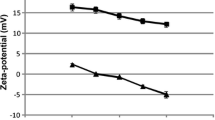
Abstract
Cystic fibrosis (CF) is an inherited childhood-onset life-shortening disease. It is characterized by increased respiratory production, leading to airway obstruction, chronic lung infection and inflammatory reactions. The most common bacteria causing persisting infections in people with CF is Pseudomonas aeruginosa. Superparamagnetic Fe3O4 iron oxide nanoparticles (NPs) conjugated to the antibiotic (tobramycin), guided by a gradient of the magnetic field or subjected to an oscillating magnetic field, show promise in improving the drug delivery across the mucus and P. aeruginosa biofilm to the bacteria. The question remains whether tobramycin needs to be released from the NPs after the penetration of the mucus barrier in order to act upon the pathogenic bacteria. We used a zero-length 1-ethyl-3-[3-dimethylaminopropyl] carbodiimide hydrochloride (EDC) crosslinking agent to couple tobramycin, via its amine groups, to the carboxyl groups on Fe3O4 NPs capped with citric acid. The therapeutic efficiency of Fe3O4 NPs attached to the drug versus that of the free drug was investigated in P. aeruginosa culture.
Similar content being viewed by others
References
R. L. Gibson, J. L. Burns, and B. W. Ramsey, “Pathophysiology and management of pulmonary infections in cystic fibrosis”, Am. J. Respir. Crit. Care Med. 168, 918–951, 2003.
Cystic Fibrosis Foundation, www.cff.org, accessed on July 20, 2013.
M. J. Welsh, B. W. Ramsey, F. Accurso, and G. Cutting, “Cystic Fibrosis”, in The Metabolic and Molecular Basis of Inherited Diseases (C. R. Scriver, A. L. Beaudet, W. S. Sly, and D. Valle, Eds.), 8th Ed., McGraw-Hill, New York 2001, pp. 5121–5188.
M. Mense, P. Vergani, D. M. White, G. Altberg, A. C. Nairn, and D. C.Gadsby, “In vivo phosphorylation of CFTR promotes formation of a nucleotide-binding domain heterodimer”, The EMBO J. 25, 4728 – 4739, 2006.
M. E. Hodson and D. M. Geddes, Cystic Fibrosis, Chapman and Hall Medical, London 1995.
J. L. Burns, B. W. Ramsey, and A. L. Smith, “Clinical manifestations and treatment of pulmonary infections in cystic fibrosis”, Adv. Pediatr. Infect. Dis. 8, 53–66, 1993.
N. Hoiby, “Antibiotic therapy for chronic infection of Pseudomonas in the lung”, Annu. Rev. Med. 44, 1–10, 1993.
L. Garcia-Contreras and A. J. Hickey, “Aerosol treatment for cystic fibrosis”, Crit. Rev. Ther. Drug Carr. Syst. 20, 317–356, 2003.
J. A. Voynow and B. K. Rubin, “Mucins, mucus, and sputum”, Chest 135 (2), 505–512, Feb. 2009.
B. K. Rubin, “Mucus structure and properties in cystic fibrosis”, Paediatric Resp. Rev. 8 (1), 4–7, March 2007.
H. Matsui, B. R. Grubb, R. Tarran, S. H. Randell, J. T. Gatzy, C. W. Davis, and R. C. Boucher, “Evidence for periciliary liquid layer depletion, not abnormal ion composition, in the pathogenesis of cystic fibrosis airway disease”, Cell 95 (7), 1005–1015, 1998.
R. C. Boucher, “New concepts of the pathogenesis of cystic fibrosis lung disease”, Eur. Respir. J. 23, 146–158, 2004.
J. Perez-Vilar and R. C. Boucher, “Reevaluating gel-forming mucins’ roles in cystic fibrosis lung disease” Free Rad. Bio. Med. 37, 1564–1577, 2004.
J. Emerson, M. Rosenfeld, S. McNamara, B. Ramsey, and R. L. Gibson, “Pseudomonas aeruginosa and other predictors of mortality and morbidity in young children with cystic fibrosis”, Pediatr. Pulmonol. 34, 91–100, 2002.
M. Fegan, P. Francis, A. C. Hayward, G. H. Davis, and J. A. Fuerst, “Phenotypic conversion of Pseudomonas aeruginosa in cystic fibrosis”, J. Clin. Microbiol. 28, 1143–1146, 1990.
J. W. Costerton, P. S. Stewart, and E. P. Greenberg, “Bacterial biofilms: A common cause of persistent infections”, Science 284, 1318–1322, 1999.
P. K. Singh, A. L Schaefer, M. R. Parsek, T. O. Moninger, M. J. Welsh, and E. P. Greenberg, “Quorum-sensing signals indicate that cystic fibrosis lungs are infected with bacterial biofilms”, Nature 407, 762–764, 2000.
D. A. Cabral, B. A. Loh, and D. P. Speert, “Mucoid Pseudomonas aeruginosa resists nonopsonic phagocytosis by human neutrophils and macrophages”, Pediatr. Res. 22, 429–431, 1987.
G. B. Pier, F. Coleman, M. Grout, M. Franklin, and D. E. Ohman, “Role of alginate O acetylation in resistance of mucoid Pseudomonas aeruginosa to opsonic phagocytosis”, Infect. Immun. 69, 1895–1901, 2001.
H. Anwar, M. Dasgupta, K. Lam, and J. W. Costerton, “Tobramycin resistance of mucoid Pseudomonas aeruginosa biofilm grown under iron limitation”, J. Antimicrob. Chemother. 24, 647–655, 1989.
N. A. Hodges and C. A. Gordon, “Protection of Pseudomonas aeruginosa against ciprofloxacin and β-lactams by homologous alginate”, Antimicrob. Agents Chemother. 35, 2450–2452, 1991.
