Coronary chronic total occlusions (CTOs) remain one of the most challenging percutaneous challenges in interventional cardiology, with technical success rates of only ~50–70 %.1,2 This lesion subset often poses the greatest risk and often requires techniques and equipment not typically utilised for more acute coronary lesions. However, successful percutaneous CTO revascularisation is associated with improved long-term survival, decreased rates of angina, improved left ventricular systolic function, and less need for coronary artery bypass surgery, as compared with unsuccessful attempts.3–5 Thus, over the last decade, much effort has been put forth to develop techniques to tackle these complex lesions and to provide operators with strategies to optimise their success rates to >90 %. Multiple reports have been published on techniques that can be used to optimise successful CTO percutaneous coronary intervention (PCI). However, only a few reports provide operators with a clear-cut algorithm, which can be used to guide the operator towards a successful CTO-PCI on a consistent basis, and even fewer include a discussion on the histopathology of CTO lesions, an important component in understanding the rationale for certain PCI strategies, which otherwise may seem counter-intuitive. In this review, we discuss the basic histopathology of CTOs followed by approaches and techniques in an algorithmic format, for consistent successful CTO recanalisation as developed by the North American Total Occlusion (NATO) group.
Histopathology of Chronic Total Occlusions
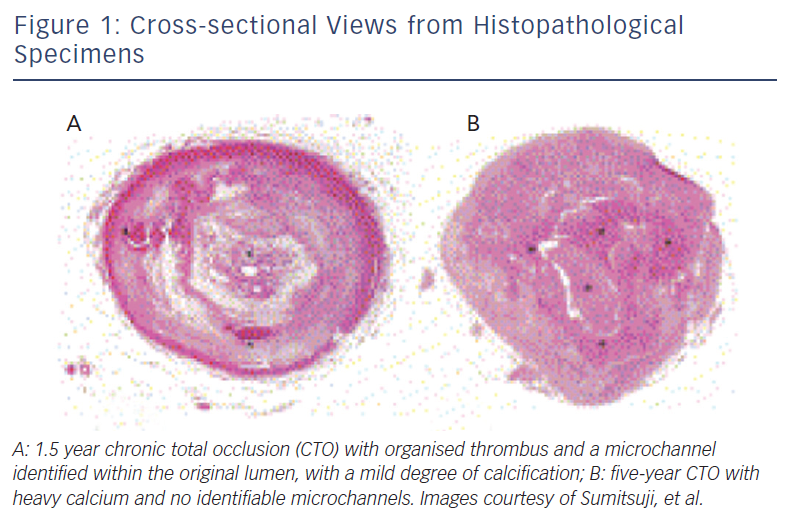
The histopathology process of CTOs is not yet fully understood. One widely accepted theory as suggested by autopsy studies is that after a coronary occlusion forms, the original thrombus becomes more organised with collagen-dense fibrous tissues at the proximal and distal caps.6 As the occlusion ages, the plaque becomes less lipid-laden, more calcified, and tends to lose its previously existent microchannels, thus making it more difficult to cross antegrade (see Figure 1).6,7 Moreover, further histological analysis has shown that the lesion is not homogenous in its rigidity. Rather, the distal fibrous cap is less rigid compared with the proximal cap.7 This is an important factor when one considers an antegrade versus retrograde approach. The proximal fibrous cap, which is the entry point of the CTO for an antegrade approach, thus is the site where a higher risk for wire migration into the subintimal space is observed, as compared with the distal cap, which serves as the entry site of the CTO when a retrograde approach is employed.
Pre-intervention Angiographic Set-up and Analysis
Undoubtedly, procedural success is highly dependent on a thorough angiographic evaluation of the target CTO lesion before the optimal PCI strategy can be determined. Such an evaluation includes assessment of the presence and location of collateral vessels and length of the CTO lesion to ultimately decide on the initial PCI strategy.
