Heart failure (HF) is a major cause of hospitalisation, morbidity and mortality. Nutritional factors are major contributors to HF precursors, including hypertension, obesity, dyslipidaemia, insulin resistance/diabetes and systemic inflammation. Multiple landmark trials, including Dietary Approaches to Stop Hypertension (DASH)1 and Prevención con Dieta Mediterránea (PREDIMED),2 have documented the profound effect of nutrition on cardiovascular disease (CVD) incidence/severity.
Most research into HF has focused on pharmacology and devices, with little attention being paid to nutrition.3 Therefore, knowledge regarding the role of nutritional factors in the pathogenesis or treatment of HF is limited3-5 and guidelines typically focus on sodium or fluid restriction. As nutritional modification is relatively low risk and low cost option, it is an attractive strategy for reducing HF incidence and severity. This review briefly summarises the evidence of the impact of dietary pattern on HF incidence and severity.
Methods
The MEDLINE, CINAHL, EMBASE and Cochrane databases were searched to identify relevant publications up to October 2017. Keywords used included ‘heart failure’ plus ‘diet’, ‘dietary pattern’, ‘nutrition’, ‘Diet And Reinfarction Trial/DART’, ‘Dietary Approaches to Stop Hypertension/DASH’, ‘Prevención con Dieta Mediterránea/PREDIMED’,‘Mediterranean’, ‘low-fat’, or ‘paleo(lithic)’. Bibliographies were searched for further references. All human studies specific to HF (not general CVD or non-HF CVD) were included.
Articles on salt/sodium or fluid restriction, omega-3/micronutrient supplementation, alcohol and over-/undernutrition (i.e. obesity or malnutrition/cardiac cachexia) were excluded as they were outside the scope of the review. Dietary components, such as fish and eggs, were not excluded but are only discussed briefly as they are not the major focus of this paper.
Results
Nutritional factors have long been appreciated to be of importance in CVD. Several epidemiological studies in the past decade have demonstrated a 45–81 % decrease in HF incidence in those following a healthy lifestyle (regular physical activity, healthy dietary pattern, normal BMI, no or moderate alcohol intake and not smoking).6–14 A dose–response relationship was found, with greater adherence to healthy behaviours being associated with a graded reduction in HF incidence. Lifestyle factors, including diet, therefore appear to be important in the primary prevention of HF. As the epidemiological studies included several lifestyle factors, the specific effect of nutrition cannot be elucidated. However, research focusing solely on nutrition and HF has been performed, with the Mediterranean diet (MedDiet) and Dietary Approaches to Stop Hypertension (DASH) being the major dietary patterns studied.
Dietary Approaches to Stop Hypertension
DASH was designed to prevent and treat hypertension.1,15 Based on early studies of lower blood pressure in vegetarians, “the diet design goals were to create patterns that would have the blood pressure lowering benefits of a vegetarian diet, yet contain enough animal products to make them palatable to non-vegetarians”.15 As such, DASH is a plant-based diet rich in carbohydrates and low in fat. It emphasises the consumption of fruit, vegetables, wholegrains and nuts with the addition of some fish, poultry and low-fat dairy products and the minimisation of red meat, sugar and processed foods.
