1Department of Diabetes and Endocrinology, Whittington Hospital, London, UK, 2Department of Endocrinology Diabetes and Metabolism, First Department of Pediatrics, National and Kapodistrian University of Athens, “Aghia Sophia” Children’s Hospital, 31st Medical Propedeutic Dept, Medical School, Aristotle University of Thessaloniki, AHEPA Hospital, 4Cardiology Department, Hippokration Hospital, Athens, 5Thalassemia Unit, First Department of Pediatrics, National and Kapodistrian University of Athens, “Aghia Sophia” Children’s Hospital, Greece, 6International Centre of Circulatory Health (ICCH) National Heart and Lung Institute (NHLI), Imperial College, London, UK
Osteoporosis is a major health problem affecting both men and women. Statins, besides their action as lipid-lowering agents, seem to have additional pleiotropic properties, among them a beneficial effect on bone mineral density. The entirety of experimental and the majority of clinical studies as well as the only relevant meta-analysis suggest that statins have an anabolic effect on bone metabolism. Statins, osteoporosis and adipogenesis share the same pathway, RANKL/OPG. It would appear that an imbalance in this pathway could be responsible for the manifestation of some metabolic disorders such as diabetes mellitus, atherogenesis, multiple myeloma, osteoporosis. Possibly in the future, drugs which can intervene in this biochemical and pathophysiological cascade, like statins, in a variety of doses, could be used for the management of ectopic ossification syndromes and other bone disorders, even as an additive treatment. Until then, further large longitudinal randomized controlled studies for each statin separately are required to confirm this hypothesis.
Adipogenesis, Bone formation, Bone mineral density, OPG, Osteoporosis, RANKL, Statins
INTRODUCTION
Osteoporosis is a serious health problem not only because it affects the quality of life but also and more importantly because it is associated with morbidity and mortality as well as economic burden. According to the WHO, osteoporosis is a “systemic skeletal disease characterized by low bone mass and microarchitectural deterioration of bone tissue which leads to bone fragility and susceptibility to fracture”. Bone mineral density (BMD) in osteoporotic patients is less than -2.5 SD compared with the BMD of young people (T-score<-2.5 SD). BMD of people without osteoporosis is usually above -1SD.1
Osteoporosis is a more frequent disease in women than in men,2 although mortality due to osteoporotic fractures is higher in men than in women.3 In addition to this, post-menopausal women suffer from osteoporosis and osteoporotic fractures at a higher frequency than pre-menopausal women.4
Through adult life there is a dynamic progress which is called “bone remodeling”. Bone remodeling is well established throughout the literature and involves both systemic and non-systemic factors.5 It is well known that in this procedure an important role is played by the system of receptor activator of nuclear factor kappa b ligand (RANKL)-osteoprotegerin (OPG),6 some cytokines and bone morphogenetic proteins (BMPs) (Figure 1).7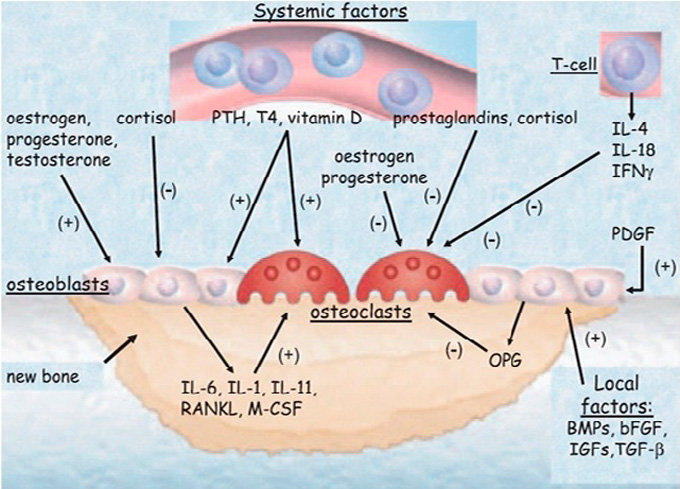
Figure 1. Bone metabolism enhanced by growth factors like bone morphogenetic proteins (BMPs), transforming growth factors beta (TGF-β), insulin growth factors (IGFs), fibroblast growth factors (FGFs). Systemic factors can also enhance osteoblast differentiation and proliferation. Systemic factors and locally produced growth factors can also induce activation of osteoclasts. Interleukins, prostaglandins and M-CSF produced from osteoblasts also induce the formation of osteoclasts. RANKL binds its receptor RANKL and induces the formation of osteoclasts. OPG inhibits RANKL binding to RANK.
