA test, followed by Tukey multiple comparisons, when appropriate. Otherwise, comparisons were performed by Kruskal-Wallis test, followed by Dunn's multiple comparisons. To compare data between mice of the same lineage infected with different doses (4 or 20 cysts) of one specific T. gondii strain we performed Student's T (for parametric data) or Mann-Whitney's test (for non-parametric data). Comparison of survival data was performed by Kaplan-Meir test. All data were tested for significance in MINITAB software, version13.0, and tests with p < 0.05 were considered statistically significant.
RESULTS
DIFFERENT VIRULENCE OF NATURAL RECOMBINANT TYPE I/III STRAINS OF T. gondii
Strains BV, EGS, EFP, D8, G2 and P-Br were isolatedin Minas Gerais and São Paulo States, in Brazil, and characterized as recombinant Type I/III strains (Fux et al. 2003, Ferreira Ade et al. 2004). Results of experimental infections of BALB/c with different tachyzoite doses of each strain are shown in Figure 1. All animals infected with BV, EGS and EFP died, independently from inoculum dose. On the other hand, all mice infected with low dose (1 tachyzoite) of EFP survived.In this case, infection was confirmed by detection ofspecific anti T. gondii antibodies in ELISA and Western Blot (data not shown). Thus, BV, EGS and EFPwere classified as highly virulent, virulent and of intermediate virulence, respectively. In contrast, all animals infected with D8, G2, and P-Br survived (Fig. 1), and those strains were considered to be avirulent.
ROLE OF TOLL-LIKE RECEPTORS (TLRS) ON INNATE IMMUNE RESPONSE AND HOST RESISTANCE TO INFECTION WITH TYPE I/III STRAINS OF T. gondii
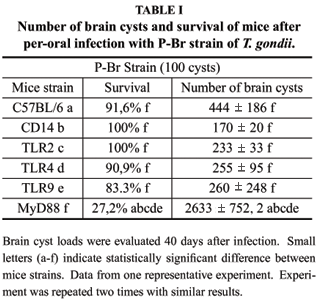
Initially, we infected MyD88-/-, TLR2-/-, TLR4-/- and TLR9-/- mice with either of the Type I/III strains of T. gondii. In addition, we infected the mice devoid of functional CD14, a co-receptor required for optimal TLR2 and TLR4 functions. Mice received an oral dose of 20 cysts of P-Br, D8 and G2 and, after 45 days, survivors were sacrificed for determination of brain cysts numbers as an indicator of host resistance to infection. As previously shown for strain ME49 (Type II) (Scanga et al. 2002), MyD88-/- mice were highly susceptible to all Type I/III strains tested (not shown). In contrast, we were unable to detect any change in host resistance/susceptibility to infection with Type I/III strains in single TLR (TLR2, TLR4 or TLR9) or CD14 knockout mice (not shown). An early study suggested that increasing infective doses of ME49 could lead to a susceptible phenotype in TLR2-/- (Mun et al. 2003). Thus, we decided to use 100 cysts of P-Br as inoculums for different knockout mice. Results were identical to those obtained with 20 cysts, i.e., enhanced susceptibility of MyD88-/- mice, but not of TLR2-/-, TLR4-/-, TLR9-/- or CD14-/- mice (Table I).
MyD88 is an adaptor of all TLR (except TLR3) functions, and is critical for induction of pro-inflammatory cytokines, including IL-12. Importantly, IL-12 isthe initiator for IFN-g synthesis by NK cells and T lymphocytes, and thus to host resistance to T. gondii. To further analyze the importance of these pro-inflammatory cytokines during initial stages of infection, we inoculated IL-12-/- and INF-g-/- mice with either 04 or 20 cysts of P-Br, D8 and G2 strains and the survival was monitored over 45 days period. Infection of IL-12-/- and INF-g-/- mice, even with the lower dose (4 cysts), resulted in 100% mortality around 10-20 days post-infection (Fig. 2).
