Abstract
Background/Objectives
The Japan-multimodal intervention trial for prevention of dementia (J-MINT) is intended to verify the effectiveness of multi-domain interventions and to clarify the mechanism of cognitive improvement and deterioration by carrying out assessment of dementia-related biomarkers, omics analysis and brain imaging analysis among older adults at high risk of dementia. Moreover, the J-MINT trial collaborates with partnering private enterprises in the implementation of relevant interventional measures. This manuscript describes the study protocol.
Design/Setting
Eighteen-month, multi-centered, randomized controlled trial.
Participants
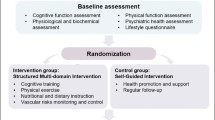
We plan to recruit 500 older adults aged 65–85 years with mild cognitive impairment. Subjects will be centrally randomized into intervention and control groups at a 1:1 allocation ratio using the dynamic allocation method with all subjects stratified by age, sex, and cognition.
Intervention
The multi-domain intervention program includes: (1) management of vascular risk factors; (2) group-based physical exercise and self-monitoring of physical activity; (3) nutritional counseling; and (4) cognitive training. Health-related information will be provided to the control group every two months.
Measurements
The primary and secondary outcomes will be assessed at baseline, 6-, 12-, and 18-month follow-up. The primary outcome is the change from baseline to 18 months in a global composite score combining several neuropsychological domains. Secondary outcomes include: cognitive change in each neuropsychological test, incident dementia, changes in blood and dementia-related biomarkers, changes in geriatric assessment including activities of daily living, frailty status and neuroimaging, and number of medications taken.
Conclusions
This trial that enlist the support of private enterprises will lead to the creation of new services for dementia prevention as well as to verify the effectiveness of multi-domain interventions for dementia prevention.
Similar content being viewed by others
Introduction
The number of people living with dementia is estimated at 46.8 million in 2015 worldwide, and is expected to increase to 131.5 million by 2050 (1). In recent years, several studies have reported a decreasing incidence of dementia in Western countries (2–4), which, however, is not the case in Japan (5). Given the high drug development failure rate in Alzheimer’s disease (AD) (6), especially for disease-modifying therapies, developing successful non-pharmacological strategies to prevent dementia is an urgent priority.
To date, several large multi-domain prevention trials have shown that interventions targeting multiple modifiable risk factors for dementia simultaneously in older adults, especially in those at increased risk of dementia, could slow cognitive decline and reduce incident dementia (7–9). The Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER) is the first large randomized controlled trial (RCT) which demonstrated that a multidomain lifestyle intervention (dietary counseling, physical exercise, cognitive training, and vascular and metabolic risk monitoring) could ameliorate cognitive decline in older adults at increased risk of developing dementia, though the effect size (difference between control and intervention groups) was very small (Cohen’s d = 0.13) (7). Sub-group analyses of the other two RCTs, the Prevention of Dementia by Intensive Vascular Care (PreDIVA) (8) and the Multidomain Alzheimer Preventive Trial (MAPT) (9) also showed the effectiveness of multidomain intervention in older adults at increased risk of dementia, although the primary outcomes were not statistically significant. These studies provide important methodological lessons: selection of at-risk individuals; appropriate timing and intensity of the interventions implemented; and selection of appropriate sensitive tools to detect changes in cognition (10, 11). However, further studies are still needed to verify the superior effectiveness of multi-domain interventions in different settings and populations (10, 11). In addition, the mechanism of effectiveness of such interventions should be further explored. In this context, the World-Wide FINGERS (WW-FINGERS) Network was launched at the 2017 Alzheimer’s Association International Conference (AAIC) in London (12). This network aims to test, adapt, and optimize the FINGER multi-domain lifestyle model for use in various populations and settings. Furthermore, data sharing is an important mission of this network, with about 30 countries around the world participating in the network (12).
In Japan, the Japan-Multimodal Intervention Trial for Prevention of Dementia (J-MINT) was started in 2019 and included in the WW-FINGERS Network (12). The aim of the J-MINT trial is to verify whether multi-domain intervention, which consists of management of vascular risk factors, group-based physical exercise and self-monitoring of physical activity, nutritional counseling, and cognitive training, could prevent the progression of cognitive decline among older adults with mild cognitive impairment. Specifically, assessment of blood-based biomarkers, omics analysis and neuroimaging make up the core of the J-MINT trial toward elucidation of the mechanism of cognitive improvement and deterioration. Additionally, the J-MINT trial collaborates with partnering private enterprises in the implementation of relevant interventional measures. This manuscript describes the study protocol for the J-MINT trial developed in accordance with the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) 2013 statement (see Appendix 1).
Methods
Study design
The J-MINT trial is designed as an 18-month, open-labeled, randomized, controlled, multicenter trial of multidomain interventions designed to target modifiable risk factors for dementia among older adults with mild cognitive impairment. Being organized by a central coordinating center at the National Center for Geriatrics and Gerontology (NCGG), this study involves the following 4 other centers located in Japan: Nagoya University; Nagoya City University, Fujita Health University, and Tokyo Metropolitan Institute of Gerontology. A total of 500 subjects being recruited at these centers will be randomized into intervention or control groups during the course of the trial. Multidomain interventions provided by partnering private enterprises will cover the following 4 domains: management of vascular risk factors (diabetes, hypertension, and dyslipidemia), group-based physical exercise and increasing physical activity, nutritional counselling, and cognitive training using Brain HQ (Posit Science Corporation). The control group will be given instructions on the management of vascular risk factors and health-related information in writing. The primary and secondary outcomes will be assessed in both groups at baseline, 6, 12, and 18 months. The study flow diagram is shown in Figure 1.
Ethic committee review and approval
All study procedures have been reviewed and approved by the Institutional Review Boards (IRBs) at each study site and the trial has been registered with the University Hospital Medical Information Network Clinical Trials Registry (UMIN-CTR) as number UMIN000038671. The purpose, nature, and potential risks of participation in the trial will be fully explained to the participants, and all participants will provide written informed consent before participating in the trial.