On the other hand, it has been well known since the 70s that bone loss in osteoporotic patients is associated with increase of adipose tissue in bone marrow.8 Mesenchymal stem cells are pluripotent cells with a high mitotic index and are involved in the differentiation of adipocytes under the regulation of genes and transcription factors. Adipose tissue is considered as a separate endocrine gland, responsible for the secretion of adipokines (leptin, adiponectin) and hormones (vitamin D3, estrogen, etc.) and is involved in the pathophysiology of some entities. Leptin controls the RANKL/OPG axis by inhibiting the expression of RANKL and inducing OPG to create pre-osteoblasts and mononuclear cells in circulation. The diversion of an adipocyte into osteoblast is considered to be a multifactorial process regulated by all these factors (Figure 2). In addition to this, it is well known that statins, osteoporosis and adipogenesis share the same pathway, RANKL/OPG.9,10 It appears that an imbalance in this pathway could be responsible for the manifestation of some metabolic disorders such as diabetes mellitus, atherogenesis, multiple myeloma, osteoporosis. We have also seen that fat and bone tissue interaction altered by activation or silencing of genes, signaling molecules and transcription factors.11
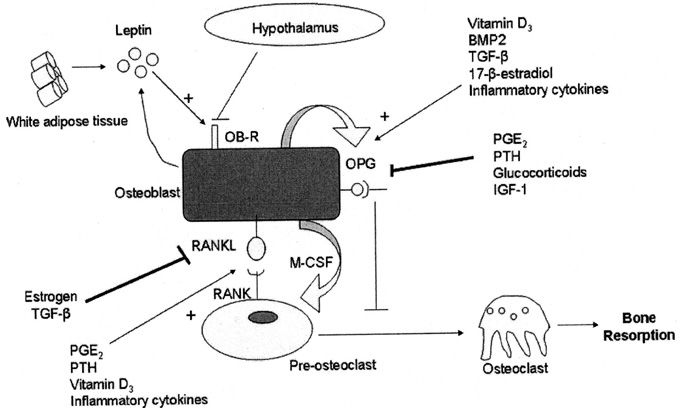
Figure 2. The interaction of adipose tissue and other factors in the differentiation of osteoblasts (Savopoulos 2011).
The purpose of our review is to investigate whether, according to the available clinical data, there is a relation between statins and osteoporosis and thus to pose queries regarding new pathways which may enhance our knowledge about the prevention and management of osteoporosis. Clinical studies, systematic reviews and meta-analyses were searched for in PUBMED and EMBASE/EXCERPTA MEDICA databases. Computerized search of the databases was accomplished by using the combination of keywords and Medical Subject Heading terms such as: statins, aminobiphosphonates, osteoporosis, RANKL, OPG, BMP, HMG-CoA reductase inhibitors, BMD, adipose tissue. We limited our search to articles published between June 2007 and October 2011 that were at least accompanied by an English abstract. We have found that there is a meta-analysis including all the clinical studies until June 2007.