INFLUENCE OF MHC HAPLOTYPE IN CYST NUMBERS OF MICE INFECTED WITH TYPE I/III STRAINS OF T. gondii
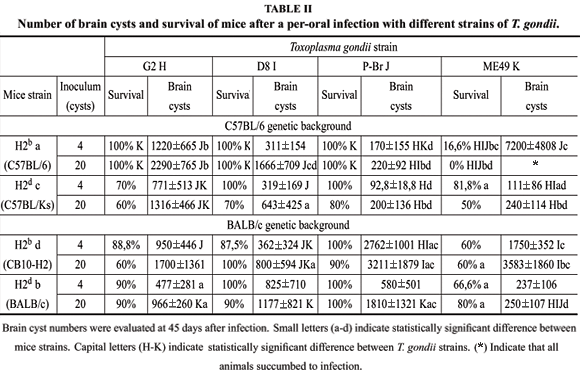
We have investigated the influence of MHC haplotype in controlling cyst numbers and mouse resistance to Type I/III strains of T. gondii. To achieve this purpose, we selected mice lineages which display H2 haplotypes "b'' and "d'', which were previously demonstrated as markers of, respectively, susceptibility and resistance to infection with T. gondii. Mice lineages involved in this experiment were parental BALB/c (H2d) and C57BL/6 (H2b) or the derivative congenic strains CB10-H2 (BALB/c genetic background with H2 haplotype) and C57BL/KsJ (C57BL/6 genetic background with H2b haplotype). Results (Table II) confirm previous observations that mice strains expressing haplotype "d'' are more resistant to ME49 than mice that express "b'' haplotype, since BALB/ c and C57BL/KsJ developed fewer brain cysts after 45 days of infection. Nevertheless, this feature was not observed in infections with D8 and G2, which induced similar cyst burdens and low mortality in the different mouse congenic strains analyzed. It is noteworthy, that the C57BL/6 genetic background, regardless of the MHC haplotype, conferred resistance to cyst formation, when infected with P-Br strain (Table II).
To depict the immune mechanisms involved in susceptibility/resistance of different mice to T. gondii, we studied the pattern of antibody response, and compared IgG1 versus IgG2a levels in infected animals. We observed that, independently of the T. gondii strain used in infection, antibody responses were consistent within a similar mouse genetic background. BALB/c and CB10-H2 produced high IgG1:IgG2a ratios, whereas the same parameter was smaller in the C57BL/6 and C57BL/KsJ, indicating a stronger Th1 response in the latter mice (data not shown). Consistently, the intense intestinal inflammatory process associated to acute toxoplasmosis - which is dependent on strong Th1 responses, with high levels of IFN-g and RNI - was only observed in mice of the C57BL/6 genetic background (Table III). The H2d haplotype expressed by the C57BL/KsJ strain resulted in increased intestinal inflammation in mice infected with D8 and P-Br strains when compared to C57BL/6 mice that express the H2b haplotype.
Usage of specific TCR Vb8 chain was previously indicated as a mechanism of resistance to severe Toxoplasmic Encephalitis in BALB/c. On the other hand, susceptible CBA mice were demonstrated to activate higher levels of Vb6 bearing T cells after infection with T. gondii, and develop greater inflammation and brain cysts than BALB/c (Wang et al. 2005). We then decided to investigate the frequency of CD4+ T and CD8+ T cells expressing Vb8, Vb6 and Vb5, in BALB/c and C57BL/6 mice infected with P-Br or ME49. According to results shown in Figure 3 and Table IV, a higher frequency of CD4+ and CD8+ T lymphocytes expressing Vb8 is naturally found in BALB/c and C57BL/6 mice. However, no alteration of frequency of any specific Vb family was detected after infection with either T. gondii strain.
EFFECTOR MECHANISMS IN CONTROL OF CYST NUMBERS AND HOST RESISTANCE TO INFECTION WITH TYPE I/III STRAINS OF T. gondii
Finally, we decided to investigate the role of different effector mechanisms in control of cyst numbers and hosts resistance to Type I/III strains of T. gondii. We infected CD8-/-, Igµ-chain-/- (B-/-) and iNOS-/- mice with 4(not shown) and 20 cysts of D8, G2 and P-Br strains (Table V). Our results indicate that CD8+ T cells and, in a lesser extent, B lymphocytes were important components controlling survival. However, regarding cyst formation, it appears that CD8+ T and B cells, individually, are not the major mediators of protection. Infection of iNOS -/- mice resulted in 40%, 80% and 40% survival with G2, D8 and P-Br strains, respectively. Further, the remaining iNOS-/- survivors developed high cyst burdens (Table V). These findings suggest that IFN-g produced by CD4+ T cells and production of RNI is indeed a key pathway for controlling cyst numbers in the CNSof mice infected with Type I/III strains.