Eligibility criteria (Inclusion/exclusion criteria)
Table 1 shows the inclusion and exclusion criteria of the J-MINT trial. In order to focus on those at increased risk of dementia, the target population for the trial was defined as older adults with mild cognitive impairment. All prospective participants will be evaluated for the presence of mild cognitive impairment by using the National Center for Geriatrics and Gerontology Functional Assessment Tool (NCGG-FAT) which has been established as a screening tool for older adults at high risk of incident dementia (13, 14). Participants are to be deemed eligible for entry in the J-MINT trial (1) if they are 65–85 years old at the time of enrollment; (2) if they have age- and education-adjusted cognitive decline with a standard deviation (SD) of 1.0 or more from the reference threshold for one or more of the four cognitive domains of memory, attention, executive function, and processing speed, as measured with the NCGG-FAT; and (3) if they are able to provide written informed consent for themselves (Table 1).
Participants are to be excluded: (1) if they need to restrict any physical exercise and/or diet due to functional decline, including severe bone or joint disease, renal failure, and unstable ischemic heart disease and cardiopulmonary disorders; (2) if they were diagnosed with dementia; (3) if they have a Mini-Mental State Examination (MMSE) score of less than 24 (15); (4) if they have a care certificate for long-term care facilities; (5) if they are unable to speak Japanese; (6) if they are unable to undergo cognitive tests; (7) if they are deemed ineligible for enrollment by the responsible investigator or co-investigator at each study site (Table 1).
Enrollment and assessment procedures
Recruitment
The NCGG and 4 other centers are to recruit participants from their hospital and/or community-based cohorts. In some cohorts, the cognitive test results to be obtained at the time of participation are to be used to select participants who are likely to meet the selection criteria, and an invitation is to be directly sent by mail to those who have met the criteria. In addition, prospective participants, i.e., patients as well as community residents, are to be informed of the call for recruitment in this study through posters, newspaper advertisements, and public relations papers. Research staff is to fully explain the purpose, nature, and potential risks of participation in this trial for prospective participants before trial participation. Then, participants who provided written informed consent are to be assessed for their cognitive function (the NCGG-FAT (13, 14), and the MMSE (15)). Participants are to be evaluated for final eligibility by the research staff and clinicians according to the trial’s inclusion and exclusion criteria. Participant recruitment was started in November, 2019 and completed in December, 2020.
Assessment
Figure 2 shows the timeline in the J-MINT trial for assessments scheduled according to the SPIRIT guidelines. All participants are to complete neuropsychological tests at baseline, as well as at 6-, 12-, and 18-month follow-up and to undergo comprehensive geriatric assessments (CGA) at baseline, as well as 6- and 18-month follow-up. Additionally, the participants are to undergo blood tests, dementia-related biomarker tests, and brain magnetic resonance imaging (MRI) or computed tomography (CT) evaluations at baseline and at 18-month follow-up. The participants are to be evaluated for adherence to and satisfaction with the intervention protocol, and all adverse events experienced by the participants are to be recorded during the trial.
Timeline for scheduled assessments of the J-MINT trial
*: Non-mandatory; †: Dementia-related biomarker testing and whole genome sequencing are to be conducted only for subjects recruited at the National Center for Geriatrics and Gerontology. Abbreviations: CT, computed tomography; J-MINT, Japan-multidomain intervention trial for prevention of dementia; MRI, magnetic resonance imaging; NfL, neurofilament light chain; SPECT, single photon emission computed tomography.
Primary outcome measure: cognitive change at 18-month follow-up (composite score)
The primary outcome measure is the change from baseline at 18 months in a global composite score using several neuropsychological tests, which include tests of global cognitive function (MMSE (15)); memory (Logical memory I and II subset of the Wechsler Memory Scale-Revised (WMS-R) (16) and the Free and Cued Selective Reminding Test (FCSRT) (17)); attention (Digit Span of the Wechsler Adult Intelligence Scale (WAIS)-III (18)); executive function/processing speed (Trail Making Test (TMT) (19), Digit Symbol Substitution Test (DSST) subset of the WAIS-III (18), Letter word fluency test (19)).
Neuropsychological tests are to be performed at each site by trained and board-certificated psychologists, occupational therapists, and/or speech therapists, who were trained in the implementation and scoring of neuropsychological tests through participation in a training session held at the NCGG. Moreover, the Logical memory I and II subset of the WMS-R is to be scored by two psychologists from the NCGG to minimize variability in scoring. Then, the composite score is to be generated by averaging the Z scores of each neuropsychological test standardized by the baseline mean and standard deviation (SD) for each test from the full-analysis set population. A Z score of -1, for example, represents a score 1 SD below the baseline mean. A 1-point decrease on the composite score indicates an average decline of 1 SD across the neuropsychological tests.
Secondary outcome measures
Secondary outcome measures of cognitive changes are: change from the baseline to 6 and 12 months in global composite score; change from baseline in score of each neuropsychological test at 6-/12-/18-month follow-up; and incident dementia. At follow-up conducted every 6 months, participants are to be advised to receive primary health care or present to a memory clinic for further evaluation (e.g., neuropsychological tests, blood tests, brain MRI, CT, or single photon emission computed tomography (SPECT)) (1) if they have complaints about cognitive decline; (2) if they have a MMSE score of less than 24 or (3) if they have a decline in MMSE score of 3 points or more from the baseline. They are to be confirmed to have incident dementia, by consensus of two or more physicians, according to the criteria of the National Institute on Aging-Alzheimer’s Association (NIA/AA) workgroups (20), with the diagnosis of incident dementia confirmed, as required, in consultation with the Endpoint (incident dementia) Committee of the J-MINT trial (see Appendix 2).
