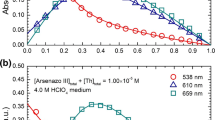
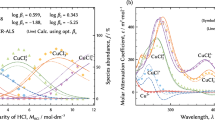
Abstract
Actinide solubilities in highly concentrated chloride solutions are about one order of magnitude higher than in similar inert electrolyte (NaClO4) solutions. This increased solubility is due to interactions between actinide and chloride ions. Contradictory results exist regarding the interaction mechanism between actinide and chloride ions. Specifically, both inner-sphere complex formation and ion pair association have been implicated in the interpretation of spectrophotometric and extraction data. To address this controversy, we investigated the interaction between actinide ions in the (III), (IV), (V) and (VI) oxidation states and chloride ions using a multi-method approach. Spectroscopie techniques (TRLFS, Raman, UV-Vis absorption, EXAFS) were used to distinguish between changes in the inner coordination sphere of the actinide ion and effects of ion pairing. X-ray absorption spectroscopy and single crystal X-ray diffraction were used to determine structural details of the actinide chloro complexes formed in solution and solid states.
Similar content being viewed by others
Literature
J. Fuger, I.L. Khodakovsky, E.I. Sergeyeva, V.A. Medvedev, J.D. Navratil, The Chemical Thermodynamics of Actinide Elements and Compounds. Part 12. The Actinide Aqueous Inorganic Compexes. IAEA, Vienna, 1992.
I. Grenthe, J. Fuger, R.J.M. Donigs, R.J. Lemire, A.B. Muller, C. Nguyen-Trung, H. Wanner, Chemical Thermodynamics of Uranium. Vol. 1. Elsevier Science Publishing Company Inc., North-Holland, 1992.
R.J. Silva, G. Bidoglio, M.H. Rand, P.B. Robouch, H. Wanner, I. Puigdomenech, Chemical Thermodynamics of Americium. Vol. 2. Elsevier Science Publishing Company Inc., North-Holland, 1995.
Y. Marcus, M. Shiloh, Israel J. Chem.7, 31 (1969).
I. Grenthe, Acat Chem. Scand. 16, 2300 (1962).
M. Bansal, L. Sommer, Z. Phys. Chem. 226, 309 (1964).
W. Runde, M.P. Neu, D.L. Cark, Geochim. Cosmochim. Acta 60 (12), 2065–2073, (1996).
D.L. Clark, S.D. Conradson, S.A. Ekberg, N.J. Hess, M.P. Neu, P.D. Palmer, W. Runde, C.D. Tait, J. Am. Chem. Soc. 118, 2089–2090, (1996).
R.D. Rogers, A.H. Bond, W.G. Hippie, A.N. Rollins, R.F. Henry, Inorg. Chem. 30, 2671, (1991).
D.L. Clark, S.D. Conradson, S.A. Ekberg, N.J. Hess, D.R. Janecky, M.P. Neu, P.D. Palmer, C.D. Tait, New J. Chem. 20, 211–220, (1996).
L. Maya, G.M. Begun, J. Inorg. Nucl. Chem. 43, 2827, (1981).
K. Nakamoto, Infrared and Raman Spectra of Inorganic and Coordination Compounds. 4th edition, Wiley-Interscience, New York, 1986, pp. 141–145.
Acknowledgement
We are grateful the Office of Basic Energy Sciences (OBES), and 94-1 Core Technology Program for Plutonium Residue Stabilization, US DOE.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Runde, W., Neu, M.P., Conradson, S.D. et al. Spectroscopic Investigation of Actinide Speciation in Concentrated Chloride Solution. MRS Online Proceedings Library 465, 693–703 (1996). https://doi.org/10.1557/PROC-465-693
Published:
Issue Date:
DOI: https://doi.org/10.1557/PROC-465-693