Percutaneous catheter-based structural heart disease procedures are a rapidly growing area of interventional cardiology, and represent a valuable option for cardiac patients with comorbidities who are ineligible for conventional surgery as well as demonstrating excellent outcomes.1,2 Catheter-based interventions include transcatheter aortic valve implantation (TAVI),3 percutaneous mitral valve (MV) repair,4 atrial septal defect (ASD) closure, percutaneous closure of paravalvular leakages (PVL)5 and left atrial appendage (LAA) closure.6 However, these techniques may involve long procedure times and steep learning curves.
During cardiac catheterisation, imaging techniques are required for intra-procedural monitoring; these include echocardiography and X-ray. Fluoroscopy is used to guide the majority of percutaneous interventions. It enables visualisation of catheters and devices but poor visualisation of cardiac anatomical structures, limiting precision in targeting soft tissue lesions in the treatment of structural heart disease.7 In addition, many experts have expressed concern about patient exposure to excess radiation in structural heart disease procedures. Ultrasound imaging using two-dimensional (2D) transesophageal echocardiography (TEE) requires neither contrast nor radiation, and provides detailed images of anatomical structures and lesions, but only provides two spatial dimensions.8 Following the development of three-dimensional (3D) echocardiography, this technique has evolved from a slow and labour-intense off-line reconstruction to real-time volumetric imaging, further enhancing the detection of cardiac pathology, especially valvular disease.7,9,10 The major limitation of echocardiography techniques is their limited ability to detect the position of catheters and devices, and therefore a combination of echocardiography and fluoroscopy is still required during interventions.
Accurately recognising the heart structures from fluoroscopic and TEE images requires considerable training and experience, and the simultaneous use and interpretation of two imaging techniques during the procedures can be challenging, especially when manipulating and steering the catheters that carry the implant devices. In addition, structural heart disease interventions involve a multidisciplinary team, typically comprising cardiac interventionalists, cardiovascular imaging specialists and specialised nurses. The action of the interventionalist depends on the images provided by the imaging specialist.10 In particular, at the key moments of device deployment, communication between the interventional cardiologist steering the catheters, and the echocardiographer operating the 3D ultrasound equipment, is demanding. There is therefore a need for an imaging system that combines anatomical information from echocardiography with catheter and device visualisation from fluoroscopy. Such an approach requires real-time image co-registration with a sufficient overlay visualisation of both imaging modalities.11
Fusion of Dynamic Imaging – The EchoNavigator System
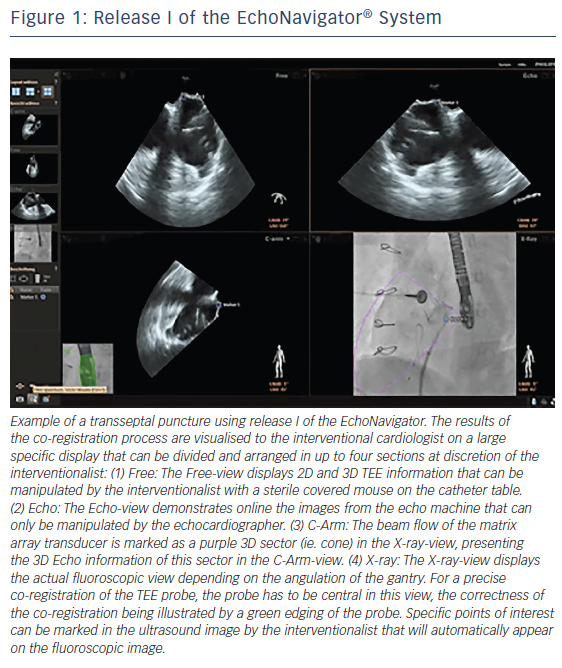
The EchoNavigator system is a multimodality approach that synchronises live echocardiography and fluoroscopy images in real time by a calibration algorithm that tracks the movement of the TEE probe using fluoroscopy based on the movement of the predefined ‘fingerprint’ of the TEE probe (see Figure 1).11,12 This is achieved via an image-based TEE probe localisation algorithm and a calibration procedure.13 After synchronisation of the images, the 3D TEE images automatically track and follow the movement of the fluoroscopy C-arm gantry.14 The results of the co-registration process are displayed in a form that allows the simultaneous visualisation of an X-ray image and up to three echocardiographic views. The X-ray view displays the actual fluoroscopic image. The probe must be central in this view in order to allow precise co-registration of the TEE probe. If this has been achieved, the probe displays a green edging; if not, for example, after substantial movement of the TEE probe, the probe displays red edging. The echo view shows images that can only be manipulated by the echocardiographer.