Other secondary outcome measures are changes in each component of CGA including activities of daily living (ADL) and frailty status, blood markers, dementia-related blood biomarkers, neuroimaging assessed using MRI or CT, and number of medications taken.
a) Comprehensive geriatric assessment
CGA includes the following self-administered questionnaires and physical and cognitive performance:
-
ADL: Participants are to be assessed for basic and instrumental ADL. Basic ADL is to be assessed using the Barthel index (21), which assesses basic self-care abilities, such as feeding, transferring from a bed to a chair, bathing, bowel control, and bladder control, with the score ranging from 0 (complete dependence) to 100 (complete independence). Instrumental ADL is to be assessed using the Lawton index (22), which includes 8 items, such as using the telephone, shopping, and handling medications, with the score ranging from 0 (low function) to 8 (high function).
-
Frailty status: Participants are to be assessed for physical frailty status based on the frailty phenotype proposed by Fried et al. in the Cardiovascular Health Study (23). The components of the frailty phenotype include shrinking, weakness, slowness, self-reported exhaustion, and low physical activity. Participants are to be assessed for social frailty status based on the following 5 items: living alone, going out less frequently than last year, not visiting friends often, not feeling like helping friends or family, and not talking with anybody every day (24). Participants are also to be assessed for oral frailty status by using the Oral Frailty Index-8 (25), which includes 8 items: subjective difficulties in eating hard food, choking on tea or soup, using denture, caring for dry mouth, going out, chewing hard food such as pickled radish or shredded and dried squid, brushing teeth at least twice a day, and regularly visiting a dental clinic.
-
Dietary diversity: The 11-item Food Diversity Score Kyoto (26) has been modified to assess each participant’s dietary diversity by asking about frequency in consumption of 14 foods for the past week (i.e., cereals, fish and shellfish, meat, eggs, milk, dairy products, beans, seaweed, potatoes, fruit, nuts, and oils and fat).
-
Nutritional status: Participants are to be assessed for nutritional status using the Mini-Nutritional Assessment Short-Form (MNA-SF) (27) composed of 6 questions, whose score ranges from 0 to 14, with a higher score indicating better nutritional status.
-
Appetite: Participants are to be assessed for their appetite using the Council on Nutritional Appetite Questionnaire (28). The total score ranges from 8 to 40, with a higher score indicating a better appetite.
-
Depressive symptoms: Participants are to be assessed for depressive symptoms using self-rated 15-item Geriatric Depression Scale (29). This scale ranges from 0 to 15, with a higher score indicating a higher level of depressive symptoms.
-
History of falls and fall risk: Participants are to be evaluated for history of falls within the past 12 months and fall risk, with the latter assessed using the Fall Risk Index (30) composed of 21 questions to detect the risk of falls, including the following 3 subcategories: physical function (8 items), geriatric syndrome (8 items) and environmental hazards (5 items). Each item receives a score of 1 (risk present) or 0 (risk absent), with a higher sum for each category indicating a higher risk of falls.
-
Social network: Participants are to be assessed for social network using the Lubben Social Network Scale 6 (31), which is composed of 6 questions. The score ranges from 0 to 30, with a higher score indicating better social network.
-
Health-related quality of life: Participants are to be assessed for health-related quality of life using a health index of the EQ-5D (32) consisting of 5 dimensions (mobility, self-care, pain/discomfort, usual activities, and anxiety/depression). The scores for these five dimensions are combined to obtain up to 3,125 possible health states, from which a single index (utility) score is computed.
-
Sleep quality: Participants are to be assessed for their sleep quality using the Pittsburgh Sleep Quality Index (PSQI) (33), which is intended to assess subjective sleep quality, sleep latency, sleep duration, sleep efficiency, sleep disturbances, use of sleeping medication, and daytime dysfunction. This index score ranges from 0 to 21 and higher score indicates poor sleep quality.
-
Social participation: Participants are to be assessed for social participation using the questionnaire about social engagement in eight types of groups (34): (1) neighborhood associations/senior citizen clubs/ fire-fighting teams (Local Community); (2) hobby groups (Hobby); (3) sports groups or clubs (Sports); (4) political organizations or groups (Politics); (5) industrial or trade associations (Industry); (6) religious organizations or groups (Religion); (7) volunteer groups (Volunteer); and (8) Others.
-
Handicap from hearing loss: Participants are to be assessed for handicap from hearing loss using Hearing Handicap Inventory for the Elderly (HHIE) (35), which consists of 25 questions, with 13 questions of these evaluating emotional aspects of hearing-related QOL, and the remaining 12 evaluating social haring-related QOL among older adults. The total score ranges from 0 to 100, and a higher total score indicates a more severe subjective hearing-related handicap.
-
Anthropometric measurements: Participants are to undergo anthropometric measurements including height, body weight, and calf circumference, with body mass index calculated as body weight in kilograms divided by height in square meters (kg/ m2). In addition, optionally, participants are to be evaluated for presence or absence of sarcopenia (36) with appendicular muscle mass (AMM) measured by bioelectrical impedance analysis (BIA), and skeletal muscle mass index calculated as AMM divided by height in square meters (kg/m2).
-
Physical performance: Participants are to be evaluated for usual gait speed over a distance of 2.4 m at the middle of the walkway that has both acceleration and deceleration zones each 1 m long, with the gait speed measured twice and the mean value calculated (37). Hand grip strength of both right and left hand are to be measured in the participants using a standard digital hand grip dynamometer (Takei Scientific Instruments Co., Ltd, Japan) in standing position with the shoulder adducted and neutrally rotated and the elbow fully extended (38). Participants are to be assessed for lower extremity muscular strength by the Five-Times-Sit-to-Stand test (39), which measures the time (seconds) required to perform five successive chair stands as quickly as possible from the initial seated position with their arms crossed on their chest and sitting with their back against a chair.
-
Visual function and vision-related QOL (optional): Participants are to be assessed for visual acuity (naked- and best corrected-visual acuity), visual refraction, and intra-ocular pressure. Slit lump examination and fundus examination are to be performed. The retinal and peripapillary nerve fiber layer thickness is to be measured by the optical coherence tomography (Model RS-3000, Nidek, Japan) without pupil dilatation. Additionally, the 25-Item National Eye Institute Visual Functioning Questionnaire (NEI-VFQ-25) (40) is to be used to assess their vision-specific health related QOL.