CURRENT MANAGEMENT OF OSTEOPOROSIS
It is now generally accepted that first-line agents for the management of osteoporosis are the aminobiphosphonates. These drugs act to decrease bone resorption by inhibition of the farnesyl diphosphate synthase, which is a step in the mevalonic acid pathway.12 3-Hydroxy-3-Methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors (statins) inhibit the same pathway at an earlier point and may also antagonize osteoclasts by increasing expression of osteoprotegerin (Figure 3).13 In order to improve BMD and prevent osteoporosis, we have used many different drugs based on two treatment strategies: i) to inhibit the osteoclast activity and ii) to stimulate the osteoblast activity. First-line agents to avoid bone resorption are the biphosphonates like alendronate, risedronate, ibandronate and zoledronic acid.14,15 Other drugs used are teriparatide, a recombinant form of parathyroid hormone,16 selective estrogen receptor modulators (SERMs), hormone replacement therapy, calcitonin, calcitriol and vitamin D analogues.17-19 We also have agents with a different mechanism of action: bone forming through osteoblast modulation and antiresorptive through osteoclastic inhibition like strontium ranelate.20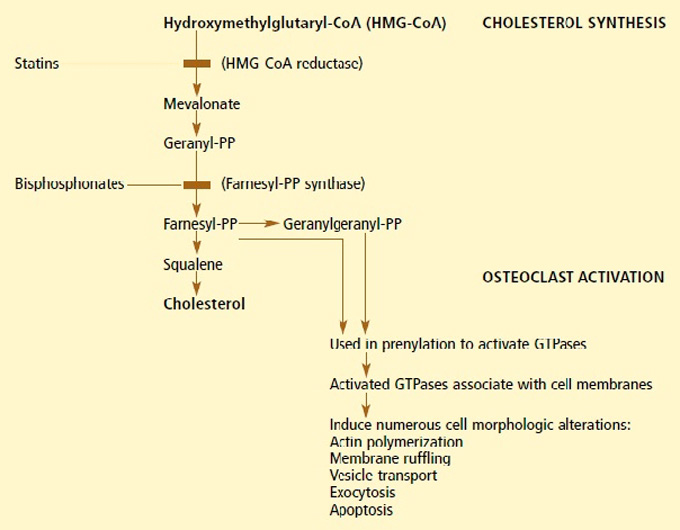
Figure 3. The interaction between HMG-CoA reductase inhibitors and bIphosphonates in the mevalonate pathway.
STATINS AND BONES: INTRIGUING INTERACTIONS
Lipid-lowering therapy and pleiotropic effects
Statins compose a drug class broadly characterized as lipid-lowering agents.21 These agents can be subgrouped according to their hydrophobic or hydrophilic nature. Hydrophobic statins (simvastatin, lovastatin) enter the liver by the hepatic portal vein, while the hydrophilic statins (rosuvastatin, pravastatin, fluvastatin) require active transport into the cell.22 Statins inhibit mevalonic acid synthesis and, as a consequence, there is a decrease in the amount of total cholesterol and decreased levels of low density lipoproteins (LDL).23 All statins have favorable effects on cardiovascular diseases, the nervous system, the immune system, the skeletal system, tumor growth.24,25 There is emerging interest in the pleiotropic effects of this class of drugs, e.g. Srivastava and colleagues26 who examined the possible action of atorvastatin in acute phase reaction in children after intravenous biphosphonate infusion, but who, however, failed to demonstrate a positive result.
Statins and bones: metabolism and clinical implications
Molecular biology and genetics reveal that both vascular and osteoblast biology have a common pathway: RANK/RANKL/OPG.27-29 With regard to this issue, there is growing interest concerning the possible mechanism and the impact of statins on bones on either the experimental or the clinical level. Mundy and colleagues in 1999 were the first to report an anabolic effect of statins in cultured mouse and human bone cells. Both simvastatin and lovastatin enhanced the expression of bone morphogenetic protein-2 (BMP-2) mRNA.30
Experimental studies
Several experimental studies illustrate the effect of statins in bone metabolism either in vitro or in vivo. Table 1 displays the main characteristics of various studies performed mostly in animal models. Although Lima et al (2011)51 demonstrated controversial, and even negative, effects of statins on bone repair, the vast majority of the studies in Table 1 support the beneficial role of this group of drugs. The administration of statins presents anabolic effects by promoting osteoblast activity and suppressing osteoclasts. As a result, statins act effectively on bone formation, inhibition of BMD decrease and, in general, on fracture healing and osteoporosis prevention. However, we should take into account that these studies refer to different animal models, they used different doses and the result was a local phenomenon, beyond the established cholesterol-lowering effect of statins. Nevertheless, certain studies underline the remarkable increase of relevant growth factors (TGFβ-1, VGF), revealing a possible explanation for the statins and bones interaction.