In order to accurately assess the lesion, we strongly advocate the use of a dual guide catheter strategy with shorter catheters (i.e. ~90 cm long) in the retrograde vessel and often the antegrade vessel. Should the operator need to switch from one technique to the other during the intervention, this would allow easy transition between antegrade and retrograde techniques. Other recommended equipment includes long (35–45 cm) sheaths and large bore guides (7 or 8 French [Fr]) for optimal support and delivery of equipment (e.g. microcatheters, balloons, stents), particularly when transfemoral arterial access is used. In situations where the operator prefers transradial access, a 6 Fr system can be successfully used depending on the operator’s experience and level of comfort.
Next, simultaneous dual injection should be performed, particularly in any case where contralateral collateral circulation exists. Dual injection is best performed on low magnification such that table panning is not needed, and is performed wherein the donor vessel is injected first followed by injection of the occluded vessel to reduce radiation exposure. This technique allows for accurate assessment of lesion length, size and location of the distal target vessel, presence of a significant bifurcation at the distal cap, and for determining the optimal PCI strategy. Furthermore, dual injection helps to reveal the presence of tortuous collateral vessels, including bridging collaterals, and can provide clarity regarding location of the angioplasty wire (true lumen versus subintimal space) during CTO crossing attempts. Evaluating the presence of bridging collaterals is needed regardless of whether an antegrade or retrograde approach is undertaken, since avoiding such vessels during crossing attempts will reduce the likelihood of vessel perforation.
Determining the Initial Percutaneous Coronary Intervention Strategy
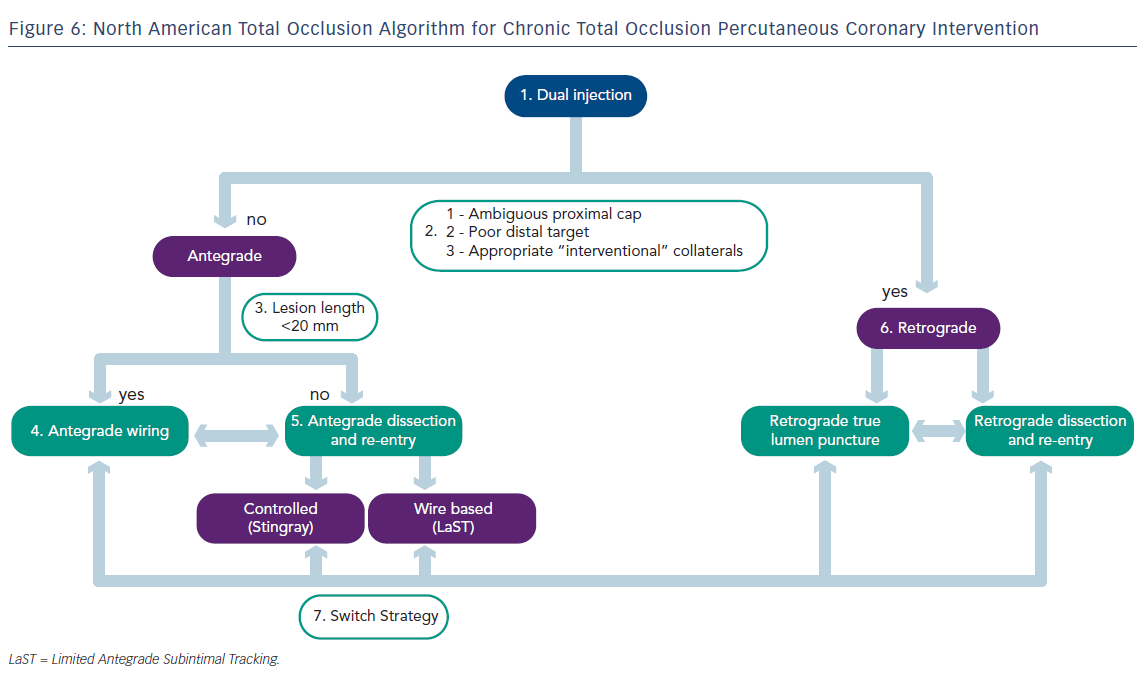
According to the NATO algorithm, the initial CTO-PCI strategy is based on four angiographic characteristics. The first of these is the proximal cap location and morphology. It is imperative to be able to localise the entry point of the CTO lesion angiographically and/or with intravascular ultrasound (IVUS). The second is lesion length. Lesions are categorised dichotomously to those <20 mm or those ≥20 mm in length. Lesion length is best determined with dual vessel injections as mentioned above. Lesions ≥20 mm are more likely to have higher failure rates and often require increased fluoroscopy time and hence increased procedure time. This complex subset of CTOs often requires early adoption of an antegrade dissection with re-entry or a primary retrograde approach. The next angiographic characteristic that needs consideration is the size of vessel lumen at the distal cap and the presence of significant side branches at the distal cap. Finally, one must evaluate the size and suitability of collateral vessels for retrograde crossing. This is visualised arising from the healthier donor vessel, which joins the CTO vessel beyond the distal cap. Ideally, the donor vessel should have minimal tortuosity, should provide more than one collateral branch to the CTO vessel, all of which increase the operator’s ability to safely pass wires and microcatheters while avoiding donor vessel damage and intraprocedural ischaemia.