-
Cognitive function (optional): Participants are to be assessed for cognitive function by CogEvo (Total Brain Care, Kobe, Japan) (41) and Japanese version of the Montreal Cognitive Assessment (MoCA-J) (42). CogEvo is a computer-aided cognitive function test battery, which allows for evaluation fo cognitive function including orientation, attention, memory, executive function and spatial cognition. MoCA-J is a brief cognitive screening tool for detecting older adults with mild cognitive impairment by assessing nine domains of cognition including attention, concentration, executive functions, memory, language, visuoconstructional skills, conceptual thinking, calculation, and orientation.
b) Blood and urinary tests
Blood markers assessed in all participants at baseline and 18-month follow-up are to include glucose, insulin, hemoglobin A1c, glycoalbumin, total protein, albumin (Alb), aspartate aminotransferase, alanine aminotransferase, γ-glutamyl transpeptidase, total cholesterol, high density lipoprotein-cholesterol, triglyceride, creatinine (Cr), estimated glomerular filtration rate, blood urea nitrogen, sodium, potassium, chloride, calcium, phosphorus, free triiodothyronine, free thyroxine, thyroid stimulating hormone, rapid plasma reagin, treponema pallidum hemagglutination, vitamin B1, vitamin B12, folate, and C-reactive protein. In addition, all participants will be assessed at baseline for their apolipoprotein E (APOE) phenotype.
Optionally, the following are to be measured: brain natriuretic peptide, interleukin-6, 25-hydroxyvitamin D, ghrelin, glucagon-like peptide-1, blood ketone bodies, carnitine, total bile acid, osmotic pressure, catecholamines, fatty acids, leptin, creatine, creatine kinase, nicotinamide phosphoribosyltransferase, and microRNAs. Urine glucose, urine protein, occult blood, urine Alb/Cr (u-Alb/Cr), and 8-Hydroxy-deoxyguanosine are also to be assessed optionally.
c) Blood-based dementia-related biomarkers
Participants recruited from the NCGG are to be assessed for dementia-related biomarkers. To assess amyloid-β (Aβ) deposition in the brain, which is the earliest pathological signature of AD, ratios of plasma Aβ-related peptides, APP669-711/Aβ1-42 and Aβ1-40/ Aβ1-42, and their composites are to be measured using the immunoprecipitation-mass spectrometry assay (43). In addition, plasma concentrations of tau phosphorylated at threonine 181 (p-tau181) and neurofilament light chain (NfL) are to be assessed using the single-molecule array (SimoaTM) platform (44, 45).
d) Whole genome sequencing
Whole genome sequencing is to be applied to blood samples from participants recruited from the NCGG, which is being used to conduct a comprehensive search for mutations within genes known to be associated with dementia and to identify novel pathogenic genes.
e) Brain MRI and CT
At baseline and 18-month follow-up, brain MRI or CT will be performed by the scanner available at each site to detect any local lesion, such as cerebral infarction, that could greatly affect cognitive function. At the NCGG, three-dimensional (3D) T1-weighted images, T2-weighted images, T2*-weighted images, 3D fluid attenuation inversion recovery (FLAIR) images, diffusion-weighted images, and diffusion kurtosis images are to be acquired on a Siemens Magnetom Skyra 3T MRI scanner (Siemens Medical Solutions, Erlangen Germany). To elucidate the mechanisms of cognitive improvement and deterioration during the intervention period, brain structural alterations, such as atrophy, cerebral small vessel disease and micro structural change in white matter and gray matter, are to be analyzed.
Adverse events and serious adverse events
To evaluate the safety of the intervention, all adverse events (AEs) and serious AEs are to be monitored during the course of the trial. Information to be collected about AEs is to include their date of onset, severity, associated treatment, consequences, and causation. Serious AEs are to be reported to the principal investigator, the IRB, and the co-investigators immediately.
Interventions procedures
Intervention arm
The intervention group is to receive multi-domain intervention programs including: (1) management of vascular risk factors; (2) group-based physical exercises and self-monitoring of physical activity; (3) nutritional counseling; and (4) cognitive training using Brain HQ. Figure 3 shows the J-MINT trial network, where the NCGG and the 4 other centers are to manage vascular risk factors and supervise the implementation of the multi-domain intervention, while working with Sompo Holdings, Inc. and Sompo Care Inc., care management companies in Japan. Group-based physical exercise programs, nutritional counseling, and cognitive training are to be provided by Konami Sports Co., Ltd., Sompo Health Support Inc., and Posit Science Corporation, respectively.
Management of vascular risk factors
Diabetes mellitus, hypertension, and dyslipidemia in the participants are to be treated according to relevant clinical practice guidelines in Japan, with diabetes managed based on the treatment guideline for elderly patients with diabetes mellitus 2017 by the Japan Diabetes Society (JDS)/Japan Geriatric Society (JGS) Joint Committee (46), Hypertension managed based on the Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2019) (47), and dyslipidemia managed based on the Japan Atherosclerosis Society (JAS) Guidelines for Prevention of Atherosclerotic Cardiovascular Diseases 2017 (48). Results of blood tests and brain MRI or CT will be mailed to all participants in both the intervention and control arms, together with letters recommending that they contact primary health care as required.
Group-based physical exercise and self- monitoring of physical activity
Participants are to engage in the group-based physical exercise session lasting 90 minutes at each site once a week for a total 78 sessions. Trained instructors are to provide group-based physical exercise sessions, each of which includes muscle stretching, muscle strength training, aerobic exercise, dual-task training combining exercise and cognitive tasks, namely “cognicise” (“cogni” for cognition + “cise” for exercise), and a group meeting to promote behavioral change, consistently with a previous trial (49). Intervention with combined cognitive and physical exercise was shown to improve cognitive and physical performance in older adults with mild cognitive impairment (49).