DISCUSSION
Although T. gondii is regarded as the only species in genus Toxoplasma, genetically different strains of the parasite have been described. The genetic variance was already extensively assessed in samples of T. gondiifound mainly in Europe and North America. According to genetic markers and virulence during acute infection in mice those strains have been divided into Type I, Type II and Type III groups (Sibley and Boothroyd 1992, Howe and Sibley 1995). Different studies have illustrated the high frequency of Type I and III, and the absence of Type II, isolates in Brazil (Dubey et al. 2002, 2003a, b, 2004). A previous study of our group revealed an unusually high frequency in Brazil (100% of isolates analyzed) of strains that shared Type I and III genetic markers (Fux et al. 2003). Such strains were separately classified as natural recombinant Type I/III, and amongst them, 85% displayed some degree of virulence and only 15% were avirulent. The highly virulent strains were more closely related to Type I genotype (Ferreira Ade et al. 2006). These findings contrast with those from North America and Europe, where most isolates associated to human infections are avirulent Type II (Sibley and Boothroyd 1992, Howe and Sibley 1995). Importantly, Grigg and colleagues (Grigg et al. 2001b) alsofound in North America an unexpected high occurrence of Type I, and recombinant Type I/III strains in patients with ocular toxoplasmosis. A high prevalence of ocular toxoplasmosis is observed in Brazilian populations, and most cases are attributed to acquired infection (Glasner et al. 1992, Holland 1999, Silveira et al. 2001, Portela et al. 2004). Although speculative, we suggest that these occurrences could be attributed in part to the dominance of Type I/III virulent strains in our territory (Glasner et al. 1992, Silveira et al. 2001, Portela et al. 2004).
In addition to intrinsic parasite virulence factors, susceptibility to Toxoplasma is determined by host genetic characteristics and environmental factors, such as co-infections, including HIV, that affect the immunological status of the host. It was observed that humansinfected with T. gondii have a parasite-specific Th1-polarized cell response, characterized by strong IFN-g production when peripheral blood mononuclear cells(PBMCs) are stimulated with parasite antigens (Gazzinelli et al. 1995) and high levels of anti-parasite specific IgG1 and IgG3 (Giraldo et al. 2000, Portela et al. 2004). Further, presence of primed CD4+ T helper and CD8+ cytotoxic T cells have been demonstrated in PBMCsfrom patients with chronic toxoplasmosis (Hunter et al. 1996). However, decreased cellular responses to T. gondii antigens, including lower IFN-g and IL-2 secretion, occur in congenitally infected patients (McLeod et al.1985, 1990, Yamamoto et al. 2000), and individualsco-infected with HIV (Hunter et al. 1996). In latter case, weaker responses were associated with development of toxoplasmic encephalitis. Regarding the influence of host genetics over toxoplasmosis, it is known the MHC contribution to disease development. It was reported, for example, an increased frequency of MHC HLA-DQ3 in infants with hydrocephalus lesions (Mack et al. 1999). A lower than expected homozygosity also suggested that this allele increased susceptibility to toxoplasmosis. Another study indicated that AIDS patients with HLA-DQ3 haplotype more often experienced reactivation of chronic toxoplasmosis (Suzuki et al. 1996). Studies with mice expressing transgenic human class II MHC alleles showed that HLA-DQ1 was associated with lower parasite burden and pathology comparing to mice with HLA-DQ3 (Mack et al. 1999). Further, mice expressing human class I molecules HLA-DB27 or HLA-Cw3 had enhanced resistance to infection (Brown et al.1994). Here we employed a murine model of toxoplasmosis to characterize the role of different immunological compartments on resistance to natural recombinant Type I/III strains of T. gondii, whose infection in mice resembles human toxoplasmosis.
In mice, cytokines such as IL-12, TNF-a and IFN-g and reactive nitrogen intermediates (RNI) are mediators of resistance to T. gondii (Suzuki et al. 1989, Gazzinelli et al. 1991, 1992, 1993a, b, 1994a, Hayashi et al. 1996a, b, Scharton-Kersten et al. 1996, 1997, Denkers and Gazzinelli 1998). Nevertheless, pathology associated with excessive stimulation of those pro-inflammatory cytokines also occur during acute toxoplasmosis in mice lacking IL-10 (Gazzinelli et al. 1996, Liesenfeld et al. 1996, Neyer et al. 1997). Results presented here show that components of innate immunity, i.e., MyD88, IL-12 and IFN-g are also critical for resistance during early stages of infection with Type I/III strains. Indeed, MyD88 and TLR11 have been shown to be critical ineliciting pro-inflammatory cytokines in mice, and development of Th1 lymphocytes during toxoplasmosis(Scanga et al. 2002, Yarovinsky et al. 2005). In addition, we have recently demonstrated that T. gondii derived glycosylphosphatidylinositol (GPI) anchors activate TLR2 and TLR4, and that mice lacking both receptors have a small increment in susceptibility. However, by testing mice deficient in a single TLR (i.e. TLR2, TLR4, or TLR9) or the related co-receptor (CD14) we were unable to define a single innate immune receptor that is critical for resistance. Together, our findings with natural recombinant Type I/III strains indicate that T. gondii parasites may engage various TLRs during infection of the intermediate host.