A 2012 cross-sectional study of 6,814 ethnically-diverse adults without CVD reported that greater consistency with DASH was associated with favourable end-diastolic volume, stroke volume and ejection fraction.16 Subsequent separate, large prospective studies reported that greater adherence to DASH was associated with 22 % decreased HF risk in men17 and 37 % decreased risk in women.18 Indeed, a 2013 systematic review and meta-analysis including >144,000 adults reported that a DASH-like diet was associated with significant reductions in CVD incidence, including CHD and stroke (19–21 %), but the greatest risk reduction was against HF (29 %).19 Another prospective observational study conducted in 3,215 women with pre-existing HF found a 16 % decrease in mortality in those with the greatest DASH adherence after 4.6 years.20
A preliminary intervention trial with 375 participants, published in 2003, suggested a natriuretic action of the DASH diet, in conjunction with a hypotensive effect.21 This trial also reported that the DASH diet was more effective with a low sodium content and in hypertensives (compared to normotensives). Follow-up was done in the DASH-Diastolic Heart Failure (DASH-DHF) pilot study. DASH-DHF was a non-blinded, non-randomised and non-controlled pilot study of 13 primarily obese, post-menopausal women with HF with preserved ejection fraction that led to three publications. All foods were prepared and served under observation by dieticians in a metabolic kitchen. After 3 weeks there was a mean weight loss of 1.7 kg, which was accompanied by decreases in 24-hour blood pressure, dyspnoea, urinary sodium, brain natriuretic peptide (BNP) and oxidative stress but increases in 24-hour urinary potassium and aldosterone levels as well as a trend towards increased exercise capacity.22 Significant increases in stroke volume, ejection fraction and cardiac contractility were noted as well as significant decreases in arterial elastance, viscoelastic/relaxation and chamber stiffness.23 There were also increases in short-chain acylcarnitines, which correlated with improved left ventricular function,24 suggesting improved myocardial energy utilisation.
A more recent randomised, controlled trial compared DASH to general HF dietary recommendations over 12 weeks in 48 patients with mild to moderate HF.25 Significant increases in large artery elasticity, exercise capacity and quality of life were reported as well as a significant decrease in BNP. All of these changes were achieved without weight loss.
Although the trials lacked investigator blinding, DASH appears to be promising for the prevention and treatment of HF. Based on consistent evidence of benefit in CVD, including HF, DASH has been called “an optimal dietary plan for symptomatic HF”26 and was included in the 2013 American College of Cardiology/American Heart Association CVD risk prevention guidelines (strong recommendation: level 1A).27
Mediterranean Diet
Similar to DASH, the MedDiet is a plant-based, carbohydrate-rich, moderate-fat diet. It is characterised by a high intake of vegetables, fruit, wholegrains and nuts with a moderate intake of extra virgin olive oil, fish and sometimes wine. It involves a low intake of dairy products, poultry, processed meat, red meat, sugar and processed foods.
The randomised, single-blind Lyon Diet Heart Study tested the hypothesis that a MedDiet would reduce CVD complications in survivors of a first MI compared to usual care plus a prudent Western diet. After 27 months there were eight cases of non-fatal HF (n=303; 1.35 %) in the usual-care group and two cases in the MedDiet group (n=302; 0.33 %).28 In an extended 46-month follow-up, the MedDiet led to a 67 % reduction in the risk of a composite endpoint including HF (p=0.0001).29
A prospective study of 1,000 adults admitted with acute coronary syndrome reported a 7 % decrease in the likelihood of developing left ventricular systolic dysfunction at hospitalisation,12 % reduction in the likelihood of recurrent CVD events, and a trend towards a 10 % lower risk of cardiac remodelling (p=0.06) in those following the MedDiet over 2 years.30 Subsequent large prospective studies reported that MedDiet adherence was associated with decreases in HF incidence of 24 % in healthy adults,31 21 % in healthy women,32 31 % in healthy men33 and 77.4 % in anticoagulated atrial fibrillation patients.34 All of these studies reported that greater adherence conferred greater protection in a dose-dependent manner.31–34 In a recent meta-analysis of six studies (n=10,950), the MedDiet was associated with significant reductions in major vascular events (37 %), coronary events (35 %) and stroke (35 %), but the greatest risk reduction was against HF (70 %).35 Despite these positive results, it should be noted that both the quantity and quality of the available evidence in this meta-analysis was limited and highly variable.