The Tools of the Trade
Before diving deep into a discussion on the techniques of CTO-PCI, it is important to first lay out the devices typically used in CTO interventions, especially those most frequently utilised according to the techniques of the NATO operators.
Guidewires
Recognising that there are a number of guidewires that are commonly utilised for CTO-PCI, the NATO operators recommend the following four wire designs:
- hydrophilic or polymer-jacketed 0.014” wires with a tapered tip, such as the Fielder XT™ (Asahi Intecc, Nagoya, Japan) and Runthrough taper™ (Terumo Corporation, Tokyo, Japan);
- non-tapered, polymer-jacketed hydrophilic 0.014” wires for retrograde approaches, such as the Fielder FC™ (Asahi Intecc, Nagoya, Japan) and Pilot 50™ (Abbot Vascular);
- high-gram polymer-jacketed such as the Pilot 200™ (Abbott Vascular) for complex, long and/or tortuous lesions; and
- high-gram tapered non-jacketed tip such as the Confianza Pro 12™ (Asahi Intecc) used for lesion penetration and true lumen re-entry.
Caution is needed in using such wires particularly if there is ambiguity regarding the target vessel pathway and/or target coronary segment. Most high-volume CTO operators recommend a 30 degree primary bend on the wire ~1 mm from the wire tip. On occasion, a secondary bend may be needed in order to help navigate through the vessel.
Microcatheters
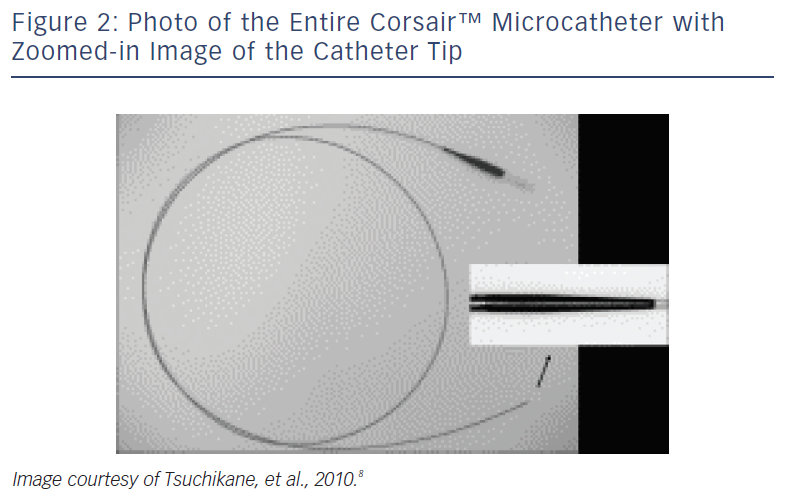
These devices are typically employed for retrograde advancement across the donor and collateral vessels.8 The Corsair™ (Asahi Intecc, Aichi, Japan) is a 2.7 Fr over-the-wire (OTW) catheter with a distal lubricious outer coating (see Figure 2). Its shaft consists of eight thin wires wound together with two larger wires, forming a spiral structure. This design allows a bidirectional rotation to be transmitted to the distal shaft to cross even tortuous collateral channels during retrograde advancing, though it can also be used during antegrade approaches.