Resistance to chronic T. gondii infection is associated with an acquired Th1-type cellular response (Gazzinelli et al. 1991, 1992, 1994a). Indeed, both class I and class II MHC molecules have been shown to influence disease outcome after T. gondii infection in mice (McLeod et al. 1989b, Brown and McLeod 1990, Suzuki et al. 1991, Blackwell et al. 1993, Johnson et al. 2002a). More precisely, H2 haplotypes "d'' and "b'' are, respectively, determinants of host resistance and susceptibility to infection with ME49 (Brown and McLeod 1990, Brown et al. 1995). Consistently, CD4+ and CD8+ T cells are essential components in host resistance to the parasite (Gazzinelli et al. 1991, Suzuki et al. 1991). Further, there is also evidence that a singular pattern of TCR Vb chain usage by anti-T. gondii T cells could influence protection. Indeed, it was demonstrated that a higher frequency of Vb8 chain in T lymphocytes was associated with BALB/c (H2d) resistance against TE causedby ME49, whereas preferential Vb6 chain usage co-related with susceptibility in CBA/Ca (H2k). Adoptive transfer of BALB/c Vb8+, but not Vb6+, T cells wascapable to prevent mortality and encephalitis in nude mice (Kang et al. 2003, Wang et al. 2005). Another evidence of the importance of cell-mediated effectormechanisms is that neutralization of IFN-g or TNF-a and inhibition of NOS2 leads to reactivation of chronic infection with ME49 and results in encephalitis anduveitis (Gazzinelli et al. 1992, 1993a, 1994a, Hayashi et al. 1996a, b). Finally, B lymphocytes have also been shown to contribute to host resistance, as demonstrated in experiments with knockout mice (Johnson and Sayles 2002). However, most of the studies described above employed strain ME49 that belongs to Type II lineageof T. gondii.
Consistently, we found that Type I/III strains also elicited strong Th1 responses with high IFN-g, and high IgG2/IgG1 ratio of specific antibodies (Fux et al. 2003). Indeed, IFN-g was shown essential for survival of animals infected with these parasite isolates. However, in contrast to results obtained with congenic mice infected with ME49, MHC haplotype H2d was not an important determinant of resistance to Type I/III isolates. Further, we were unable to find any association between usage of specific TCR Vb chains and resistance/susceptibility to Type I/III strains. While not essential, B lymphocytes, CD8+ T cells and NOS2 seamed to be involved in mechanisms controlling cyst numbers and host survival during infection with those natural recombinant strains of T. gondii isolated in Brazil.
In conclusion, the elements MyD88, IL-12 and IFN-g from the cellular compartment of innate immunity are critical during early stages of infection with Type I/III strains of T. gondii. However, we found a discrepancy on the role of MHC haplotypes in host resistance/susceptibility to Type I/III Brazilian isolates, when comparing to the standard ME49, which is used in many laboratories for immunological studies. Results suggest that an immunodominant T cell epitope expressed by Type II strains, but not by Type I/IIIisolates, may be involved on induction of protective acquired immunity during T. gondii infection. Identification of parasite antigen that encodes this particular Tcell epitope may contribute to better understanding the different biological behavior of Type II versus Type I/III strains of T. gondii and eventually the pathogenesis ofocular toxoplasmosis.
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health (RO1 AI071319-01) and by the Millennium Institute of Technology and Vaccine Development - Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). RTG, MNM, and RWAV are Research Fellows from CNPq. BF had a post-doctoral fellowship from the Fundação Oswaldo Cruz (FIOCRUZ/ CNPq program. BCC have a fellowship from FIOCRUZ/ PDTIS - Vacinas program.
Manuscript received on November 11, 2007; accepted for publication on January 21, 2008; contributed by RICARDO T. GAZZINELLI* * Member Academia Brasileira de Ciências
- BAHIA-OLIVEIRA LM, JONES JL, AZEVEDO-SILVA J, ALVES CC, OREFICE F AND ADDISS DG.2003. Highly endemic, waterborne toxoplasmosis in north Rio de Janeiro state, Brazil. Emerg Infect Dis 9: 5562.