Further studies performed in vitro, in cell cultures (Table 2 ), support the previous findings, thus clarifying the potential mechanism of the beneficial effect of statins on bone metabolism. The expression of genes as BMP-2, COLLIA1, osteocalcin (OC) (which demonstrate an anabolic effect) and depression of others like RANKL (leading to suppression of osteoclast activity), all stimulated by statins, may regulate the role of this class of drugs in bone formation. Hughes A et al in 200752 found that hydrophobic and hydrophylic statins can inhibit osteoclast function in vitro, thereby showing a possible class effect, although stronger evidence supports the role of lipophilic agents as simvastatin (Pagkalos et al60).
Observational studies
An interesting meta-analysis of clinical studies since 2007 by Uzzan et al61 showed that statins have a positive effect on BMD in various sites. In particular, the better effect on BMD was found by lipophylic statins (simvastatin, lovastatin). The authors proposed that statins could be used for the management of osteoporosis, but the minimum concentration required for the beneficial effects on bone remains to be determined.
Several clinical studies since then have demonstrated the positive effect of statins on bones. Table 3 displays the main characteristics of these studies and their effect on BMD and bone biochemical markers. All these studies found a significant change either in BMD or in bone markers, except for three studies which found no statistically significant correlation.
Among these studies, there are four controlled studies, one cross-sectional, three open-label, one open randomized and one cohort study. Although not all of them are characterized by a strictly controlled study design, the vast majority reveal a positive effect on BMD and bone biochemical markers. Furthermore, as shown in Table 1 the controlled studies, even though not establishing the effects on BMD, showed significant changes in bone markers, which corroborates the hypothesis of the correlation between statins and bone formation.
DISCUSSION
The pleiotropic effect of statins has led clinicians to investigate their potential use among other entities, such as bone metabolism. Uzzan et al,61 in their aforementioned meta-analysis, found that statins have a positive effect on BMD in various sites. Although the authors concluded that there was a modest but statistically significant favorable effect of statins on BMD, thus confirming the results of previous studies, we are as yet far from an evidence-based recommendation of statins as a useful therapeutic modality in osteoporotic patients, even as a complementary one. In addition, more data are needed to support the use of statins for prevention of bone fracture.
This perception has mainly been developed by experimental studies, there being a lack of observational studies to clarify the field. The majority of the literature showed an increase in BMD or in bone markers. Several reasons might be advocated to explain the discrepancies. In fact, the doses used in experimental models which provided a favorable effect were much higher than the doses used in clinical practice. In addition, implementation of treatment was in a short-term perspective. Although obesity and physical activity were associated with prevention of fracture risk, they were neither controlled nor quantified. Thus, the control groups in most of the studies were small, thereby not reflecting an equal, comparative population and thus leading to bias.
On the other hand, statins could be prescribed in people with lower risk for fractures. Moreover, most of the studies attribute the interaction of statins with bone metabolism to a class-effect mechanism rather than to an individual drug effect. We have to stress here that we do not have extensive data on the pharmacological effects of statins in non-hypercholesterolemic patients.
All the available data from the literature, including evidence from experimental studies as well as from the vast majority of observational studies and the results of a single meta-analysis, suggested that there is a positive effect of statins on BMD, although another meta-analysis by Bauer et al72 showed evidence that the beneficial effects on BMD and on fracture risk are observational, while many limitations and the placebo-controlled trials did not demonstrate any clear-cut benefit. However, the in vitro and some clinical studies (Chuengsamarn et al71) suggest that statins inhibit bone resorption and stimulate bone formation, having a dual action on bone metabolism. Therefore, in the future statins might gain a position among drugs used for the prevention and management of osteoporosis, taking into account that clinicians already have a good deal of experience in prescribing statins, for other indications, and feel familiar with this drug family. Their anabolic and anti-resorptive effects on bone make them an ideal candidate for osteoporosis treatment.