Over-the-wire Microcatheters and Balloons
Examples of OTW microcatheters include the Finecross™ (Terumo Corporation) and Quick-Cross™ (Spectranetics Corporation, Colorado Springs, CO, US). Such devices are useful for wire exchanges. The Tornus™ (Asahi Intecc, Nagoya, Japan) is a uniquely designed OTW microcatheter with a braided wire mesh used for lesion crossing in particularly resistant CTOs. Finally, OTW balloons are helpful in providing wire support.
Lesion Crossing and Re-entry Devices
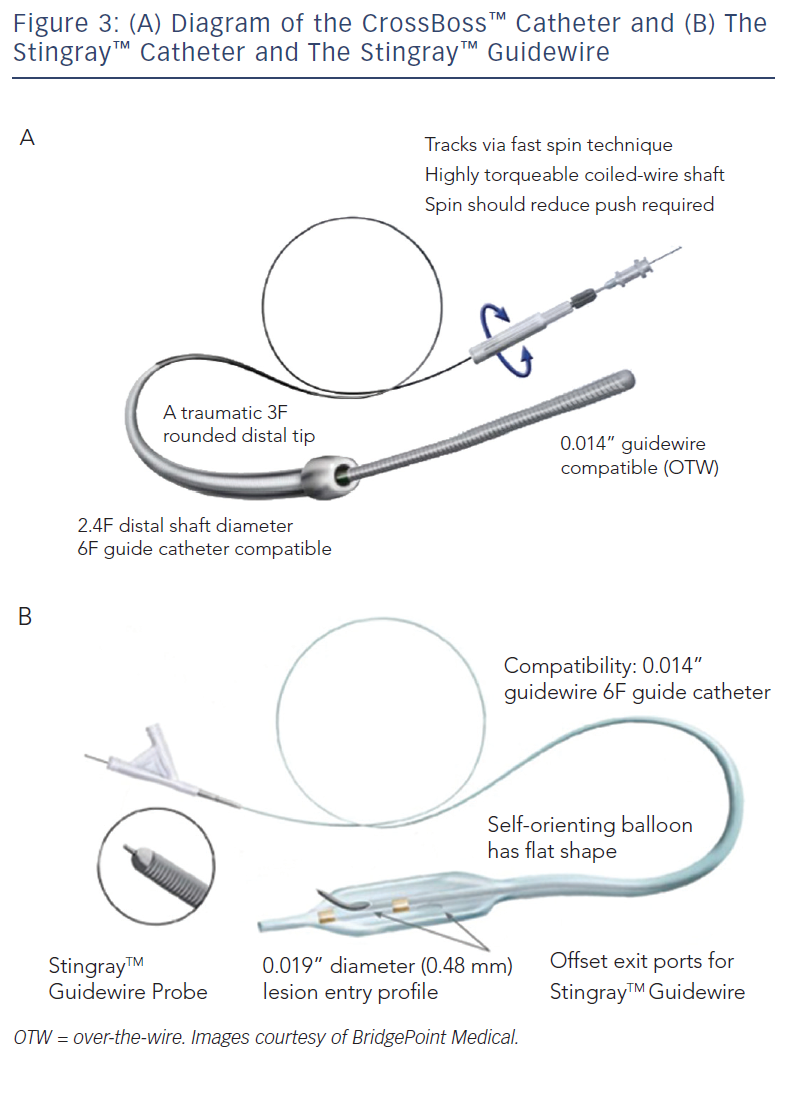
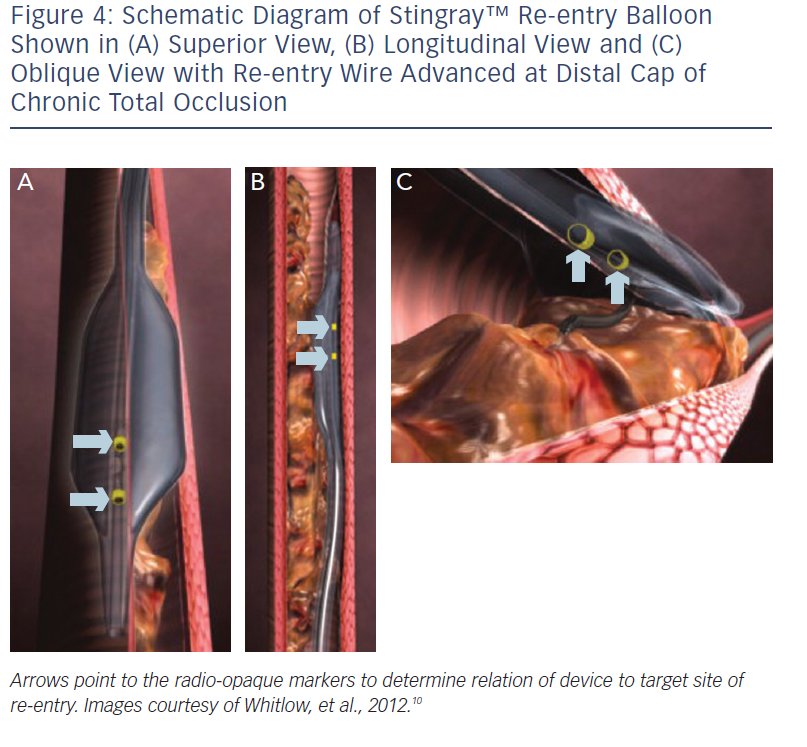
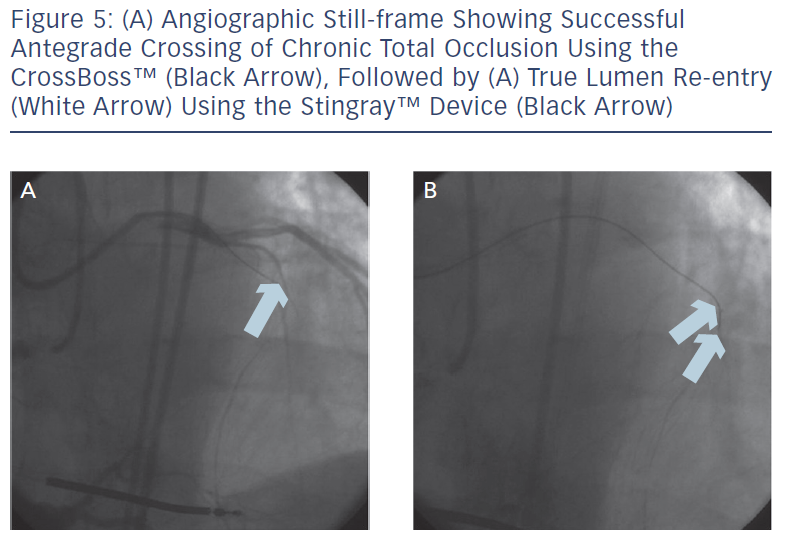
These include the Crossboss™ catheter (Bridgeport Medical, Plymouth, MN), a metal OTW microcatheter with a blunt tip that pierces through the lesion preventing extraluminal entry, without the need for a leading wire, but requires a ‘rapid-spinning’ motion technique (see Figure 3).6 As with many of the aforementioned devices, there is an associated learning curve for the operator with this microcatheter. In instances where the device leaves the true lumen, re-entry is then accomplished using the Stingray™ balloon and Stingray™ guidewire (BridgePoint Medical). This uniquely designed balloon is a 1 mm flat balloon containing three ports all connected to a single guidewire lumen (see Figure 4).6 The most distal port is used to position the balloon. The remaining two ports are oriented 180 degrees from each other such that when the balloon is inflated, one port is aimed at the lumen while the other to the adventitial layer of the vessel. Under fluoroscopic guidance, the 0.014” Stingray guidewire with its distal tapered end is used to then re-enter the true lumen.
General Considerations and Initial Plan of Attack
Regardless of whether an antegrade or retrograde approach is initially chosen, the NATO operators recommend strategic planning using a ‘hybrid’ approach, which allows the operator to switch between the two approaches should the initial approach fail. The purpose of the hybrid approach is to maximise procedural efficiency by achieving vessel recanalisation while minimising radiation and contrast dye exposure. This does require the operator to change strategies early on if the current approach is proving to be fruitless. Decidingon the initial direction (antegrade versus retrograde) is based on the patient’s coronary anatomy from angiographic visualisation using dual injection (discussed earlier). The NATO operators recommend considering the following characteristics when evaluating coronary anatomy:
- ability to identify and safely engage the proximal cap;
- lesion length;
- presence of significant branches and collaterals;
- size and location of the distal cap; and
- ability to use collateral vessels for a retrograde approach.