- BLACKWELL JM, ROBERTS CW AND ALEXANDER J.1993. Influence of genes within the MHC on mortality and brain cyst development in mice infected with Toxoplasma gondii: kinetics of immune regulation in BALBH-2 congenic mice. Parasite Immunol 15: 317324.
- BROWN CR AND MCLEOD R. 1990. Class I MHC genes and CD8+ T cells determine cyst number in Toxoplasma gondii infection. J Immunol 145: 34383441.
- BROWN CR, DAVID CS, KHARE SJ AND MCLEOD R.1994. Effects of human class I transgenes on Toxoplasmagondii cyst formation. J Immunol 152: 45374541.
- BROWN CR, HUNTER CA, ESTES RG, BECKMANN E, FORMAN J, DAVID C, REMINGTON JS AND MCLEOD R. 1995. Definitive identification of a gene that confers resistance against Toxoplasma cyst burden and encephalitis. Immunology 85: 419428.
- CAI G, KASTELEIN R AND HUNTER CA.2000. Interleukin-18 (IL-18) enhances innate IL-12-mediated resistance to Toxoplasma gondii Infect Immun 68: 69326938.
- COLOMBO FA, VIDAL JE, PENALVA DE OLIVEIRA AC, HERNANDEZ AV, BONASSER-FILHO F, NOGUEIRA RS, FOCACCIA R AND PEREIRA-CHIOCCOLA VL. 2005. Diagnosis of cerebral toxoplasmosis in AIDS patients in Brazil: importance of molecular and immunological methods using peripheral blood samples. J Clin Microbiol 43: 50445047.
- DARDE ML, BOUTEILLE B AND PESTRE-ALEXANDRE M. 1992. Isoenzyme analysis of 35 Toxoplasma gondii isolates and the biological and epidemiological implications. J Parasitol 78: 786794.
- DE MOURA L ET AL. 2006. Waterborne toxoplasmosis, Brazil,from field to gene. Emerg Infect Dis 12: 326329.
- DENKERS EY AND GAZZINELLI RT. 1998. Regulation and function of T-cell-mediated immunity during Toxoplasma gondii infection. Clin Microbiol Rev 11: 569588.
- DESMONTS G AND COUVREUR J. 1974. Toxoplasmosis in pregnancy and its transmission to the fetus. Bull NYA cad Med 50: 146159.
- DUBEY JP. 1996. Strategies to reduce transmission of Toxoplasma gondii to animals and humans. Vet Parasitol 64: 6570.
- DUBEY JP. 1998. Advances in the life cycle of Toxoplasma gondii Int J Parasitol 28: 10191024.
- DUBEY JP ET AL. 2002. Biological and genetic characterisation of Toxoplasma gondii isolates from chickens (Gallus domesticus)from São Paulo, Brazil: unexpected findings. Int J Parasitol 32: 99105.
- DUBEY JP, GRAHAM DH, DA SILVA DS, LEHMANN T AND BAHIA-OLIVEIRA LM. 2003a. Toxoplasma gondii isolates of free-ranging chickens from Rio de Janeiro, Brazil: mouse mortality, genotype and oocyst shedding by cats. J Parasitol 89: 851853.
- DUBEY JP, NAVARRO IT, GRAHAM DH, DAHL E, FREIRE RL, PRUDENCIO LB, SREEKUMAR C, VIANNA MC AND LEHMANN T. 2003b. Characterization of Toxoplasma gondii isolates from free range chickens from Paraná, Brazil. Vet Parasitol 117: 229234.
- DUBEY JP ET AL. 2004. Toxoplasma gondii infections in cats from Parana, Brazil: seroprevalence, tissue distribution, and biologic and genetic characterization of isolates. J Parasitol 90: 721726.
- FERREIRA ADE M, VITOR RW, CARNEIRO AC, BRANDÃO GP AND MELO MN. 2004. Genetic variability of Brazilian Toxoplasma gondii strains detected by random amplified polymorphic DNA-polymerase chain reaction (RAPD-PCR) and simple sequence repeat anchored-PCR (SSR-PCR). Infect Genet Evol 4: 131142.
- FERREIRA ADE M, VITOR RW, GAZZINELLI RT AND MELO MN. 2006. Genetic analysis of natural recombinant Brazilian Toxoplasma gondii strains by multilocus PCR-RFLP. Infect Genet Evol 6: 2231.
- FUENTES I, RUBIO JM, RAMIREZ C AND ALVAR J. 2001. Genotypic characterization of Toxoplasma gondii strains associated with human toxoplasmosis in Spain: direct analysis from clinical samples. J Clin Microbiol 39: 15661570.