In conclusion, statins, osteoporosis and adipogenesis share a major pathway, that of RANKL/RANK/OPG. Moreover, fat and bone tissue interaction is altered by activation or silencing of genes signaling molecules and transcription factors. Possibly in the future drugs which intervene in this biochemical and pathophysiological cascade, like statins, in a variety of doses, could be used for the management of ectopic ossification syndromes and other bone disorders like osteoporosis and multiple myeloma, even as an adjuvant therapy. Until then, further large longitudinal randomized controlled studies for each statin separately are required to confirm this hypothesis.
ACKNOWLEDGEMENTS
We are indebted to Dr Michael Schachter MD, PhD, Senior Lecturer at the International Centre for Circulatory Health and the National Heart & Lung Institute, Imperial College London. His lecture inspired us to produce this review.
REFERENCES
1. Data from the World Health Organization Assessment of osteoporosis at the primary health care level. Summary report of a WHO scientific Group 2007; WHO, Geneva.
2. Cole ZA, Dennison EM, Cooper C, 2008 Osteoporosis epidemiology update. Curr Rheumatol Rep 10: 92-96.
3. Nguyen ND, Ahlborg HG, Center JR, Eisman JA, Nguyen TV, 2007 Residual lifetime risk of fractures in women and men. J Bone Miner Res 22: 781-788.
4. Pinheiro MM, Reis Neto ET, Machado FS, et al, 2010 Risk factors for osteoporotic fractures and low bone density in pre and postmenopausal women. Rev Saude Publica 44: 479-485.
5. Painter SE, Kleerekoper M, Camacho PM, 2006 Secondary osteoporosis: a review of the recent evidence. Endocr Pract 12: 436-445.
6. Vega D, Maalouf NM, Sakhaee K, 2007 Clinical review: the role of receptor activator of nuclear factor kappa B(RANK)/RANK ligand/osteoprotegerin clinical implications. J Clin Endocrinol Metab 92: 4514-45121.
7. Cristenson RH, 1997 Biochemical markers of bone metabolism: an overview. Clin Biochem 30: 573-593.
8. Meunier P, Aaron J, Edouard C, et al, 1971 Osteoporosis and the replacement of cell populations of the marrow by adipose tissue. A quantitative study of 84 iliac bone biopsies. Clin Orthop Relat Res 80: 147-154.
9. Savopoulos Ch, Dokos Ch, Kaiafa Get, et al, 2011 Adipogenesis and osteoblastogenesis: trans-differentiation in the pathophysiology of bone disorders. Hippokratia 15: 18-21.
10. Song C, Guo Z, Ma Q, et al, 2003 Simvastatin induces osteoblastic differentiation and inhibits adipocytic differentiation in mouse bone marrow stromal cells. Biochem Biophys Res Commun 308: 458-462.
11. Nuttall ME, Gimble JM, 2004 Controlling the balance between osteoblastogenesis and adipogenesis and the consequent therapeutic implications. Curr Opin Pharmacol 4: 290-294.
12. Van Beek E, Pieterman E, Cohen L, et al, 1999 Farnesyl pyrophosphate synthase is the molecular target of nitrogen-containing biphosphonates. Biochem Biophys Res Commun 264: 108-111.
13. Cruz AC, Gruber LB, 2002 Statins and osteoporosis: can these lipid-lowering drugs also bolster bones? Cleve Clin J Med 69: 277-288.
14. Papapoulos SE, 2011 Use of biphosphonates in the management of postmenopausal osteoporosis. Ann N Y Acad Sci 1218: 15-32.
15. Rakel A, Boucher A, Ste-Marie LG, 2011 Role of zoledronic acid in the prevention and treatment of osteoporosis. Clin Interv Aging 6: 89-99.
16. Jakob F, Oertel H, Lanqdahl B, et al, 2012 Effects of terparatide in postmenopausal women with osteoporosis pre-treated with bisphosphonates: 36-months results from the European Forsteo Observational study. Eur J Endocrinol 166: 87-97, Epub 2011 Nov 2.
