
Journal of Inorganic Materials ›› 2016, Vol. 31 ›› Issue (10): 1087-1093.DOI: 10.15541/jim20160207
• Orginal Article • Previous Articles Next Articles
QU Jiao1, 2, ZHU Qi2, Li Ji-Guang2, SUN Xu-Dong1
Received:2016-03-30
Revised:2016-05-24
Published:2016-10-20
Online:2016-09-23
About author:QU Jiao. E-mail: qujiaomail@163.com
Supported by:CLC Number:
QU Jiao, ZHU Qi, Li Ji-Guang, SUN Xu-Dong. Eu3+ Activated Y2O3 Red Phosphor Monospheres: Size-controlled Processing and Luminescence Property[J]. Journal of Inorganic Materials, 2016, 31(10): 1087-1093.
| Sample ID | Ln3+ (Ln=Y, Eu) conentration /(mol·L-1) | Urea concentration /(mol·L-1) | NH4NO3 concentration /(mol·L-1) | Urea/Ln3+ molar ration, R |
|---|---|---|---|---|
| S1 | 0.015 | 0.3 | 0 | 20 |
| S2 | 0.015 | 0.5 | 0 | 33.3 |
| S3 | 0.015 | 0.3 (adding in three times: 0.1, 0.1, 0.1) | 0 | 20 |
| S4 | 0.015 | 0.5 (adding in three times: 0.15, 0.15, 0.20) | 0 | 33.3 |
| S5 | 0.015 | 0.5 | 0.015 | 33.3 |
| S6 | 0.015 | 0.5 | 0.045 | 33.3 |
Table 1 UBHP conditions for precursor synthesis
| Sample ID | Ln3+ (Ln=Y, Eu) conentration /(mol·L-1) | Urea concentration /(mol·L-1) | NH4NO3 concentration /(mol·L-1) | Urea/Ln3+ molar ration, R |
|---|---|---|---|---|
| S1 | 0.015 | 0.3 | 0 | 20 |
| S2 | 0.015 | 0.5 | 0 | 33.3 |
| S3 | 0.015 | 0.3 (adding in three times: 0.1, 0.1, 0.1) | 0 | 20 |
| S4 | 0.015 | 0.5 (adding in three times: 0.15, 0.15, 0.20) | 0 | 33.3 |
| S5 | 0.015 | 0.5 | 0.015 | 33.3 |
| S6 | 0.015 | 0.5 | 0.045 | 33.3 |
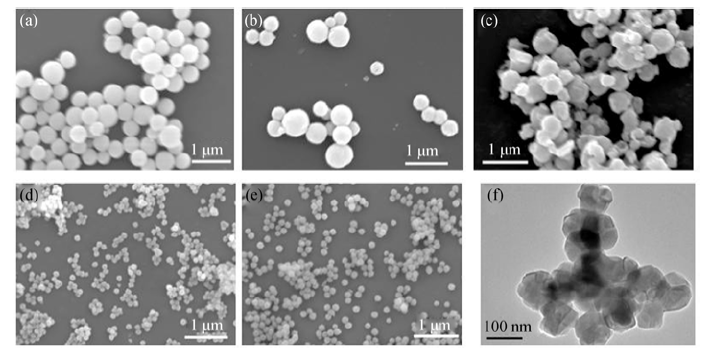
Fig. 4 FE-SEM images of the products calcinated from precursor S6 at (a) 800℃, (b) 1000℃, (c) 1300℃; (d-e) are FE-SEM and TEM micrographs of the products calcinated from precursors S1 and S4, at 1000℃ for 4 h respectively
| [1] | LI J G, LI X D, SUN X D, et al.Uniform colloidal spheres for (Y1-xGdx)2O3 (x=0-1): formation mechanism, compositional impacts, and physicochemical properties of the oxides.Chemistry of Materials, 2008, 20(6): 2274-2281. |
| [2] | LI J G, LI X D, SUN X D, et al.Monodispersed colloidal spheres for uniform Y2O3: Eu3+ red-phosphor particles and greatly enhanced luminescence by simultaneous Gd3+ doping.Journal of Physical Chemistry C, 2008, 112(31): 11707-11716. |
| [3] | LI J G, ZHU Q, LI X D, et al.Colloidal processing of Gd2O3: Eu3+ red phosphor monospheres of tunable sizes: solvent effects on precipitation kinetics and photoluminescence properties of the oxides.Acta Materialia, 2011, 59(9): 3688-3696. |
| [4] | ZHU Q, LI J G, LI X D, et al.Monodisperse colloidal spheres for (Y, Eu)2O3 red-emitting phosphors: establishment of processing window and size-dependent luminescence behavior.Science and Technology of Advanced Materials, 2011, 12(5): 055001. |