These five factors help determine the initial strategy in addition to a ‘plan B’ should the initial approach fail. In general, lesions <20 mm can be successfully treated with an antegrade approach using a wire escalation strategy (discussed below). Longer and/or more complex lesions but with identification of proximal and distal caps tend to be best treated with a primary dissection re-entry technique. When an operator is faced with a more complex lesion with uncertainty as to the proximal and/or distal cap, such lesions are more likely to be successfully treated with an initial retrograde approach.
In general, given the complexity of this lesion subset, when performing CTO-PCI, one must be mindful of fluoroscopy time during the procedure. Exposure of >10 Gray markedly increases the risk of radiation-induced skin injury. Thus, once this threshold is reached, unless the lesion has been successfully crossed and there is only a minimal amount of additional procedural time remaining, the procedure should be aborted. Certain techniques can be employed to limit radiation exposure to the patient, operator and catheterisation laboratory staff. These include the use of viewing on the lowest magnification, fluoroscopy storing rather than cine acquisition, coning and use of shutters, and decreasing frame rates. Another important consideration is the amount of radiocontrast dye already used.
Chronic Total Occlusions Percutaneous Coronary Intervention According to the North American Total Occlusion Algorithm
Now that the general concepts of CTO-PCI have been laid out along with a description of the typical devices, the remainder of this discussion will focus on the specific technical aspects of the hybrid strategy of CTO-PCI as developed by the NATO operators – wire escalation and dissection and re-entry techniques for both antegrade and retrograde approaches.
Wire Escalation
The technique of wire escalation is typically employed as an initial strategy for ‘less complex’ CTOs <20 mm in length with good visualisation of both the proximal and distal caps. The initial choice of wire is a low-gram, polymer-jacketed wire (e.g. Fielder XT). Due to the typical morphology of a CTO (concave-shaped proximal cap with convex distal cap), a tapered wire is recommended from the antegrade direction while a non-tapered wire is recommended with a retrograde approach. The lesion is then probed with emphasis on keeping the wire tip free. Should the wire buckle, an OTW support catheter can be advanced to provide column strength for the tip, followed by further lesion probing. Should the operator continue to experience wire buckling or the wire simply remains ineffective despite multiple attempts, which should occur for no more than a few minutes, wire exchange should occur. Choice of subsequent wire is dictated on lesion characteristics; for longer and/or tortuous lesions, a high-gram, non-tapered, polymer-jacketed wire is recommended (e.g. Pilot 200). This wire design helps prevent exit from the true lumen and is optimal for vessel tracking. In contrast, a high-gram tapered wire (e.g. Confianza Pro 12) can be used for shorter lesions whose course is well understood. Once wire substitution has occurred, it is again recommended that lesion probing be performed with the new wire for a few minutes, just as was done with the initial wire. If the lesion still remains unable to be crossed, conversion to a dissection/re-entry technique should be performed or at least considered.