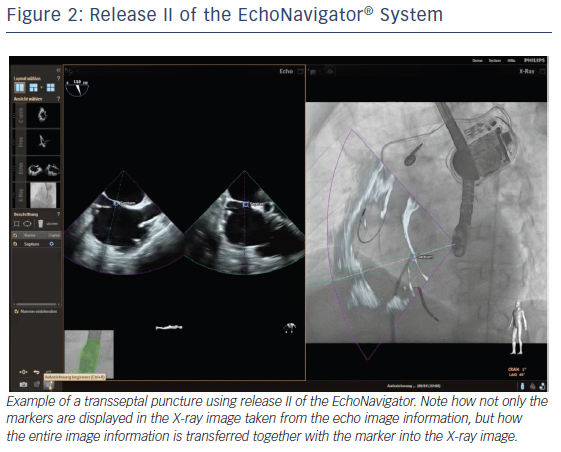
The C-arm view shows the beam flow (ie echo cone) of the matrix array transducer as a purple sector corresponding to the position of the TEE probe. Changes in the angulation, rotation or position of the TEE probe automatically appear in this view. The free view displays 2D and 3D information that may be rotated, cropped, zoomed or segmented by the interventional cardiologist by steering with a sterile covered tableside mouse. Multiplanar reconstruction (MPR) software provides tools for 3D volume segmentation along the three axes (x, y, z) in real time or during post-processing and also for quantitative analysis. Specific point of interest can be marked, and are immediately displayed on the fluoroscopic image. These marks then serve as targets to direct catheter manipulations.15 The first release (release I) of the EchoNavigator was only capable of co-registering echocardiography and fluoroscopy data (see Figure 1). The second release (release II) became commercially available in late 2014 (see Figure 2). Release II allows for real-time fusion of both imaging modalities.
To date few publications have described the value of the EchoNavigator system (Philips Healthcare), a novel software solution that enables the necessary merging of echocardiographic and fluoroscopic images on the same display in real time, during interventions in structural heart disease. This paper aims to describe the initial experience with this innovative software solution using both the combination of co-registration of markers and the novel fusion imaging technology in the catheter laboratory in structural heart disease interventions. Until now only case reports have demonstrated the value of the new system (release II) during percutaneous interventions.16,17 Recently, our group demonstrated that the application of this innovative fusion imaging EchoNavigator system is effectively capable to reduce radiation exposure and fluoroscopy time both for the patient and the involved medical team.18
Advantages of Fusion Imaging in Cardiac Interventions
Another recent single-centre study performed by our group evaluated the use of the EchoNavigator software release I in 127 percutaneous interventions for structural heart disease (three paravalvular leaks, 11 atrial septal defects, 31 transapical TAVI procedures, 35 left atrial appendage occlusions, and 47 MitraClip® procedures).19 In this study, due to the fact that we worked with release I, we were only capable of transferring information from the echocardiographic image into fluoroscopy by the means of markers. A particular point of interest was designated in the echocardiographic view and then appeared in fluoroscopy. In order to control the correctness of the marker we moved the marker within the fluoroscopic image. This led to malposition of the marker within the echocardiographic image. By working with the release I of the system during several different types of structural heart disease interventions we can conclude that the information given by the markers is quite reliable, as long as the TEE probe with its 2D and 3D sector view is within the dimension of the gantry. Results of this, and other studies employing the EchoNavigator system are detailed below. Additionally the application of the new release II of the EchoNavigator and its implementation during interventions in structural heart disease is described in detail below.