A cross-sectional study conducted in 372 adults with pre-existing HF found that a higher MedDiet score was positively correlated with log skeletal muscle ventricles, left atrial ejection fraction and several measures of ventricular function.36 A prospective study demonstrated a 45 % greater decrease in mortality risk was reported in men who went on to develop HF and had the highest MedDiet adherence compared to those who developed HF and had the lowest adherence.33 In a similar study including 3,215 women with pre-existing HF, after 4.6 years of follow up there was a 15 % decrease in mortality risk in the highest versus lowest quartile of MedDiet adherence (p=0.006).20
Similar to DASH, there is a lack of interventional data. A preliminary analysis of the PREDIMED trial reported decreased plasma N-terminal pro-BNP and oxidised LDL as well as a lower increase in lipoprotein(a) in participants on a MedDiet plus extra virgin olive oil or nuts compared to those on a low to moderate fat diet.37 After 4.8 years, data from PREDIMED demonstrated that the MedDiet plus extra virgin olive oil or nuts had no significant protective effect on HF incidence compared to the low fat diet. However, HF incidence was not significantly lower (22–32 %) in point estimates throughout the trial in the MedDiet group. The lack of significance may be due to low HF incidence, short follow up and moderate to high baseline adherence to the MedDiet.38
Only one randomised, controlled trial has assessed the effects of the MedDiet on cardiovascular events and mortality. This trial, which included first MI survivors, compared the effects of a MedDiet with a low-fat diet (both with saturated fat ≤7 % calories and dietary cholesterol ≤200 mg/day) with usual care. No significant difference was found between the diets. However, there was significantly increased survival at a median follow-up of 46 months in the MedDiet and low-fat diet groups (84 %) compared to the usual care group (60 %).39
DASH Versus the Mediterranean Diet
Both dietary patterns are plant-based and low in red/processed meat, refined carbohydrates and processed foods. A prospective study involving 3,215 women with pre-existing HF reported 16 % and 15 % decreases in mortality rates in women with HF with adherence to DASH and the MedDiet, respectively, but these observations were only statistically significant for DASH.20
High-protein Diets
There are two interventional trials that have utilised high-protein diets in HF. A small, three-arm trial compared 12 weeks of high-protein, hypoenergetic diet to a standard protein, hypoenergetic diet or a normocaloric American Heart Association-recommended diet among 14 overweight/obese subjects with mild to moderate HF and diabetes. The higher protein diet resulted in significantly greater reductions in weight, body fat, total/LDL cholesterol and triglycerides, as well as significantly greater improvements in exercise capacity, HDL, quality of life and a trend towards increased muscle mass.40 Interestingly, patients in the high-protein group were encouraged to increase their intake of plant- as opposed to animal-based proteins. A subsequent randomised trial compared the high-protein Nordic Nutrition Recommendation diet to a high protein ‘paleo’ diet (both ad libitum) in 68 overweight postmenopausal women. After 2 years, there were non-significant decreases in weight but significant decreases in left ventricular mass and end diastolic volume in both high-protein groups.41 Conversely, excess dietary protein may be harmful in HF. One pilot intervention trial reported a decrease in stroke volume and cardiac output over time with high-protein diets.42 Further, protein-bound uremic toxins are derived from colonic microbiota metabolism of dietary amino acids, and recent reviews suggest that a low-protein diet may reduce protein-bound uremic toxins with beneficial effects on CVD.,44
The Rice Diet
In 2014, two articles were published that recounted Dr Walter Kempner and his rice diet.,46 This diet, which was proposed in the 1940s, consists of white rice, sugar, fruit, fruit juices, vitamins and iron. It provides ~2,000 calories, 20 g protein, 2–3 % fat, 1,000 ml liquid and 150–250 mg sodium daily. High blood pressure and its related symptoms reportedly improved markedly and rapidly in people following the rice diet. A 1949 editorial stated that “results are little short of miraculous […] practically speaking, there is probably no more effective diet for obese decompensated cardiac patients”.47 There has been no original research on the rice diet since 1975,48 but its remarkable reported success combined with similarities to other therapeutic (plant-based and low-sodium) regimens suggest that this may be an interesting concept.