Two types of exercise session are to be provided: (1) one involving muscle strength training, which consists of 10 minutes of muscle stretching, 15–20 minutes of muscle strength training, 5 minutes of rest, 20–30 minutes of aerobic exercise, 5 minutes of rest, 20–30 minutes of dual-task training; (2) the other involving group meeting, which consist of 10 minutes of stretching, 20–30 minutes of aerobic exercise, 5 minutes of rest, 20–30 minutes of dual-task training, 5 minutes of rest, and 15–20 minutes of group meeting. The sessions involving muscle strength training and group meeting are to be provided at a ratio of 1:1 and 3:1 per month from baseline to 6 months and from 6 months to 18 months, respectively. Aerobic exercise is to be set at moderate intensity and to be progressively intensified from 40% to 80% of maximum heart rate (HR) over the study period. The maximum HR is to be estimated by age-predicted maximal HR formula (207 − 0.7 × age). HR during aerobic exercise is to be monitored by using the wrist-worn device (Fitbit® Inspire HR activity monitor). Instructors are also to monitor their HR and exercise intensity using an iPad Air tablet computer synchronizing with participant’s Fitbit® Inspire HR activity monitor. These devices are to be distributed to all participants in the intervention arm.
In group meetings, health-related information including that on prevention of dementia, frailty, knee and low back pain, malnutrition, sleep disorder, and falls and fall-related fracture, and the beneficial effect of social participation and physical activity is to be provided to promote healthy behaviors. Moreover, participants are also to be assessed for their subjective physical activity, perceived benefit and barrier to exercise, and satisfaction with the intervention programs by using the Global Physical Activity Questionnaire (50), the Exercise Benefits/Barriers Scale (51), and the Client Satisfaction Questionnaire (52), respectively, at the start of the intervention, as well as at 6-, 12-, and 18-month follow-up.
Participants are to receive several exercise videos/ messages every week via an iPad Air tablet computer to promote home-based muscle strengthening exercises, aerobic exercise, and dual-task training three or more times a week. Moreover, participants are also to be advised to monitor their daily steps, HR, and sleep using an iPad Air tablet computer synchronizing with their Fitbit® Inspire HR activity monitor.
Nutritional counseling
Nutritional counseling is to be offered individually by qualified health consultants (registered dieticians, nurses, or public health nurses). Participants are to receive face-to-face counseling (60 minutes per session) once and telephone counseling 4 times during a 6-month period, with face-to-face counseling 3 times and twelve times telephone counseling are scheduled to occur 3 and 12 times, respectively, during the course of the 18-month intervention period. In face-to-face counseling, health consultants are to assess each participant’s current situation and problems, propose coping methods, and set individual goals together with the participant. In telephone counseling, health consultants are to assess changes in lifestyle and track goal achievement. Participants are to be requested to monitor their body weight and to track goal achievement everyday using a study-provided booklet.
For the first 6 months, nutritional counseling is to focus on improvement of lifestyle (sleep and waking times) and dietary behavior (dietary timing, frequency, and regularity) based on the body’s circadian rhythm (chrononutrition). From the next 7 to 18 months, nutritional counseling is to include guidance on dietary intake required to improve cognitive and physical condition, including frailty and sarcopenia and on chewing and swallowing function and oral care (assessment of oral frailty (25), brushing teeth, and regular visits to a dental clinic). Appropriate dietary intakes are to be determined based on the Dietary Reference Intakes for Japanese (2020) (53), which provide the reference intakes of proteins, fats, carbohydrates, vitamins, and minerals and guidance on energy-providing nutrient balance for older adults 65–74 years old and 75 years or older, respectively. Moreover, participants are to be instructed to take a well-balanced diet and increase dietary diversity, which is reported to be associated with better physical and cognitive performance in Japanese older community-dwelling adults (54, 55). Participants are also to be requested to monitor their dietary diversity every day using a study-provided booklet. Additionally, based on the evidence from previous cohort studies and clinical trial (56–60), intake of fish and seafood containing eicosapentaenoic and docosahexaenoic acids, milk and dairy products, fruits, vegetables, and green tea is to be also recommended.
Cognitive training
Participants are to be instructed to engage in cognitive training individually using an iPad Air tablet computer wherein the Brain HQ (Japanese version) has been installed. The Brain HQ (Japanese version) is customized for the J-MINT trial and consists of 13 visual exercises focusing on specific cognitive abilities, such as attention, processing speed, memory, mental flexibility, and visuospatial ability. Exercise difficulty is adjusted based on cognitive abilities of each individual to ensure and sustain engagement of attention and motivation. Brain HQ exercise is reported to be beneficial for several cognitive domains including processing speed and memory in an RCT conducted specifically in older adults (61).
During the 4–6, 10–12, and 16–18 months (intensive training periods) in the 18-month intervention period, participants are to be instructed to engage in intensive training lasting at least 30 minutes per day for 4 or more days per week. Participants are to be monitored for adherence during each intensive training period as well as in the entire 18-month intervention period. Lower adherence may also be expected in cognitive training due to its use of tablet computers involving operational difficulties. Therefore, continuous support is to be provided not only in terms of instructions on the tablet computer operations at the start of the program, but in the form of a free consultation desk to be put in place during the intervention period.
Delivery of intervention during the coronavirus disease-19 (COVID-19) pandemic
The declaration by the Japanese government of a state of emergency due to the COVID-19 pandemic is expected to result in travel restrictions, social distancing, and avoidance of “3Cs” (closed spaces, crowded places, and close-contact settings, particularly their combination) and is likely to lead to part of the J-MINT intervention being restricted, especially group-based physical exercise and face-to-face nutritional counseling. In such situation, the J-MINT trial is to provide on-line intervention programs for physical exercise and nutritional counseling.
Price sensitivity measurement
At the completion of the intervention programs, a price sensitivity measurement approach is to be drawn on to discuss the appropriate price of each intervention program in view of its future implementation in the real-world setting.