Transcatheter Aortic Valve Implantation
TAVI is a valuable alternative to surgery for high risk or inoperable patients with severe, symptomatic aortic stenosis.3 TEE has limited utility in peri-interventional guidance during TAVI, as general anaesthesia may be required and the probe may obstruct the optimal view. However, TEE is useful before and after valve implantation.20 The use of 3D TEE facilitates precise aortic annular sizing and exact delineation of the hinge points during valve sizing and implantation.21
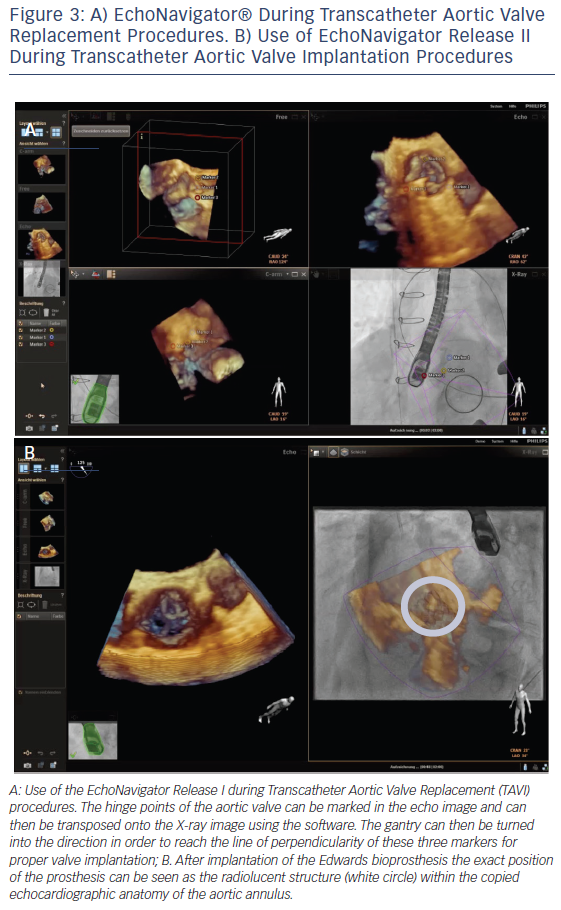
In order to optimise TAVI procedural safety and effectiveness, multimodality imaging enables a precise knowledge of the anatomy of the aortic root and its surrounding structures.22 During TAVI interventions, important landmarks of the aortic root such as the leaflets, the coronary cusps, the sinotubular junction, the anatomic ventriculo-arterial junction, the aortic-mitral curtain and the virtual ring formed by the hinge points of the aortic valvar leaflets are not visible on fluoroscopy but on echocardiography. In addition, the orientation of the prosthesis is crucial for procedural success. The EchoNavigator system allows the transfer of specific echocardiographic markers onto the fluoroscopic image. This enables marking of the level of the annulus and correction of the position of the gantry to the point where all three hinge point markers derived from echocardiography create one orthogonal plane, and thus facilitating catheter guidance and prosthesis placement.16,23 It is important to generate a straight line of perpendicularity in order to reach the correct image plain for implantation of the valve.24 This can be achieved by means of the first release of the EchoNavigator (see Figure 3A). Release II of the system allows the interventionalist to virtually fuse both images and therefore transpose the echo image into the X-ray image (see Figure 3B).
Percutaneous Mitral Valve Repair with MitraClip
Manoeuvring the MitraClip® (Abbott Vascular) device during mitral valve repair is a procedure that requires precision to avoid complications such as accidental puncture of the aortic root and perforation of the left atrial wall.10 The optimum means of demonstrating the effects of the MitraClip system is visualisation of the mitral valve by 3D TEE with various offline reconstruction techniques.25 The use of 3D TEE can define the correct height above the mitral valve that is sufficient to allow movement of the delivery guide and device, information that may not be adequately provided by 2D TEE alone.26 However, while TEE allows peri-interventional evaluation of the mitral valve leaflets and annulus, as well as the subvalvular apparatus, fluoroscopy is a superior technique for determining the orientation of the guiding system and the structures of the MitraClip and its grippers. Therefore a multimodality imaging approach during the intervention is of particular value in this technique.