17. Gallagher JC, Levine JP, 2011 Preventing osteoporosis in symptomatic postmenopausal women. Menopause 18: 109-118.
18. Bhalla AK, 2010 Management of osteoporosis in a pre-menopausal women. Best Pract Res Clin Rheumatol 24: 313-327.
19. De Nijs RN, Jacobs JW, Alqra A, et al, 2004, Prevention and treatment of glucocorticoid-induced osteoporosis with active vitamin D3 analogues: a review with meta-analysis of randomized controlled trials including organ-transplantation studies. Osteoporos Int 15: 589-602.
20. Cesare R, Napolitano C, Lozzino M, 2010, Strontium ranelate in postmenopausal osteoporosis treatment: a critical appraisal. Int J Womens Health 2: 1-6.
21. Hatzitolios AI, Athyros VG, Karagiannis A, et al, 2009 IMPROVE Collaborative Group Implementation of strategy for the management of overt dyslipidemia: the IMPROVE-dyslipidemia study. Int J Cardiol 134: 322-329.
22. Ellsworth JA, Witt MD, Dugdale D, et al, Medical Drug Reference. Philadelphia: Elsevier Mosby, 2007.
23. Hmelin BA, Turgeon J, 1998 Hydrophilicity/lipophlicity: relevance for the pharmacology and clinical effects of HMG-CoA reductase inhibitors. Trends Pharmacol Sci 19: 26-37.
24. Brunton L, Lazo J. Goodman, et al, 2005 The pharmacological basis of therapeutics. McGraw-Hill.
25. Yeung CA, Tsao P, 2002 Statin therapy: beyond cholesterol lowering and antiinflamantory effects. Circulation 105: 2937-2938.
26. Srivastava T, Haney CJ, Alon US, 2009 Atorvastatin May Have No Effect on Acute Phase Reaction in Children After Intravenous Bisphosphonate Infusion. J Bone Miner Res 24: 334-337.
27. Liao KJ, 2004 Statin therapy: having the good without the bad. Hypertension 43: 1171-1172.
28. Hofbauer LC, Brueck CC, Shanahan CM, et al, 2007 Vascular calcification and osteoporosis - from clinical observation towards molecular undestanding. Osteoporos Int 18: 251-259.
29. Bagger YZ, Rasmussen HB, Alexandersen P, et al, 2007 PERF Study Group. Links between cardiovascular disease and osteoporosis in postmenopausal women: serum lipids or atherosclerosis per se? Osteoporos Int 18: 505-512.
30. Mundy G, Garrett R, Harris S, et al, 1999 Stimulation of bone formation in vitro and in rodents by statins. Science 286: 1946-1949.
31. Saraf SK, Singh A, Garbyal RS, et al, 2007 Effect of simvastatin on fracture healing--an experimental study. Indian J Exp Biol 454: 444-4449.
32. Wang JW, Xu SW, Yang DS, et al, 2007 Locally applied simvastatin promotes fracture healing in ovariectomized rat.Osteoporos Int 18:1641-1650.
33. Nyan M, Sato D, Oda M, et al, 2007 Bone Formation with the Combination of Simvastatin and Calcium.Sulfate in Critical-Sized Rat Calvarial Defect. J Pharmacol Sci 104: 384-386.
34. Skoglund B, Aspenberg P, 2007 Locally applied Simvastatin improves fracture healing in mice. BMC Musculoskelet Disord 8: 98.
35. Du Z, Chen J, Yan F, et al, 2009 Effects of Simvastatin on bone healing around titanium implants in osteoporotic rats. Clin. Oral Implants Res 20: 145-150.
36. Funk JL, Chen J, Downey KJ, et al, 2008 Bone Protective Effect of Simvastatin in Experimental Arthritis. J Rheumatol 35: 1083-1091.
37. Pengde K, Fuxing P, Bin S, et al, 2008 Lovastatin inhibits adipogenesis and prevents osteonecrosis in steroid-treated rabbits Joint Bone Spine 75: 696-701.
38. Gutierrez GE, Edwards JR, Garrett IR, et al, 2008 Transdermal Lovastatin Enhances Fracture Repair in Rats. J Bone Miner Res 23: 1722-1730.
39. Kaji H, Naito J, Inoue Y, et al, 2008 Statin suppresses apoptosis in osteoblastic cells: role of transforming growth factor-beta-Smad3 pathway. Horm Metab Res 40: 746-751.