Control arm
The control group is to receive health-related information in writing every 2 months on how to prevent/manage dementia, frailty, low back pain, malnutrition, health-related disease, sleep disorder and falls and fall-related fracture, as well as on the beneficial effect of social participation and physical activity and how to manage vascular risk factors according to current clinical practice guidelines.
Statistical considerations
Sample size
Since, to date, no multi-domain intervention trials have detected changes in our global composite score consisting of several neuropsychological tests, sample size calculation was based on the previous randomized controlled trial that evaluated the effect of an exercise program combined with physical and cognitive tasks in 308 older adults with mild cognitive impairment shown to have an age-adjusted cognitive decline (an SD of 1.5 or more from the reference threshold in any of the cognitive domains determined by using the NCGG-FAT (49)). In this trial, the intervention group showed a significantly greater score change on the MMSE than the control group (Intervention group vs. control group, 0.0 ± 2.48 vs. −0.8 ± 2.48 after the 40-week trial. Based on this trial, we hypothesized that the present study is also likely to detect a difference of change in cognitive function between the intervention and control groups. With a two-sided significance level of 5% and a statistical power of 80%, the total sample size required by the t-test was calculated as 302. In addition, the dropout rate at final follow-up is estimated to be 40% at each trial site. Thus, it was assumed that the trial required to enroll a total of 500 patients.
Randomization and blinding
In this trial, participants are to be centrally randomized at a 1:1 allocation ratio into intervention and control groups using the dynamic allocation method through stratification by the following variables:
-
1)
Age at enrollment (65–74 years vs. 75–85 years).
-
2)
Sex (female vs. male).
-
3)
MMSE (24–27 vs. 28–30)
-
4)
Presence of memory impairment as determined by the NCGG-FAT (amnestic vs. non-amnestic)
Participants are to be randomized electronically via a web-based system constructed by a trial statistician (F. K.) and a co-investigator (A. H.). Study participants and research staff including investigators and clinicians at each site are to be blinded to the next assignment in the sequence using electronic assignment and a dynamic allocation algorithm.
Data collection forms and data monitoring
All variables are to be measured by trained research staff at each site, and most of the measured outcome data are to be collected in the form of hard copy forms. Then, these data are to be collected through entry of these data by assessors using an electronic data capture (EDC) system. All hard copy forms are to be retained as backups as required at each site.
On-site monitoring is to be conducted at each site to ensure that the patient rights are protected, the reported data are accurate, and the conduct of the trial is compliant with the current approved protocol. The monitor is to ensure that (1) written informed consent is obtained from all participants before their participation in the trial; (2) primary outcome data reported in the EDC are complete and accurate; and (3) all AEs and serious AEs are reported appropriately.
Data analyses
In this trial, we defined the following four analysis sets: (1) the intention-to-treat (ITT) analysis set, which includes all subjects randomized regardless of whether or not they received the intervention programs/health-related information; (2) the full analysis set (FAS), which includes subjects who received the intervention program/ health-related information at least once and had at least one post-baseline assessment of neuropsychological tests; (3) the per protocol set (PPS), which includes subjects who received the intervention program/health-related information for 18-month or more; (4) the safety analysis set (SAF), which includes subjects who received the intervention program/health-related information at least once. The primary efficacy analysis is to be performed using the FAS, and the secondary efficacy analysis is to be performed using the ITT and the PPS.
Data are to be presented as means, medians, standard deviations, ranges and interquartile ranges for continuous and ordinal variables, and counts and percentages for categorical variables. Differences in baseline clinical characteristics between the intervention and control groups are to be examined for significance by using the t-test or the Mann-Whitney U test for continuous variables and by the chi-squared test or the Fisher’s exact test for categorical variables, as appropriate.
To evaluate differences from baseline in cognitive changes at 18-month follow-up between the intervention and control groups, the mixed-effects model for repeated measures (MMRM) with an unstructured covariance structure is to be used with groups, time of visit, group by time interaction, age at randomization, sex, presence of memory impairment at randomization (amnestic or non-amnestic), and baseline composite cognitive score as covariates. For the secondary continuous variables, the same analyses are to be performed using MMRM. For the secondary categorical variables, logistic regression analyses or chi-squared tests will be used as appropriate. Frequencies of serious AEs are to be summarized for the intervention and control groups.
All statistical analyses are to be performed using SAS (SAS Institute, Inc., Cary, NC, USA). P-values of < 0.05 are to be considered statistically significant.
Discussion
The J-MINT trial will be the first not only to demonstrate the effectiveness of multi-domain interventions but to clarify the mechanism of cognitive improvement and deterioration through assessment of dementia-related biomarkers, omics analysis and brain image analysis. Moreover, along with the creation of evidence for prevention of dementia, collaborative research with partnering companies is expected to allow creation of new services and manuals for proposed ideal services. The J-MINT trial raises some concern that high-intensity intervention programs may result in an insufficient level of adherence, which lead to minimal beneficial effect. To address this issue, group-based physical exercise and nutritional counseling have been designed, in light of encouraging results from the FINGER and MAPT trials, to include face-to-face contacts, which may increase adherence (62). Moreover, there is still lack of consensus about the level of sustained adherence deemed acceptable or sufficient for prevention of cognitive decline. Given that all participants are to be monitored for adherence to its intervention programs during the intervention period, the J-MINT may provide insight into the level of sustained adherence deemed acceptable or sufficient for each intervention program among older adults at higher risk of dementia.
Finally, the J-MINT Prime trial incorporating two RCTs, Japan-multimodal Intervention Trial for Prevention of Dementia in Kanagawa (UMIN000041887) and Japan-multimodal Intervention Trial for Prevention of Dementia PRIME Tamba Study (UMIN000041938), was initiated in Japan to clarify the effectiveness of multi-domain intervention in different settings and populations in Japan. Both the J-MINT trial and the J-MINT Prime trial are intended to evaluate cognitive and other outcomes measures, with their combined data-analyses being planned. These results are expected to contribute to the development of a mechanism through which a sustainable dementia prevention service may be made widely available to those in need.