Fusion imaging using the EchoNavigator software in percutaneous edge-to-edge repair of mitral valve regurgitation with the MitraClip enhances understanding of the morphological and functional changes during the procedure. In this procedure, the exact site of transseptal puncture within the fossa ovalis for optimal height selection of the delivery system is crucial for successful device delivery. Using the EchoNavigator system the optimal location for the transseptal puncture can be marked on the TEE images. The same marker simultaneously appears on fluoroscopy (release I, see Figure 2A) together with the echo overlay (release II, see Figure 2B), facilitating precise targeting puncture site and assuring correct crossing of the device through the interatrial septum, in particular allowing safe guidance of the clip delivery system, precise positioning of the clip delivery system and accurate alignment of the clip arms.10,11,27
One of the first studies to employ the EchoNavigator system, demonstrated its advantages in terms of facilitating transseptal puncture, understanding mitral valve anatomy, sheath exchange, clip advancement, and post-deployment visualization.28 In a study of 21 patients undergoing MitraClip interventions, the use of the EchoNavigator software was safe and feasible in all patients.12 In addition, a recent study found that the EchoNavigator software improved the visualisation of the complex relationship between catheter devices and anatomical structures during interventions using the MitraClip.19
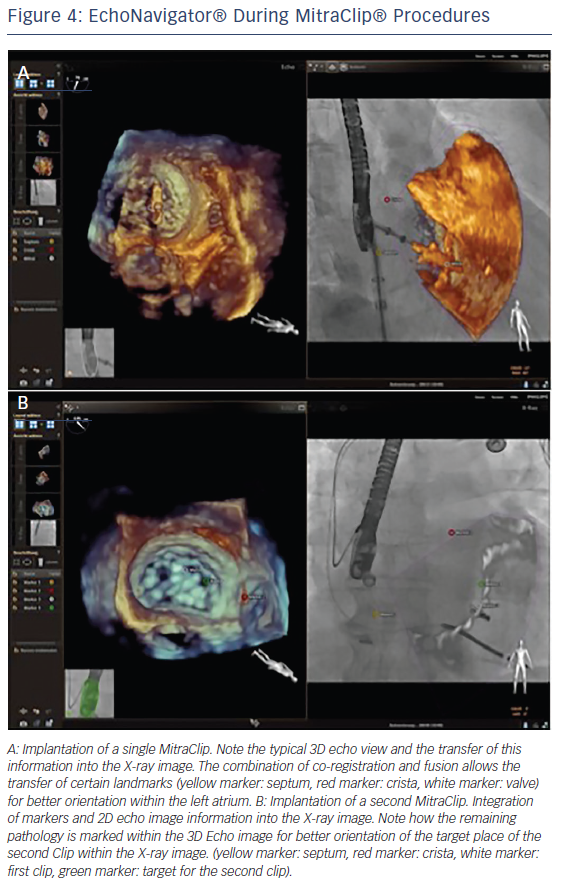
Fusion imaging using the EchoNavigator system also enhances the safety of the MitraClip procedure. By enabling the interventionalist to designate three echocardiographic orientation points (interatrial septum at puncture site, crista terminalis between pulmonary vein and the LAA and the centre of the mitral valve) into the fluoroscopic image, the system reduces the risk of injury of the left atrium (see Figure 4A). Perforation of the atrial septal wall by the MitraClip can be associated with severe complications resulting in cardiac tamponade.29 In addition, when the deployment of more than one MitraClip is required, it is easy to misjudge the relative positions of the clips as a result of blooming artefacts of the echocardiographic image.30 The EchoNavigator system enables the translation of the residual pathology from the echocardiographic image into the fluoroscopic image, allowing precise implantation of multiple MitraClips (see Figure 4B).10
Treatment of Left Atrial Appendage Occlusion
In non-valvular atrial fibrillation, LAA closure has been shown to be safe and effective in patients for whom systemic oral anticoagulation is contraindicated.31 This procedure involves transseptal crossing of the guiding catheter into the left atrium and the placement of the occluder into the LAA. During this procedure, perforation of the LAA wall and laceration of the pulmonary artery can lead to fatal complications.32 Precise knowledge of LAA orifice size is essential to ensure the correct sizing of LAA closure devices, and optimal catheter alignment and precise positioning of the closure device can be challenging.33 A study has demonstrated that real-time 3D TEE is more accurate than 2D TEE for the assessment of LAA orifice size.34 In addition, 3D measurements of the perimeter enable precise definition of the landing zone and correct device selection.33