Low-fat Diets
Low-fat diets have been and remain the cornerstone of cardiovascular dietary advice. An early, single-blind randomised controlled trial comparing a low-fat diet with added fruit, vegetables, nuts and grain products to a standard low-fat diet in 406 patients with acute MI or unstable angina reported that the supplemented low-fat diet resulted in greater weight loss and significantly increased HDL as well as significantly greater reductions in total/LDL cholesterol, triglycerides, fasting blood glucose, blood pressure, cardiovascular events – including HF incidence – and total mortality after 1 year.49 A more recent controlled trial among subjects after a first MI demonstrated significantly increased survival (84 %) with a low-fat, low saturated fat (7 % calories) and low dietary cholesterol (200 mg/day) diet compared to usual care (60 %).39
Low-fat, Plant-based Diet
A low-fat, plant-based diet remains the only dietary pattern objectively proven to reverse CHD.,51 This diet has yet to be subjected to a trial specific to HF incidence or outcome. However, an early randomised trial comparing a low-fat, plant-based diet with exercise and stress management to usual care in 46 CHD patients reported significant increases in exercise capacity and left ventricular ejection fraction as well as significant decreases in total cholesterol and angina frequency in the intervention group after just 24 days.52 Subsequent trials from the same group demonstrated that 3 months of the same regimen significantly decreased BMI, LDL, inflammation (C-reactive protein), apolipoprotein-B, angina frequency/severity and physical limitations in subjects with CHD or risk factors.42 It also reduced BMI, body fat, blood pressure, resting heart rate and total/LDL-cholesterol in subjects with impaired left ventricular function while increasing their exercise capacity and quality of life.53 Importantly, these benefits were maintained after 1 year. A follow-up trial in 27 CHD patients with asymptomatic reduced left ventricular ejection fraction reported that, compared to a low-fat, plant-based diet with exercise and stress management, usual care with revascularisation resulted in marked increases in cardiovascular events after 3 months (1,227 %) and 3 years (175 %). However, 88 % of those in the lifestyle change group did not require primary revascularisation at 3 years.54 This 3-year follow-up demonstrates that comprehensive lifestyle changes are achievable, sustainable and effective. Further, a recent case report demonstrated the effects of a plant-based diet in a 79-year-old male with documented triple vessel disease (80–95 % stenosis) and left ventricular systolic dysfunction (ejection fraction 35 %) in the context of progressive dyspnoea. Two months of the diet led to clinically-significant reductions in body weight and lipids, with improved exercise tolerance and ejection fraction (+15 %).55
Studies Based on Overall Dietary Quality
Alternative Healthy Eating Index
The alternative healthy eating index (AHEI) is a nine-component index. It includes vegetables, fruit, nuts, soy protein, cereal fibre and multivitamin use. It is low in trans-fat and alcohol. It also has high ratios of polyunsaturated to saturated fatty acids and of white to red meat.