M. Whiteley, M. G. Bangera, R. E. Bumgarner, M. R. Parsek, G. M. Teitzel, S. Lory, and E. P. Greenberg, “Gene expression in Pseudomonas aeruginosa biofilms”, Nature 413 (6858) 860–864, 25 Oct. 2001.
R. M. Harshey, “Bacterial motility on a surface: Many ways to a common goal”, Annu. Rev. Microbiol. 57, 249–273, 2003.
H. P Schweizer, “Efflux as a mechanism of resistance to antimicrobials in Pseudomonas aeruginosa and related bacteria: Unanswered questions”, Genet. Mol. Res. 2, 48–62, 2003.
F. Ratjen, G. Döring, and W. H. Nikolaizik, “Effect of inhaled tobramycin on early Pseudomonas aeruginosa colonisation in patients with cystic fibrosis”, Lancet 358, 983–984, 2001.
M. Griese, I. Müller, and D. Reinhardt, “Eradication of initial Pseudomonas aeruginosa colonization in patients with cystic fibrosis”, Eur. J. Med. Res. 7 (2), 79–80, 21 Feb. 2002.
R. R. Qiao, C. H. Yang, and M. Y. Gao, “Superparamagnetic iron oxide nanoparticles: From preparations to in vivo MRI applications”, J. Mater. Chem. 19, 6274–6293, 2009.
R. T. Castaneda, A. Khurana, R. Khan, and H. E. Daldrup-Link, “Labeling stem cells with ferumoxytol, an FDA-approved iron oxide nanoparticle”, J. Vis. Exp. 57, Art. e3482, 4 Nov. 2011.
Y. Sahoo, A. Goodarzi, M. T. Swihart, T. Y. Ohulchanskyy, N. Kaur, E. P. Furlani, and P. N. Prasad, “Aqueous ferrofluid of magnetite nanoparticles: Fluorescence labeling and magnetophoretic control”, J. Phys. Chem. B 109, 3879–3885, 2005.
H. Iida, K. Takayanagi, T. Nakanishi, and T. Osaka, “Synthesis of Fe3O4 nanoparticles with various sizes and magnetic properties by controlled hydrolysis”, J. Colloid. Interface Sci. 314, 274–280, 2007.
L. M. Armijo, Y. I. Brandt, N. J. Withers, J. B. Plumley, N. C. Cook, A. C. Rivera, S. Yadav, G. A. Smolyakov, T. Monson, D. L. Huber, H. D. C. Smyth, and M. Osiński, “Multifunctional superparamagnetic nanocrystals for imaging and targeted drug delivery to the lung”, Colloidal Nanocrystals for Biomedical Applications VII (W. J. Parak, M. Osiński, and K. Yamamoto, Eds.), SPIE International Symp. on Biomedical Optics BiOS 2012, San Francisco, CA, 21–23 Jan. 2012, Proc. SPIE 8232, Paper 82320M (11 pp.).
D. J. Herman, P. Ferguson, S. Cheong, I. F. Hermans, B. J. Ruck, K. M. Allan, S. Prabakar, J. L. Spencer, C. D. Lendrum, and R. D. Tilley, “Hot-injection synthesis of iron/iron oxide core/shell nanoparticles for T2 contrast enhancement in magnetic resonance imaging”, Electr. Suppl. Material (ESI), Chem. Communic. 47, 9221–9223, 2011.
G. T. Hermanson, Bioconjugate Techniques, 2nd Ed, Academic Press 2008, p. 598.
S. Shakil, R. Khan, R. Zarrilli, and A. U. Khan, “Aminoglycosides versus bacteria - A description of the action, resistance mechanism, and nosocomial battleground”, J. Biomed. Sci. 15 (1), 5–14, Jan. 2008.
L. Saiman, “Microbiology of early CF lung disease”, Paediatr. Respir. Rev. 5 (Suppl A), S367–S369, 2004.
F. Le Goffic, M. L. Capmau, F. Tangy, and M. Baillarge, “Mechanism of action of aminoglycoside antibiotics. Binding studies of tobramycin and its 6’-n-acetyl derivative to the bacterial ribosome and its subunits”, Eur. J. Biochem. 102, 73–81, 1979.
H. Nikaido and R. E. W. Hancock, “Outer membrane permeability of Pseudomonas aeruginosa”, in The Bacteria: A Treatise on Structure and Function (J. R. Sokatch, Ed.), Academic Press, London 1986, pp. 145–193.
H. Nikaido, “Nonspecific and specific permeation channels of the Pseudomonas aeruginosa outer membrane”, in Pseudomonas. Molecular Biology and Biotechnology (E. Galli, S. Silver, and B. Witholt, Eds.), Am. Soc. Microbiol., Washington, DC 1992, pp. 146–154.
R. Y. Stanier, N. J. Palleroni, and M. Doudoroff, “The aerobic pseudomonads: A taxonomic study”, J. Gen. Microbiol. 43 (2), 159–271, May 1966.
J. B.Morrow, C. P. Arango, and R. D. Holbrook, “Association of quantum dot nanoparticles with Pseudomonas aeruginosa biofilm”, J. Environ. Qual. 39, 1934–1941, 2010.
J. McQuillan, Bacterial-Nanoparticle Interactions, Ph.D. Dissertation, Univ. of Exeter, UK, 2010.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Osiński, M., Brandt, Y.I., Armijo, L.M. et al. Efficacy of Tobramycin Conjugated to Superparamagnetic Iron Oxide Nanoparticles in Treating Cystic Fibrosis Infections. MRS Online Proceedings Library 1617, 127–137 (2013). https://doi.org/10.1557/opl.2013.1175
Published:
Issue Date:
DOI: https://doi.org/10.1557/opl.2013.1175