- FUX B, RODRIGUES CV, PORTELA RW, SILVA NM, SU C, SIBLEY D, VITOR RW AND GAZZINELLI RT. 2003. Role of cytokines and major histocompatibility complex restriction in mouse resistance to infection with a natural recombinant strain (type I-III) of Toxoplasma gondii Infect Immun 71: 63926401.
- GAZZINELLI R, XU Y, HIENY S, CHEEVER A AND SHER A. 1992. Simultaneous depletion of CD4+ and CD8+ T lymphocytes is required to reactivate chronic infection with Toxoplasma gondii J Immunol 149: 175180.
- GAZZINELLI RT, HAKIM FT, HIENY S, SHEARER GM AND SHER A. 1991. Synergistic role of CD4+ and CD8+ T lymphocytes in IFN-gamma production and protective immunity induced by an attenuated Toxoplasma gondii vaccine. J Immunol 146: 286292.
- GAZZINELLI RT, ELTOUM I, WYNN TA AND SHER A. 1993a. Acute cerebral toxoplasmosis is induced by in vivo neutralization of TNF-alpha and correlates with the down-regulated expression of inducible nitric oxide synthase and other markers of macrophage activation. J Immunol 151: 36723681.
- GAZZINELLI RT, HIENY S, WYNN TA, WOLF S AND SHER A.1993b. Interleukin 12 is required for the T-lymphocyteindependent induction of interferon gamma by an intracellular parasite and induces resistance in T-cell-deficient hosts. Proc Natl Acad Sci USA 90: 61156119.
- GAZZINELLI RT, GIESE NA AND MORSE HC 3RD. 1994a. In vivo treatment with interleukin 12 protects mice from immune abnormalities observed during murine acquired immunodeficiency syndrome (MAIDS). J Exp Med 180: 21992208.
- GAZZINELLI RT, WYSOCKA M, HAYASHI S, DENKERS EY, HIENY S, CASPAR P, TRINCHIERI G AND SHER A. 1994b. Parasite-inducedIL-12 stimulates early IFN-gamma synthesis and resistance during acute infection with Toxoplasma gondii J Immunol 153: 25332543.
- GAZZINELLI RT, BALA S, STEVENS R, BASELER M, WAHL L, KOVACS J AND SHER A. 1995. HIV infection suppresses type 1 lymphokine and IL-12 responses to Toxoplasma gondii but fails to inhibit the synthesis of other parasite-induced monokines. J Immunol 155: 15651574.
- GAZZINELLI RT, WYSOCKA M, HIENY S, SCHARTONKERSTEN T, CHEEVER A, KUHN R, MULLER W, TRINCHIERI G AND SHER A.1996. In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent on CD4+ T cells and accompanied by overproduction of IL-12, IFN-gamma and TNF-alpha. J Immunol 157: 798805.
- GIRALDO M, CANNIZZARO H, FERGUSON MA, ALMEIDA IC AND GAZZINELLI RT. 2000. Fractionation of membrane components from tachyzoite forms of Toxoplasma gondii: differential recognition by immunoglobulin M (IgM) and IgG present in sera from patients with acute or chronic toxoplasmosis. J Clin Microbiol 38: 14531460.
- GLASNER PD, SILVEIRA C, KRUSZON-MORAN D, MARTINS MC, BURNIER JUNIOR M, SILVEIRA S, CAMARGO ME, NUSSENBLATT RB, KASLOW RA AND BELFORT JUNIOR R. 1992. An unusually high prevalence of ocular toxoplasmosis in southern Brazil. Am J Ophthalmol 114: 136144.
- GRIGG ME, BONNEFOY S, HEHL AB, SUZUKI Y AND BOOTHROYD JC. 2001a. Success and virulence in Toxoplasma as the result of sexual recombination between two distinct ancestries. Science 294: 161165.
- GRIGG ME, GANATRA J, BOOTHROYD JC AND MARGOLIS TP. 2001b. Unusual abundance of atypical strains associated with human ocular toxoplasmosis. J Infect Dis 184:633639.
- HAYASHI S, CHAN CC, GAZZINELLI R AND ROBERGE FG. 1996a. Contribution of nitric oxide to the host parasite equilibrium in toxoplasmosis. J Immunol 156: 14761481.
- HAYASHI S, CHAN CC, GAZZINELLI RT, PHAM NT, CHEUNG MK AND ROBERGE FG.1996b. Protectiverole of nitric oxide in ocular toxoplasmosis. Br J Ophthalmol 80: 644648.
- HOLLAND GN. 1999. Reconsidering the pathogenesis of ocular toxoplasmosis. Am J Ophthalmol 128: 502505.