In a large prospective study, a healthy lifestyle including a high AHEI score was associated with a 77 % reduction in HF incidence.11 Large prospective studies focusing on AHEI score alone observed a 52 % reduction in the risk of HF in women56 and 28 % reduction in HF risk in those with pre-existing CVD or diabetes over 4.5–10-year follow up.57 One of these studies was a prospective analysis of two combined trials of antihypertensive medication. Higher AHEI score had a protective effect regardless of which medications were prescribed (ACE inhibitors or angiotensin II receptor antagonist) or the presence of co-morbidities (CVD or diabetes). This result suggests that diet can be protective in the absence of pharmacology but can also act synergistically.57 This is noteworthy, as an additive benefit of nutrition to pharmacology was reported in one of the first reports of the MedDiet and CVD.29
Dietary Inflammatory Index
The dietary inflammatory index was developed to characterise dietary intake, from maximally anti- to pro-inflammatory. A large, cross-sectional study (n=15,693) reported that subjects eating a pro-inflammatory diet were 30 % more likely to have a circulatory disorder (including HF) compared to those on a less inflammatory diet.58
Dietary Modification Index
The dietary modification index score is based on percentage of total energy intake from fat, vegetable and fruit servings, grain servings, percentage of energy intake from saturated fat, percentage of energy intake from trans-fat and dietary cholesterol intake. There was a significant (39 %) decrease in HF risk in women in the highest compared to the lowest dietary modification index quintile in a large prospective study with 10-year follow up.56
Dietary Risk Score
The dietary risk score is based on food items that are considered predictive of or protective against CVD. Foods predictive of CVD include meat, salty snacks and fried foods. Protective foods include fruit, leafy green vegetables and other cooked and raw vegetables. A large, prospective study with 4.5-year follow up observed a 43 % decrease in HF risk in adults with pre-existing CVD or type 2 diabetes in the highest versus the lowest dietary risk score quartile.57
Evidence Linking Dietary Components with Heart Failure
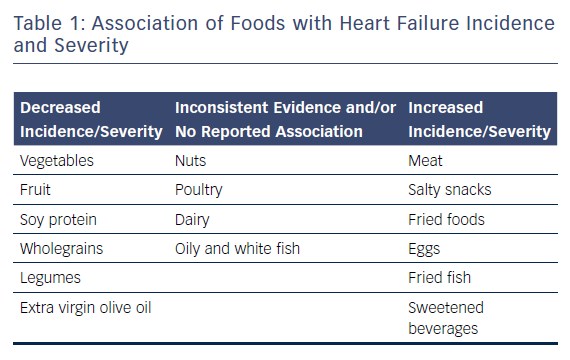
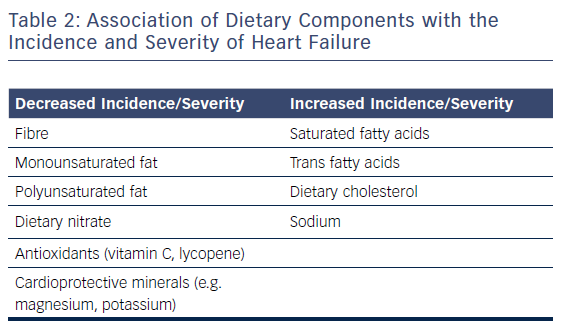
Nutritional research has traditionally focused on single foods or nutrients. Although individual dietary components are not the focus of this review, they are covered here as the effect of a single food or nutrient may be confounded by overall dietary habits and patterns.59 Table 1 provides an overview of the associations of different types of food with HF, and Table 2 lists the association of different dietary components with this condition.
Several studies of dietary patterns that have already been discussed also assessed the effects of individual foods and/or food components. Multivariate analysis of the Women’s Health Initiative Observational Study suggested that a diet low in cholesterol (p=0.001) and high in fibre (p=0.026) had a protective association with CVD.56 Interestingly, dietary cholesterol is found only in animal products, whereas dietary fibre is found only in unprocessed plant products. In the prospective analysis of AHEI and HF incidence from two combined pharmacology trials, the authors further analysed each component of the AHEI.57 They noted that all types of vegetables, green leafy vegetables, other raw vegetables, fruit, soy protein and nuts were inversely associated with HF incidence; while meat, poultry and eggs were positively associated with increased risk. There was no association with fish.57 A prospective study including 201 women with suspected myocardial infarction reported a 13 % decrease in CVD event risk (including HF) with increased consumption of fibre and a 64 % decrease with increased fruit, vegetable and legume consumption.60 Finally, a 2013 cross-sectional study of 312 HF patients awaiting heart transplantation reported that dietary habits typically considered unhealthy (i.e. infrequent consumption of fruits/vegetables/legumes and frequent intake of foods high in saturated fats) were related to enhanced physical quality of life, but only among overweight and obese patients.61 However, this study contrasts with other evidence and may represent reverse causation, whereby patients with severe HF changed their dietary habits to less healthy ones as opposed to an improved diet causing severe HF. A separate prospective study by the same group involving 318 heart transplant candidates reported that frequent intake of salty foods and saturated fat was associated with a 290 % increase in high-urgency transplantation, whereas frequent intake of foods rich in mono- and polyunsaturated fats was associated with 51 % reduction in the risk of HF deterioration/death. There was also a trend towards delisting for transplantation due to HF improvement with more frequent consumption of fruit, vegetables and legumes.62
Dietary Fats