40. Alam S, Ueki K, Nakagawa K, et al, 2009 Statin-induced bone morphogenetic protein (BMP) 2 expression during bone regeneration: an immunohistochemical study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 107: 22-29.
41. Uyar Y, Baytur Y, Inceboz U, et al, 2009 Comparative effects of risedronate, atorvastatin, estrogen and SERMs on bone mass and strength in ovariectomized rats. Maturitas 63: 261-267.
42. Hanayama R, Shimizu H, Nakagami H, et al, 2009 Fluvastatin improves osteoporosis in fructose-fed insulin resistant model rats through blockade of the classical mevalonate pathway and antioxidant action. Int J Mol Med 23: 581-588.
43. Ayukawa Y, Yasukawa E, Moriyama Y, et al, 2009 Local application of statin promotes bone repair through the suppression of osteoclasts and the enhancement of osteoblasts at bone-healing sites in rats. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 107: 336-342.
44. Ho ML, Chen YH, Liao HJ, et al, 2009 Simvastatin increases osteoblasts and osteogenic proteins in ovariectomized rats. Eur J Clin Invest 39: 296-303.
45. Pauly S., Luttosch F, Morawski M, et al, 2009 Simvastatin locally applied from a biodegradable coating of osteosynthetic implants improves fracture healing comparable to BMP-2 application. Bone 45: 505-511.
46. Liu C, Wu Z, Sun HC, 2009 The Effect of Simvastatin on mRNA Expression of Transforming Growth Factor-β1, Bone Morphogenetic Protein-2 and Vascular Endothelial Growth Factor in Tooth Extraction Socket Int J Oral Sci 1: 90-98.
47. Chen SH, Chou FF, Ko JY, 2010 The Use of Simvastatin with Aromasin in an Ovariectomized Rat Model: Effects on the Skeletal System. Chang Gung Med J 33: 509-514.
48. Nyan M, Sato D, Kihara H, et al, 2009 Effects of the combination with alpha-tricalcium phosphate and simvastatin on bone regeneration. Clin Oral Implants Res 20: 280-287.
49. Wang W, Nyman JS, Moss HE, et al, 2010 Local Low-Dose Lovastatin Delivery Improves the Bone-Healing Defect Caused by Nf1 Loss of Function in Osteoblasts J Bone Miner Res 25: 1658-1667.
50. Goes P, Lima AP, Melo IM, et al, 2010 Effect of Atorvastatin in radiographic density on alveolar bone loss in wistar rats. Braz Dent J 21: 193-198.
51. Lima CE, Calixto JC, Anbinder AR, 2011 Influence of the association between simvastatin and demineralized bovine bone matrix on bone repair in rats Braz Oral Res 25: 42-48.
52. Hughes A, Rogers MJ, Idris AI, et al, 2007 A Comparison between the Effects of Hydrophobic andHydrophilic Statins on Osteoclast Function In Vitro and Ovariectomy-Induced Bone Loss In Vivo. Calcif Tissue Int 81:403-413.
53. Ruiz-Gaspa S, Nogues X, Enjuanes A, et al, 2007 Simvastatin and atorvastatin enhance gene expression of collagen type 1 and osteocalcin in primary human osteoblasts and MG-63 cultures. J Cell Biochem 101: 1430-1438.
54. Ahn KS, Sethi G, Chaturvedi MM, et al, 2008 Simvastatin. 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor, suppresses osteoclastogenesis induced by receptor activator of nuclear factor-kappaB ligand through modulation of NF-kappaB pathway. Int J Cancer 123: 1733-1740.
55. Yamashita M, Otsuka F, Mukai T, et al, 2008 Simvastatin antagonizes tumor necrosis factor-a inhibition of bone morphogenetic proteins-2-induced osteoblast differentiation by regulating Smad signaling and Ras/Rho-mitogen-activated protein kinase pathway. J Endocrinol 196: 601-613.
56. Monjo M, Rubert M, Ellingsen JE, et al, 2010 Rosuvastatin promotes osteoblast differentiation and regulates SLCO1A1 transporter gene expression in MC3T3-E1 cells. Cell Physiol Biochem 26: 647-656.