- HOWE DK AND SIBLEY LD. 1995. Toxoplasma gondii comprises three clonal lineages: correlation of parasite genotype with human disease. J Infect Dis 172: 15611566.
- HOWE DK, SUMMERS BC AND SIBLEY LD. 1996. Acute virulence in mice is associated with markers on chromosome VIII in Toxoplasma gondii Infect Immun 64: 51935198.
- HOWE DK, HONORE S, DEROUIN F AND SIBLEY LD.1997. Determination of genotypes of Toxoplasma gondii strains isolated from patients with toxoplasmosis. J Clin Microbiol 35: 14111414.
- HUNTER CA, SUBAUSTE CS, VAN CLEAVE VH AND REMINGTON JS. 1994. Production of gamma interferon by natural killer cells from Toxoplasma gondii-infected SCID mice: regulation by interleukin-10, interleukin-12, and tumor necrosis factor alpha. Infect Immun 62: 28182824.
- HUNTER CA, SUZUKI Y, SUBAUSTE CS AND REMINGTON JS. 1996. Cells and cytokines in resistance to Toxoplasma gondii Curr Top Microbiol Immunol 219: 113125.
- JAMRA LMF AND VIEIRA MPL. 1991. Isolamentodo Toxoplasma gondii de exudato peritoneal e órgãos de camundongos com infecção experimental. Rev Inst Med Trop SP 33: 435441.
- JOHNSON J, SUZUKI Y, MACK D, MUI E, ESTES R, DAVID C, SKAMENE E, FORMAN J AND MCLEOD R. 2002a. Genetic analysis of influences on survival following Toxoplasma gondii infection. Int J Parasitol 32: 179185.
- JOHNSON JJ ET AL. 2002b. In vitro correlates of Ld-restricted resistance to toxoplasmic encephalitis and their critical dependence on parasite strain. J Immunol 169: 966973.
- JOHNSON LL AND SAYLES PC. 2002. Deficient humoral responses underlie susceptibility to Toxoplasma gondii in CD4-deficient mice. Infect Immun 70: 185191.
- KANG H, LIESENFELD O, REMINGTON JS, CLAFLIN J, WANG X AND SUZUKI Y. 2003. TCR V beta 8+ T cells prevent development of toxoplasmic encephalitis in BALB/c mice genetically resistant to the disease. J Immunol 170: 42544259.
- LIESENFELD O, KOSEK J, REMINGTON JS AND SUZUKI Y. 1996. Association of CD4+ T cell-dependent, interferongamma-mediated necrosis of the small intestine with genetic susceptibility of mice to peroral infection with Toxoplasma gondii J Exp Med 184: 597607.
- LUNDE MN AND JACOBS L. 1983. Antigenic differences between endozoites and cystozoites of Toxoplasma gondii J Parasitol 69: 806808.
- MACK DG, JOHNSON JJ, ROBERTS F, ROBERTS CW, ESTES RG, DAVID C, GRUMET FC AND MCLEOD R. 1999. HLA-class II genes modify outcome of Toxoplasma gondii infection. Int J Parasitol 29: 13511358.
- MCLEOD R, BEEM MO AND ESTES RG.1985. Lymphocyte anergy specific to Toxoplasma gondii antigens in a baby with congenital toxoplasmosis. J Clin Lab Immunol 17: 149153.
- MCLEOD R, EISENHAUER P, MACK D, BROWN C, FILICE G AND SPITALNY G. 1989a. Immune responses associated with early survival after peroral infection with Toxoplasma gondii J Immunol 142: 32473255.
- MCLEOD R, SKAMENE E, BROWN CR, EISENHAUER PB AND MACK DG. 1989b. Genetic regulation of early survival and cyst number after peroral Toxoplasma gondii infection of A × B/B × A recombinant inbred and B10 congenic mice. J Immunol 143: 30313034.
- MCLEOD R ET AL. 1990. Phenotypes and functions of lymphocytes in congenital toxoplasmosis. J Lab Clin Med 116: 623635.
- MCLEOD R, BUSCHMAN E, ARBUCKLE LD AND SKAMENE E. 1995. Immunogenetics in the analysis of resistance to intracellular pathogens. Curr Opin Immunol 7: 539552.
- MCLEOD R, JOHNSON J, ESTES R AND MACK D. 1996. Immunogenetics in pathogenesis of and protection against toxoplasmosis. Curr Top Microbiol Immunol 219: 95112.