Cross-sectional studies suggest that fatty acids may have beneficial or detrimental effects, depending on the type of fatty acid. One study reported that HF patients with higher saturated and trans fat intake had higher systemic inflammation (tumour necrosis factor-α).63 Consistent with this, another study reported that plasma trans fatty acids were strongly associated with multiple markers of systemic inflammation as well as BNP levels in HF patients.64 In contrast with this, a study of 651 acute coronary syndrome patients reported a 65 % decrease in the risk of left ventricular systolic dysfunction with exclusive olive oil consumption.65 Conversely, a large prospective study of 15,362 male physicians reported a dose–response association between fried food and HF incidence and a 103 % increase in HF risk in those with the highest versus the lowest levels of fried food consumption.66
Dairy
A cross-sectional study of 86 adults with HF noted that dairy intake was associated with both poorer memory and higher pulsatility index in the medial cerebral artery.67 Consistent with this, a later prospective study noted that dairy was associated with an 8 % increase in HF risk.68 However, a recent systematic review and meta-analysis of prospective studies noted an inverse relationship between biomarkers of dairy intake and HF incidence.69
Eggs
Prospective studies have reported an increased incidence of HF associated with egg consumption (28–64 %).68,70,71 However, one of these studies reported no association in women or people with diabetes, and the positive association in men was limited to those consuming more than six eggs weekly.70 Nevertheless, a 2017 meta-analysis reported a 25 % increase in the risk of incident HF in those with the highest compared to the lowest egg consumption.72
Fish
There are no randomised trials of fish consumption in HF. Several prospective studies have reported that fish consumption is associated with decreased HF risk.31,73–77 However, further studies have reported no protective effect,79 and two studies have reported a U-shaped association between HF risk/event rates and dietary marine omega-3 fatty acids77 or fish consumption.80 The effect of fish consumption may be influenced by its preparation (fried versus non-fried) and type (oily versus non-oily). For example, fried fish has been associated with reduced ejection fraction, lower cardiac output and higher systemic vascular resistance in older adults81 and prospective studies have reported an increased risk of HF with fried fish consumption.73,76
There are three meta-analyses of fish intake and HF risk, two of which reported an inverse association between HF risk and oily fish consumption,83 and one that reported a positive association with fried fish but no significant associations between intake and HF.84 These seemingly inconsistent observations may be partially related to toxins such as mercury. Alternatively, it is possible that dietary displacement may explain the inconsistencies. For example, if consumed in place of red/processed meat or eggs, fish may appear protective; however, if consumed in place of vegetables or wholegrains, fish may not be protective.
One potentially important aspect of fish is the presence of omega-3 fatty acids. Further, a recent meta-analysis of randomised controlled trials reported that omega-3 fatty acids conferred a benefit in HF.85 A 2017 science advisory from the American Heart Association regarding omega-3 – based mostly on secondary prevention trials in those at high risk of CVD – suggested that individuals with recent MI or current HF may benefit from supplementation.86
Meat
There are five prospective studies examining the association between meat consumption and HF incidence in separate medium to large, middle-aged cohorts. All of these studies found increased HF risk with meat consumption.31,68,87–89 One study reported a dose–response relationship whereby higher meat consumption seemed to confer higher HF risk,87 while another reported increased HF and non-HF mortality with increased meat consumption.88 However, one study stated that there was no association after multivariate adjustment68 and another that although processed meat increased HF risk, unprocessed red meat was not detrimental.89
Fruit and Vegetables
A 2008 prospective study of 14,153 healthy white and African-American adults reported that neither fruit nor vegetable consumption had a benefit on HF incidence.68 However, a subsequent larger prospective study (n=34,319) reported a 20 % decrease in HF risk in the highest versus the lowest consumers after multivariate adjustment.90 Interestingly, vegetables (mutually adjusted for fruit) were protective but not total fruit (mutually adjusted for vegetables). However, the consumption of apples, pears and berries and of green leafy vegetables was inversely associated with HF risk in a dose–response manner.90 A retrospective study reported marked decreases in both white blood cell count and HF incidence with increasing tomato consumption.91 Consistent with this, a prospective study among adults with pre-existing HF reported that higher lycopene intake was associated with significantly longer cardiac event-free survival.92 Lycopene is a major phytonutrient found in tomatoes and other fresh produce, such as watermelon and papaya.