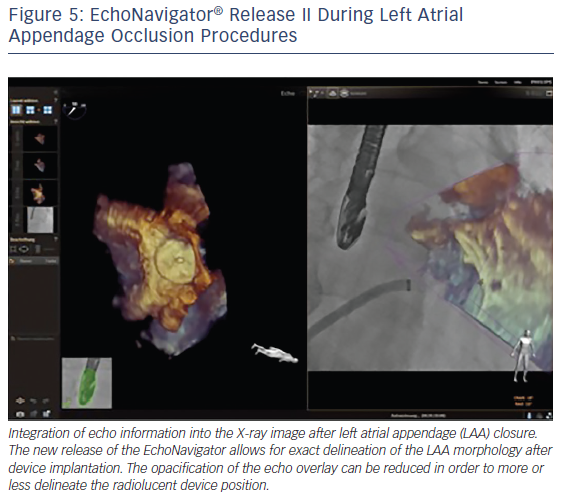
The EchoNavigator’s use of markers in the echocardiographic images that simultaneously appear in the co-registered X-ray images, facilitates positioning and alignment of the catheter in the LAA and the secure placement of the closure device.11,19,27 The use of markers is helpful to locate otherwise X-ray invisible LAA structures, maximize procedural safety and decrease radiation exposure for the patient and the staff.18 Markers can be placed at the LAA orifice at the level of the circumflex artery, the orifice of the left upper pulmonary vein or at the tip of the LAA.10 The 3D orientation within the LAA can often be difficult, especially due to the nature of its complex structure as described earlier by our group.35 The direct overlay of the echocardiographic 3D information before, during and after the procedure can here be very helpful to better understand the complex relationship between the device and the anatomy of the LAA (see Figure 5).
Interatrial Septum Defect Occlusion
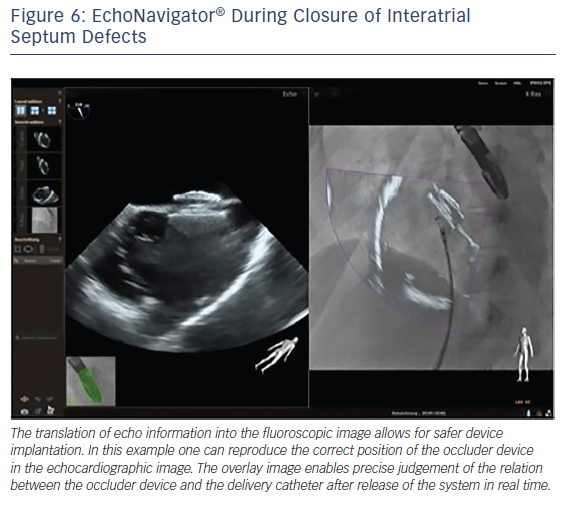
The interventional closure of defects of the interatrial septum with transcatheter techniques is used in children and adults. This procedure is safe and effective if guided using TEE alone.36 The use of TEE provides fast and complete information regarding the appropriate deployment and position of the device with regard to the surrounding structures, reducing procedure time and hence radiation exposure.37 However, good communication between the imaging operator and interventional cardiologists is crucial for the success of the procedure. The EchoNavigator allows easy visualisation of the defect size and catheter course. A recent study has successfully used the EchoNavigator system in ASD closure, and found that it facilitates safe device implantation (see Figure 6).19
Closure of Paravalvular Leaks
Paravalvular leak (PVL) is a known complication of surgical and transcatheter valve replacement procedures and, as a result, percutaneous approaches to PVL have been developed. However, this is a particularly challenging procedure, requiring precise information about the shape and dimensions of the defect to enable selection of the appropriate type, size, and number of devices.38 Multimodality imaging is particularly advantageous in PVL closure, providing both diagnostic and procedural guidance.39 The use of 3D TEE enables improved spatial resolution of the defect, especially during placement of the guide wire through the defect after transseptal puncture.38
The EchoNavigator software has been successfully employed in PVL closure procedures. It reduces procedure time, facilitating location of the lesion and evaluation of the surrounding structures.40 It also facilitates the manipulation of the guidewire through the defect.19 In addition, colour imaging fused with X-ray can be exploited to indicate the location of the leak in real time for efficient device guidance.