| [5] | KANG Y C, ROH H S, PARK S B.Preparation of Y2O3: Eu phosphor particles of filled morphology at high precursor concentrations by spray pyrolysis.Advanced Materials, 2000, 12(6): 451-453. |
| [6] | KANG Y C, ROH H S, PARK S B.Sodium carbonate flux effects on the luminescence characteristics of (Y0.5Gd0.5)2O3: Eu phosphor particles prepared by spray pyrolysis.Journal of the American Ceramic Society, 2001, 84(2): 447-449. |
| [7] | WANG H, YU M, LIN C K, et al.Synthesis and luminescence properties of monodispersed spherical Y2O3: Eu3+@SiO2 particles with core-shell structure.Journal of Physical Chemistry C, 2007, 111(30): 11223-11230. |
| [8] | MATIJEVIC E, HSU W P.Preparation and properties of monodispersed colloidal particles of lanthanide compounds: I. Gadolinium, europium, terbium, samarium, and cerium (III).Journal of Colloid and Interface Science, 1987, 118(2): 506-523. |
| [9] | AIKEN B, HSU W P, MATIJEVIC E.Preparation and properties of monodispersed colloidal particles of lanthanide compounds: III, Yttrium (III) and mixed Yttrium(III)/Cerium(III) systems.Journal of the American Ceramic Society, 1988, 71(10): 845-853. |
| [10] | SORDELET D, AKINC M.Preparation of spherical, monosized Y2O3 precursor particles.Journal of Colloid and Interface Science, 1988, 122(1): 47-59. |
| [11] | ZHU Q, XIONG M, LI J G, et al.(Y, Tb, Eu)2O3 monospheres for highly fluorescent films and transparent hybrid films of color tunable emission.RSC Advances, 2015, 5: 36122-36128. |
| [12] | ZONG L, XU P F, DING Y, et al.Y2O3: Yb3+/Er3+ hollow spheres with controlled inner structures and enhanced upconverted photoluminescence.Small, 2015, 11(23): 2768-2733. |
| [13] | MIAO H, JI R, HU X Y, et al.Y2O3: Eu3+/Tb3+ spherical particles based anti-reflection and wavelength conversion bi-functional films: synthesis and application to solar cells.Journal of Alloys and Compounds, 2015, 629: 74-79. |
| [14] | LV R, YANG P, HE F, et al.Hollow structure Y2O3: Yb/Er-CuxS nanospheres with controllable size for simultaneous chemo/photothermal therapy and bioimaging.Chemistry of Materials, 2015, 27(2): 483-496. |
| [15] | ZHU Q, LI J G, MA R, et al.Well-defined crystallites autoclaved from the nitrate/NH4OH reaction systemas the precursor for (Y, Eu)2O3 red phosphor: crystallization mechanism, phase and morphology control, and luminescent property.Journal of Solid State Chemistry, 2012, 192: 229-237. |
| [16] | ZHU Q, LI J G, LI X D, et al.Morphology-dependent crystallization and luminescence behavior of (Y, Eu)2O3red phosphors.Acta Materialia, 2009, 57(20): 5975-5985. |
| [17] | PENG H, SONG H, CHEN B, et al.Temperature dependence of luminescent spectra and dynamics in nanocrystalline Y2O3: Eu3+.Journal of Chemical Physics, 2003, 118(7): 3277-3282. |