Several cross-sectional studies have suggested that diverse benefits are associated with greater consumption of fruit and vegetables in those with pre-existing HF. These benefits include decreased inflammation,93 decreased oxidative stress,94 increased ejection fraction95 and improved functional capacity.96
Nuts
Despite reported non-HF cardio-protective effects, two independent prospective studies found no association between nut consumption and HF incidence.68,97
Wholegrains
An early prospective study with 19.6-year follow-up period reported a 29 % decrease in HF risk in people consuming seven servings of breakfast cereal weekly compared to none. This protective effect was limited to wholegrain cereals.98 Support for this finding was provided by a subsequent prospective study, which reported a 7 % decrease in HF risk with each additional wholegrain serving.68
Other Carbohydrates
In contrast to the protective effect of wholegrains, a large prospective study of men (n=42,400) reported a 23 % increase in HF risk with the consumption of ≥400 ml sweetened beverages daily compared to non-consumers.99 Only a single study has assessed glycaemic index or glycaemic load and HF. The 9-year prospective study of 36,019 healthy adults reported no significant association between dietary glycaemic index or glycaemic load and HF events,100 perhaps suggesting that fibre content and the overall composition of carbohydrates is more important than absolute glycaemic index or glycaemic load.
Potential Mechanisms of Action
Plant-based diets are typically rich in fibre, dietary nitrates and cardioprotective micronutrients such as magnesium, potassium and antioxidants (e.g. vitamin C and lycopene) but low in saturated/trans fat. In contrast, animal foods are typically much lower in nitrate, magnesium, potassium and antioxidants. Therefore plant-based diets may increase antioxidant status and nitric oxide bioavailability and decrease reactive oxygen species, inflammation, homocysteine, blood pressure, hyperglycaemia, obesity, lipids and even atherosclerosis.
Another potential mechanism of action is dietary modulation of the gut microbiome. A series of well-conduced human studies demonstrated that intestinal microbiota metabolise choline/phosphatidylcholine and L-carnitine to produce trimethylamine, which is oxidised to pro-atherogenic trimethylamine-N-oxide (TMAO).101,102 TMAO levels are elevated in people with HF.103 Further, TMAO levels have been correlated with BNP104 and associated with HF severity102–106 and HF mortality.103,104,106 Foods rich in L-carnitine (e.g. red meat)31,68,87-89 and choline/phosphatidylcholine (e.g. eggs)72 have been linked with HF incidence/severity. TMAO production may also explain the inconsistent effects observed with fish, dairy and poultry (all rich sources of choline). Interestingly, those eating primarily plant-based diets, with limited choline/phosphatidylcholine and L-carnitine ingestion, do not seem to produce significant quantities of TMAO, even after ingestion of L-carnitine/choline.101 Consistent with the data presented herein, plant-based diets appear to have beneficial effects on the gut microbiome while Western diets that are high in animal products appear to have deleterious effects on the gut.