57. Yamashita M, Otsuka F, Mukai T, et al, 2010 Simvastatin inhibits osteoclast differentiation induced by bone morphogeneticprotein-2 and RANKL through regulating MAPK, AKT and Src signaling Regulatory Peptides 162: 99-108.
58. Chen PY, Sun JS, Tsuang YH, et al, 2010 Simvastatin promotes osteoblast viability and differentiation via Ras/Smad/Erk/BMP-2 signaling pathway. Nutr Res 30: 191-199.
59. Zhou Y, Ni Y, Liu Y, et al, 2010 The role of simvastatin in the osteogenesis of injectable tissue-engineered bone based on human adipose-derived stromal cells and platelet-rich plasma. Biomaterials 31: 5325-5335.
60. Pagkalos J, Cha JM, Kang Y, et al, 2010 Simvastatin induces osteogenic differentiation of murine embryonic stem cells. J Bone Miner Res, 25: 2470-2478.
61. Uzzan B, Cohen R, Nicolas P, et al, 2007 Effects of statins on bone mineral density: a meta-analysis of clinical studies. Bone 40: 1581-1587.
62. Uysal AR, Delibasi T, Erdogan MF, et al, 2007 Effect of simvastatin use on bone mineral density in women with type 2 diabetes. Endocr Pract 13: 114-116.
63. Majima T, Komatsu Y, Fukao A, et al, 2007 Short term effects of atorvastatin on bone turnover in male patients with hypercholesterolemia. Endocr J 54: 145-151.
64. Majima T, Shimatsu A, Komatsu Y, et al, 2007 Short-term effects of pitavastatin on biochemical markers of bone turnover in patients with hypercholesterolemia. Intern Med, 46: 1967-1973.
65. Safaei H, Janghorbani M, Aminorroaya A, et al, 2007 Lovastatin effects on bone mineral density in postmenopausal women with type 2 diabetes mellitus. Acta Diabetol 44: 76-82.
66. Bone HG, Kiel DP, Lindsay RS, et al, 2007 Effects of Atorvastatin on Bone in Postmenopausal Women with Dyslipidemia: A Double-Blind, Placebo-Controlled, Dose-Ranging Trial. J Clin Endocrinol Metab 92: 4671-4677.
67. Perez-Castrillon JL, Vega G, Abad L, et al, 2008 Effect of the TNFalpha-308 G/A polymorphism on the changes produced by atorvastatin in bone mineral density in patients with acute coronary syndrome. Ann Nutr Metab 53: 117-121.
68. Patil S, Holt G, Raby N, et al, 2009 Prospective, double blind, randomized, controlled trial of simvastatin in human fracture healing. J Orthop Res 27: 281-285.
69. Yavuz B, Ertugrul DT, Cil H, et al, 2009 Increased levels of 25 hydroxyvitamin D and 1,25-dihydroxyvitamin D after rosuvastatin treatment: a novel pleiotropic effect of statins? Cardiovasc Drugs Ther 23: 295-299.
70. Kanazawa I, Yamaguchi T, Yamauchi M, et al, 2009 Rosuvastatin Increased Serum Osteocalcin Levels Independent of Its Serum Cholesterol-Lowering Effect in Patients with Type 2 Diabetes and Hypercholesterolemia. Intern Med 48: 1869-1873.
71. Chuengsamarn S, Rattanamongkoulgul S, Suwanwalaikorn S, et al, 2010 Effects of statins vs. non-statin lipid-lowering therapy on bone formation and bone mineral density biomarkers in patients with hyperlipidemia. Bone 46: 1011-1015.
72. Bauer DC, Mundy GR, Jamal SA, et al, 2004 Use of Statins and Fracture. Arch Intern Med 164: 146-152.
Address for correspondence:
Christos Savopoulos, Medical School of Thessaloniki, Aristotle University, AHEPA Hospital, Thessaloniki, Greece
e-mail: chrisavop@hotmail.com
Received 25-10-11, Revised 08-01-12, Accepted 30-01-12