| [18] | CHRISTENSEN H P, GABBE D R, JENSSEN H P.Fluorescence lifetimes for neodymium-doped yttrium aluminum garret and yttrium-oxide powders.Physical Review B, 1982, 25(3): 1467-1473. |
| [1] | MAO Qi-Nan, LI He, JI Zhen-Guo, XI Jun-Hua, ZHANG Jun, KONG Zhe. Influence of Eu2+ and Dy3+ Concentrations on Fluorescence and Phosphorescence of Sr2MgSi2O7 Phosphors [J]. Journal of Inorganic Materials, 2016, 31(8): 819-826. |
| [2] | LI Jin-Kai, TENG Xin, CAO Bing-Qiang, LIU Zong-Ming. Synthesis and Property of Spherical Lu3Al5O12:Eu3+ Phosphor [J]. Journal of Inorganic Materials, 2016, 31(6): 634-640. |
| [3] | YIN Hai-Rong, LIU Jing, QIAO Yin-Po, LI Yan-Xiao, ZHANG Pan, ZHOU Qin, YANG Chen. Effect of Strontium on Luminescence Properties and Substitution Site of Eu Doped Sr-Ca-Hydroxyapatite [J]. Journal of Inorganic Materials, 2016, 31(10): 1103-1109. |
| [4] | WANG Cui-Feng, CHIOU Shi-Yung, OU Keng-Liang, CAI Zhang-Ting. Optimal Process Parameters for 3Y-TZP/TiN Conductive Polycrystal by Taguchi Method [J]. Journal of Inorganic Materials, 2012, 27(5): 529-535. |
| [5] | HUANG Yi-Hua,JIANG Dong-Liang,ZHANG Jing-Xian,LIN Qing-Ling. Fabrication of Transparent Yttria Ceramics through Gel-freezing Dry Method [J]. Journal of Inorganic Materials, 2008, 23(6): 1135-1140. |
| [6] | LI Xiao-Dong,XIU Zhi-Meng,BAI Li-Li,GAO Tie,LIU Yi-Nong,HU Xiao-Zhi,SUN Xu-Dong. Synthesis of (Y,Gd)2O3:Eu (YGO:Eu) Nano-Powder and Fabrication of Transparent Ceramics [J]. Journal of Inorganic Materials, 2007, 22(6): 1089-1094. |
| [7] | JIAN Jia-Wen,YANG Bang-Chao,ZHANG Yi-Kang. Aging Characteristic of Pt/YSZ Electrode Structure [J]. Journal of Inorganic Materials, 2004, 19(1): 93-100. |
| [8] | WANG Jie-Qiang,ZHENG Shao-Hua,YUE Yun-Long,TAO Zhen-Dong,SUN Xu-Dong. Fabricating Transparent Yttria Ceramics at Low Temperature [J]. Journal of Inorganic Materials, 2003, 18(6): 1222-1228. |
| [9] | HUANG Xiao-Li,MA Qing-Zhi,LI Fa,LIU Hui-Qing. Influence of CaO-Y2O3 as Sintering Aid on the Microstructure and Properties of AlN Ceramics [J]. Journal of Inorganic Materials, 2002, 17(2): 277-282. |
| [10] |
LI Jian-Jun,JIANG Chang-Yin,WAN Chun-Rong,WU Yu-Ping,HAO Jian-Min.
|
| [11] | SUN Cheng-Wen,CHEN Shen,YANG Zhi-Zhou. Low Vacuum Measurement of Zirconia-Based Oxygen Sensor for an Evacuated (Air) System [J]. Journal of Inorganic Materials, 1999, 14(6): 963-968. |
| [12] | SUN Cheng-Wen,LI Qi,CHEN Shen,YANG Zhi-Zhou. Electrode Resistance of Pt/YSZ Oxygen Sensor and Response Behaviour [J]. Journal of Inorganic Materials, 1998, 13(4): 561-567. |
| Viewed | ||||||||||||||||||||||||||||||||||||||||||||||||||
|
Full text 1412
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||
|
Abstract 796
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||