Specific to HF, the MedDiet has been associated with decreased oxidative stress.35 Further interventional data have reported decreased plasma N-terminal pro BNP levels, lower oxidised LDL and the prevention of lipoprotein(a) increase.37
Discussion
There is growing evidence that nutrition might be a critical factor in HF incidence and progression. Although existing research is limited, it appears that plant-based diets high in antioxidants, micronutrients, dietary nitrate and fibre but low in saturated/trans fats and sodium are associated with decreased HF incidence/severity. It is likely that these dietary features contribute to decreased oxidative stress, lower homocysteine levels and reduced inflammation as well as to higher antioxidant defence, nitric oxide bioavailability and gut microbiome modulation. Based on studies such as these and mechanistic data, a 2005 review suggested that certain lifestyle measures – including plant-based diets (moderate to low in bioavailable phosphate) – might modulate parathyroid hormone secretion and reduce left ventricular hypertrophy as well as HF risk.107 Similarly, a 2014 editorial regarding a prospective study of processed/unprocessed red meat consumption and HF risk88 suggested that plant-rich diets could lower HF incidence and severity.108
Limitations
This review has several important limitations. Although individual foods and nutrients were briefly introduced, the focus was on data relating to dietary pattern. Further, although some studies relating to overall CVD and its components (e.g. hypertension) were included, they were only included if HF was specifically mentioned and the overall focus was on data relating specifically to HF. Therefore, it is possible that relevant studies were not included in the review. Evidence regarding salt/sodium or fluid restriction, omega-3/micronutrient supplementation, chocolate, supplements, alcohol, over- or undernutrition and animal/cell model data was omitted. These major topics are outside the scope of this review. Further, studies of nutritional biomarkers were not included.
Study designs, cohorts and outcome measures were heterogeneous, limiting the ability to make comparisons across studies and draw conclusions. In addition, many of the studies included were pilot studies and may not have been adequately powered to see significance in the outcomes of interest.
Most available data are observational. Although reported observations could be real and are plausible, confounding is possible. Many reports included here were re-analyses of the same cohorts. Although many of these studies were large and prospective, they often enrolled limited cohorts, for example male physicians. Many existing studies included only one measurement of dietary intake. These studies can only provide associations as opposed to causation and cannot determine whether participants changed their diet during follow up. Many studies assessed dietary intake via self-report, which is prone to misreporting.
There is a notable lack of interventional trials regarding HF. Existing interventional trials of plant-based diets in HF have reported improvements in cardiac function, myocardial energetics, functional capacity and quality of life, inferring a remarkable response. Nevertheless, many existing intervention studies were pilot studies with small samples and short follow up.
Future Recommendations
The potential role of nutrition in HF prevention and treatment was first suggested in the 1940s.46,47 The field has grown but remains limited. Future studies should take account of HF type and severity, pharmacology and co-morbidities. Adequately-powered sample sizes and relevant follow-up periods with investigator-blinded, randomised and controlled trials are urgently required. Dietary intake should ideally be assessed subjectively (e.g. with a food diary) and objectively (e.g. using nutritional biomarkers). Further, future studies should include women, older people and diverse ethnic groups, which have been largely neglected in existing HF nutritional research.
Conclusion
Considering the relative safety and low cost of dietary intervention, clinical trials are urgently needed to help elucidate the effect of dietary patterns and components on HF incidence and severity. A seminal 1999 editorial regarding the famous Lyon Diet Heart Study stated that “relatively simple dietary changes achieved greater reductions in risk of all-cause and coronary heart disease mortality in a secondary prevention trial than any of the cholesterol-lowering studies to date”.109 The editorial details the cost-effectiveness and high benefit-to-risk ratio of dietary manipulation compared to drugs and invasive procedures and concludes that dietary factors must be very important. The current review suggests that diet is important and, agrees with an expert editorial that states: “in our search for the silver bullet, we have overlooked the silver plate. It is regrettable that we remain so imprecise and ill-informed about a cornerstone in patient care. Diet is important. We can and should know more.”110
The existing but limited human evidence suggests that a plant-based diet rich in fruit, vegetables, legumes and wholegrains is likely beneficial. The role of nuts, dairy and poultry is controversial, while red/processed meats, eggs and refined carbohydrates appear to be detrimental.