Intimal Dissection/Re-entry Technique – Antegrade Approach
Antegrade dissection is defined as when the guidewire or microcatheter enter the subintimal space (area within vessel wall) from the antegrade direction. This is deliberately performed using either a guidewire in the so-called ‘knuckle-wire’ technique or the blunt-tipped CrossBoss catheter (BridgePoint Medical). The knuckle-wire technique involves a loop formation of the wire (typically, polymer-jacketed), which is advanced through the lesion. This technique has proven to be a safe method to traverse the lesion because of its decreased likelihood of piercing through the adventitial layer of the vessel wall. Moreover, a knuckled wire is unlikely to enter side branches or small collateral channels and cause perforation. Still, caution is needed to control the size of the knuckle such that it is not larger than the expected target vessel size. Alternatively, the CrossBoss catheter offers a second and highly effective method to cross the CTO segment when a wire escalation strategy is unsuccessful.9 This device, with its 3 Fr blunted distal tip, creates a small channel in between the intima and adventitia while avoiding the formation of a false lumen haematoma. In doing so, it creates the optimal environment for the Stingray system to allow reaccess of the true lumen, as the accidental creation of a false lumen haematoma would hinder contact of the vessel wall with the Stingray balloon. The Stingray balloon is inflated to low pressures (3–4 atmospheres [atm]) followed by insertion of a dedicated re-entry wire such as the Stingray guidewire or a Confianza Pro 12 guidewire.10 Multiple angiographic views from orthogonal angles may be needed to determine the orientation of the exit ports of the Stingray balloon (i.e. right versus left, above versus below) to the target site of re-entry (see Figures 4 and 5). Following this, re-entry of the guidewire into the true lumen is performed and confirmed fluoroscopically by either ipsilateral injection of the collateral vessels or via contralateral injection. In instances where there is difficulty or inability to advance the Stingray wire into the distal true lumen, a plastic-jacketed wire can be substituted in and then positioned in the distal vessel true lumen.
Another technique for true lumen re-entry, aside from the Stingray device, is the wire-based Limited Antegrade Subintimal Tracking (LaST) and redirection method. This technique is used as a bailout strategy when antegrade wiring and the Stingray both fail or are deemed inappropriate. It involves positioning a microcatheter near the site of re-entry followed by insertion of a stiff polymer-jacketed wire or a stiff tapered wire into the distal true lumen.
Intimal Dissection/Re-entry Technique – Retrograde Approach
The most common dissection/re-entry technique to connect the proximal and distal true lumens via a retrograde approach is reverse Controlled Antegrade and Retrograde Tracking (reverse CART). In reverse CART, the guidewire and microcatheter (e.g. Corsair, Asahi Intecc) are advanced from the donor vessel, across the collateral vessel and into the CTO segment. An antegrade guidewire is placed in the CTO segment (either alone or along with a crossing catheter or balloon) adjacent to the retrograde microcatheter and then inflated. Once a channel has been created between the proximal and distal CTO caps, the retrograde wire is then advanced into the proximal CTO vessel followed by wire externalisation. A 300 cm or longer wire is required for wire externalisation via the antegrade guide catheter. At this point, PCI can then be performed from the antegrade approach during a reverse CART technique.
The North American Total Occlusion Algorithm and Summary
Ultimately, the goal of any CTO-PCI is to provide a safe and successful recanalisation of the diseased vessel without compromising important side branches. The NATO algorithm (see Figure 6) was designed as a strategy to aid operators achieve maximal success in CTO-PCI, and has been validated in a small prospectively studied cohort.11 This report was written to serve as a general guide for CTO-PCI among operators who are familiar with the equipment discussed above. For those who wish to adopt and implement these techniques in their practice, but have little or no familiarity with the techniques of CTO-PCI and the various technologies discussed above, the NATO operators encourage attending combined didactic and hands-on training programmes supplemented by attendance at local/national CTO conferences to become proficient in CTO-PCI techniques.
Appendix: The North American Total Occlusion (NATO) operators include: Emmanouil S Brilakis (VA North Texas Healthcare System and UT Southwestern Medical Center, Dallas, TX, US), J Aaron Grantham (Mid-America Heart Institute, Kansas City, MO, US), Stephane Rinfret (Quebec Heart and Lung Institute, Laval University, Quebec City, Canada), R Michael Wyman (Torrance Memorial Medical Center, Torrance, CA, US), M Nicholas Burke (Minneapolis Heart Institute, Mineapolis, MN, US), Dimitris Karmpaliotis, Nicholas Lembo, David E Kandzari (Piedmont Heart Institute, Atlanta, GA, US), Ashish Pershad (Banner Good Samaritan Medical Center, Phoenix, AZ, US), Christopher E Buller (University of Toronto, Toronto, Canada), Tony DeMartini (Prairie Cardiovascular, Springfield, IL, US), William L Lombardi (PeaceHealth Cardiology, Bellingham, WA, US) and Craig A Thompson (Yale University School of Medicine, New Haven, CT, US).