- MUN HS, AOSAI F, NOROSE K, CHEN M, PIAO LX, TAKEUCHI O, AKIRA S, ISHIKURA H AND YANO A. 2003. TLR2 as an essential molecule for protective immunity against Toxoplasma gondii infection. IntImmunol 15: 10811087.
- NEYER LE, GRUNIG G, FORT M, REMINGTON JS, RENNICK D AND HUNTER CA. 1997. Role of interleukin-10 in regulation of T-cell-dependent and T-cell-independent mechanisms of resistance to Toxoplasma gondii Infect Immun 65: 16751682.
- PENA HF, GENNARI SM, DUBEY JP AND SU C. In press. Population structure and mouse-virulence of Toxoplasma gondii in Brazil. Int J Parasitol.
- PORTELA RW, BETHONY J, COSTA MI, GAZZINELLI A, VITOR RW, HERMETO FM, CORREA-OLIVEIRA R AND GAZZINELLI RT. 2004. A multihousehold study reveals a positive correlation between age, severity of ocular toxoplasmosis and levels of gly coinositolphospholipidspecific immunoglobulin A. J Infect Dis 190: 175183.
- SCANGA CA, ALIBERTI J, JANKOVIC D, TILLOY F, BENNOUNA S, DENKERS EY, MEDZHITOV R AND SHER A. 2002. Cutting edge: MyD88 is required for resistance to Toxoplasma gondii infection and regulates parasite-induced IL-12 production by dendritic cells. JImmunol168: 59976001.
- SCHARTON-KERSTEN TM, WYNN TA, DENKERS EY, BALA S, GRUNVALD E, HIENY S, GAZZINELLI RT AND SHER A. 1996. In the absence of endogenous IFN-gamma, mice develop unimpaired IL-12 responses to Toxoplasma gondii while failing to control acute infection. J Immunol 157: 40454054.
- SCHARTON-KERSTEN TM, YAP G, MAGRAM J AND SHER A.1997. Inducible nitric oxide is essential for host control of persistent but not acute infection with the intracellular pathogen Toxoplasma gondii J Exp Med 185: 12611273.
- SIBLEY LD AND BOOTHROYD JC. 1992. Virulent strains of Toxoplasma gondii comprise a single clonal lineage. Nature 359: 8285.
- SIBLEY LD, LEBLANC AJ, PFEFFERKORN ER AND BOOTHROYD JC. 1992. Generation of a restriction fragment length polymorphism linkage map for Toxoplasma gondii Genetics 132: 10031015.
- SILVEIRA C, BELFORT JR R, MUCCIOLI C, ABREU MT, MARTINS MC, VICTORA C, NUSSENBLATT RB AND HOLLAND GN. 2001. A follow-up study of Toxoplasma gondii infection in southern Brazil. Am J Ophthalmol 131: 351354.
- SUZUKI Y, CONLEY FK AND REMINGTON JS.1989. Importance of endogenous IFN-gamma for prevention of toxoplasmicencephalitisinmice. J Immunol 143: 20452050.
- SUZUKI Y, JOH K, ORELLANA MA, CONLEY FK AND REMINGTON JS. 1991. A gene(s) within the H-2D region determines the development of toxoplasmic encephalitis in mice. Immunology 74: 732739.
- SUZUKI Y ET AL. 1996. Evidence for genetic regulation of susceptibility to toxoplasmic encephalitis in AIDS patients. J Infect Dis 173: 265268.
- TENTER AM, HECKEROTH AR AND WEISS LM. 2000. Toxoplasma gondii: from animals to humans. Int J Parasitol 30: 12171258.
- WANG X, CLAFLIN J, KANG H AND SUZUKI Y. 2005. Importance of CD8(+)Vbeta8(+) T cells in IFN-gamma-mediated prevention of toxoplasmic encephalitis in genetically resistant BALB/c mice. J Interferon Cytokine Res 25: 338344.
- YAMAMOTO JH, VALLOCHI AL, SILVEIRA C, FILHO JK, NUSSENBLATT RB, CUNHA-NETO E, GAZZINELLI RT, BELFORT JR R AND RIZZO LV. 2000. Discrimination between patients with acquired toxoplasmosis and congenital toxoplasmosis on the basis of the immune response to parasite antigens. J Infect Dis 181: 20182022.
- YAROVINSKY F ET AL. 2005. TLR11 activation of dendritic cells by a protozoan profilin-like protein. Science 308: 16261629.
Correspondence to:
Publication Dates
-
Publication in this collection
10 Mar 2008 -
Date of issue
Mar 2008
History
-
Accepted
21 Jan 2008 -
Received
11 Nov 2007