Summary and Concluding Remarks
As a result of the ageing population and increasing technological advances, the use of percutaneous structural heart interventions is likely to increase. In order to optimise procedural efficacy and safety, accurate imaging of the three-dimensional structure of the heart is essential. Real-time fusion of imaging modalities is essential to facilitate communication between members of the intervention team and increase procedural success.
The EchoNavigator system is the first software to allow the merging of echocardiographic and fluoroscopic imaging data in real time during percutaneous interventions. This can reduce procedural time, and our group also recently demonstrated that this innovative fusion imaging technology reduces radiation exposure and fluoroscopic time due to better and faster understanding of the combination of soft tissue anatomy and catheter devices, resulting in less need to X-ray during the procedure.40 18 Typically, interventional cardiologists are more familiar with fluoroscopic than ultrasound images. Information from TEE images is therefore transposed onto the fluoroscopic images. Fusion imaging using the EchoNavigator system facilitates improved understanding of anatomical structures and the spatial relation between the X-ray and ultrasound images, improved communication between imaging and interventional cardiologists, ability of the interventionalist to manipulate the 3D TEE images and improved confidence when positioning the interventional device and guiding interventional procedures. Target markers can be used to direct catheter access through a specific defect. With proper ‘calibration’ (ie. using three views), these markers can be very accurate. The rotation and cropping features allow assessment of the relationship of the closure device and the intra-atrial anatomy prior to and after device release. These features can reduce procedural time and increase safety in the performance of procedural steps.
An increasing body of data has demonstrated the safety and efficacy of interventions performed using the system but further studies are warranted to fully assess its clinical utility. At present, although qualitative data supports the benefits of this system, there are few clinical data, and no randomized trial to establish the superiority of fusion imaging over standard techniques. Therefore, it is not yet known whether the use of fusion imaging results in increased procedural success.
In conclusion, fusion imaging using the EchoNavigator system has the potential to increase the safety, accuracy and effectiveness of percutaneous interventions in structural heart disease, which we could already demonstrate during LAA closure. The system can be applied in any procedure where echocardiographic information is important or even essential for safe and efficient accomplishment. Needless to say, in patients with contraindications for TEE or bad echocardiographic image quality, the benefit of the hybrid imaging technology is limited. Prospectively the integration of various imaging modalities during interventions may even have an impact upon periprocedural success.41
Limitations
This manuscript reviews our initial clinical experience using an innovative fusion imaging technology in the context of the current literature. Only few centres so far are using this technology and we tried to emphasise the cutting-edge technology of truly fusing echocardiography and fluoroscopy during structural heart disease interventions. Our review lacks information about the clinical benefit of this technology for the patient in the context of reducing procedure length or radiation dose. Data demonstrating these effects are currently missing for release II of the EchoNavigator system, but could be demonstrated for release I by our own group. Prospective randomised multi-centre studies with a larger sample size are necessary to demonstrate veritable benefits of this promising technology for the patient. Another limitation might be the fact that due to a certain learning curve the process of co-registration of the TEE probe with the gantry implements radiation and therefore might even lead to a temporary gain in radiation exposure.