References
Prince M, Wimo A, Guerchet M, Ali GC, Wu YT, Prina M. World Alzheimer Report 2015 -The Global Impact of Dementia: Alzheimer’s Disease International; 2015
Matthews FE, Arthur A, Barnes LE, Bond J, Jagger C, Robinson L, Brayne C. A two-decade comparison of prevalence of dementia in individuals aged 65 years and older from three geographical areas of England: results of the Cognitive Function and Ageing Study I and II. Lancet 2013;382, 1405–1412.
Satizabal CL, Beiser AS, Chouraki V, Chene G, Dufouil C, Seshadri S. Incidence of Dementia over Three Decades in the Framingham Heart Study. N Engl J Med 2016;374, 523–532.
Ding M, Qiu C, Rizzuto D, Grande G, Fratiglioni L. Tracing temporal trends in dementia incidence over 25 years in central Stockholm, Sweden. Alzheimers Dement 2020;16, 770–778.
Ohara T, Hata J, Yoshida D, Mukai N, Nagata M, Iwaki T, Kitazono T, Kanba S, Kiyohara Y, Ninomiya T. Trends in dementia prevalence, incidence, and survival rate in a Japanese community. Neurology 2017;88, 1925–1932.
Cummings J, Lee G, Ritter A, Sabbagh M, Zhong K. Alzheimer’s disease drug development pipeline: 2019. Alzheimers Dement (N Y) 2019;5, 272–293.
Ngandu T, Lehtisalo J, Solomon A, Levalahti E, Ahtiluoto S, Antikainen R, Backman L, Hanninen T, Jula A, Laatikainen T, Lindstrom J, Mangialasche F, Paajanen T, Pajala S, Peltonen M, Rauramaa R, Stigsdotter-Neely A, Strandberg T, Tuomilehto J, Soininen H, Kivipelto M. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet 2015;385, 2255–2263.
Moll van Charante EP, Richard E, Eurelings LS, van Dalen JW, Ligthart SA, van Bussel EF, Hoevenaar-Blom MP, Vermeulen M, van Gool WA. Effectiveness of a 6-year multidomain vascular care intervention to prevent dementia (preDIVA): a cluster-randomised controlled trial. Lancet 2016;388, 797–805.
Andrieu S, Guyonnet S, Coley N, Cantet C, Bonnefoy M, Bordes S, Bories L, Cufi MN, Dantoine T, Dartigues JF, Desclaux F, Gabelle A, Gasnier Y, Pesce A, Sudres K, Touchon J, Robert P, Rouaud O, Legrand P, Payoux P, Caubere JP, Weiner M, Carrie I, Ousset PJ, Vellas B. Effect of long-term omega 3 polyunsaturated fatty acid supplementation with or without multidomain intervention on cognitive function in elderly adults with memory complaints (MAPT): a randomised, placebo-controlled trial. Lancet Neurol 2017;16, 377–389.
Andrieu S, Coley N, Lovestone S, Aisen PS, Vellas B. Prevention of sporadic Alzheimer’s disease: lessons learned from clinical trials and future directions. Lancet Neurol 2015;14, 926–944.
Rosenberg A, Mangialasche F, Ngandu T, Solomon A, Kivipelto M. Multidomain Interventions to Prevent Cognitive Impairment, Alzheimer’s Disease, and Dementia: From FINGER to World-Wide FINGERS. J Prev Alzheimers Dis 2020;7, 29–36.
Kivipelto M, Mangialasche F, Snyder HM, Allegri R, Andrieu S, Arai H, Baker L, Belleville S, Brodaty H, Brucki SM, Calandri I, Caramelli P, Chen C, Chertkow H, Chew E, Choi SH, Chowdhary N, Crivelli L, Torre R, Du Y, Dua T, Espeland M, Feldman HH, Hartmanis M, Hartmann T, Heffernan M, Henry CJ, Hong CH, Håkansson K, Iwatsubo T, Jeong JH, Jimenez-Maggiora G, Koo EH, Launer LJ, Lehtisalo J, Lopera F, Martínez-Lage P, Martins R, Middleton L, Molinuevo JL, Montero-Odasso M, Moon SY, Morales-Pérez K, Nitrini R, Nygaard HB, Park YK, Peltonen M, Qiu C, Quiroz YT, Raman R, Rao N, Ravindranath V, Rosenberg A, Sakurai T, Salinas RM, Scheltens P, Sevlever G, Soininen H, Sosa AL, Suemoto CK, Tainta-Cuezva M, Velilla L, Wang Y, Whitmer R, Xu X, Bain LJ, Solomon A, Ngandu T, Carrillo MC. World-Wide FINGERS Network: A global approach to risk reduction and prevention of dementia. Alzheimers Dement 2020;16, 1078–1094.
Makizako H, Shimada H, Park H, Doi T, Yoshida D, Uemura K, Tsutsumimoto K, Suzuki T. Evaluation of multidimensional neurocognitive function using a tablet personal computer: test-retest reliability and validity in community-dwelling older adults. Geriatr Gerontol Int 2013;13, 860–866.
Shimada H, Makizako H, Park H, Doi T, Lee S. Validity of the National Center for Geriatrics and Gerontology-Functional Assessment Tool and Mini-Mental State Examination for detecting the incidence of dementia in older Japanese adults. Geriatr Gerontol Int 2017;17, 2383–2388.
Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12, 189–198.
Wechsler D. Wechsler Memory Scale-Revised. San Antonio, TX: Psychological Corporation, 1981.
Grober E, Buschke H. Genuine memory deficits in dementia. Developmental Neuropsychology 1987;3, 13–36.
Wechsler D. Manual for the Wechsler Adult Intelligence Scale, Psychological Corp., Oxford, England, 1955.
Lezak MD, Lezak MD. Neuropsychological assessment, Oxford University Press, Oxford; New York, 2004.
McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr., Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 2011;7, 263–269.
Mahoney FI, Barthel DW. Functional evaluation: The Barthel Index. Md State Med J 1965;14, 61–65.
Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist 1969;9, 179–186.
Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56, M146–156.
Makizako H, Shimada H, Tsutsumimoto K, Lee S, Doi T, Nakakubo S, Hotta R, Suzuki T. Social Frailty in Community-Dwelling Older Adults as a Risk Factor for Disability. J Am Med Dir Assoc 2015;56, M146–156.
Tanaka T, Hirano H, Ohara Y, Nishimoto M, Iijima K. Oral Frailty Index-8 in the risk assessment of new-onset oral frailty and functional disability among community-dwelling older adults. Arch Gerontol Geriatr 2021;94: 104340.
Kimura Y, Wada T, Ishine M, Ishimoto Y, Kasahara Y, Konno A, Nakatsuka M, Sakamoto R, Okumiya K, Fujisawa M, Otsuka K, Matsubayashi K. Food diversity is closely associated with activities of daily living, depression, and quality of life in community-dwelling elderly people. J Am Geriatr Soc 2009;57, 922–924.
Rubenstein LZ, Harker JO, Salva A, Guigoz Y, Vellas B. Screening for undernutrition in geriatric practice: developing the short-form mininutritional assessment (MNA-SF). J Gerontol A Biol Sci Med Sci 2001;56, M366–372.
Wilson MM, Thomas DR, Rubenstein LZ, Chibnall JT, Anderson S, Baxi A, Diebold MR, Morley JE. Appetite assessment: simple appetite questionnaire predicts weight loss in community-dwelling adults and nursing home residents. Am J Clin Nutr 2005;82, 1074–1081.
Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO (1982) Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res 1982;17, 37–49.
Kikuchi R, Kozaki K, Iwata A, Hasegawa H, Toba K. Evaluation of risk of falls in patients at a memory impairment outpatient clinic. Geriatr Gerontol Int 2009;9, 298–303.
Lubben J, Blozik E, Gillmann G, Iliffe S, von Renteln Kruse W, Beck JC, Stuck AE. Performance of an abbreviated version of the Lubben Social Network Scale among three European community-dwelling older adult populations. Gerontologist. 2006;46, 503–513.
Kunz S. Psychometric properties of the EQ-5D in a study of people with mild to moderate dementia. Qual Life Res 2010;19, 425–434.
Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 1989;28, 193–213.
Kanamori S, Kai Y, Aida J, Kondo K, Kawachi I, Hirai H, Shirai K, Ishikawa Y, Suzuki K. Social participation and the prevention of functional disability in older Japanese: the JAGES cohort study. PLoS One 2014;9, e99638.
Ventry IM, Weinstein BE. TThe hearing handicap inventory for the elderly: a new tool. Ear Hear 1982;3, 128–134.
Chen LK, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K, Jang HC, Kang L, Kim M, Kim S, Kojima T, Kuzuya M, Lee JSW, Lee SY, Lee WJ, Lee Y, Liang CK, Lim JY, Lim WS, Peng LN, Sugimoto K, Tanaka T, Won CW, Yamada M, Zhang T, Akishita M, Arai H. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J Am Med Dir Assoc 2019;21, 300–307.
Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, Scherr PA, Wallace RB. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol 1994;49, M85–94.
Watanabe T, Owashi K, Kanauchi Y, Mura N, Takahara M, Ogino T. The short-term reliability of grip strength measurement and the effects of posture and grip span. J Hand Surg Am 2005;30, 603–609.
Whitney SL, Wrisley DM, Marchetti GF, Gee MA, Redfern MS, Furman JM. Clinical measurement of sit-to-stand performance in people with balance disorders: validity of data for the Five-Times-Sit-to-Stand Test. Phys Ther 2005;85, 1034–1045.
Mangione CM, Lee PP, Gutierrez PR, Spritzer K, Berry S, Hays RD; National Eye Institute Visual Function Questionnaire Field Test Investigators. Development of the 25-item National Eye Institute Visual Function Questionnaire. Arch Ophthalmol 2001;119, 1050–1058.
Ichii S, Nakamura T, Kawarabayashi T, Takatama M, Ohgami T, Ihara K, Shoji M. CogEvo, a cognitive function balancer, is a sensitive and easy psychiatric test battery for age-related cognitive decline. Geriatr Gerontol Int 2020;20, 248–255.
Fujiwara Y, Suzuki H, Yasunaga M, Sugiyama M, Ijuin M, Sakuma N, Inagaki H, Iwasa H, Ura C, Yatomi N, Ishii K, Tokumaru AM, Homma A, Nasreddine Z, Shinkai S. Brief screening tool for mild cognitive impairment in older Japanese: validation of the Japanese version of the Montreal Cognitive Assessment. Geriatr Gerontol Int 2010;10, 225–232.
Nakamura A, Kaneko N, Villemagne VL, Kato T, Doecke J, Doré V, Fowler C, Li QX, Martins R, Rowe C, Tomita T, Matsuzaki K, Ishii K, Ishii K, Arahata Y, Iwamoto S, Ito K, Tanaka K, Masters CL, Yanagisawa K. High performance plasma amyloid-β biomarkers for Alzheimer’s disease. Nature 2018;554, 249–254.
Tatebe H, Kasai T, Ohmichi T, Kishi Y, Kakeya T, Waragai M, Kondo M, Allsop D, Tokuda T. Quantification of plasma phosphorylated tau to use as a biomarker for brain Alzheimer pathology: pilot case-control studies including patients with Alzheimer’s disease and down syndrome. Mol Neurodegener 2017;12, 63.
Shinomoto M, Kasai T, Tatebe H, Kondo M, Ohmichi T, Morimoto M, Chiyonobu T, Terada N, Allsop D, Yokota I, Mizuno T, Tokuda T. Plasma neurofilament light chain: A potential prognostic biomarker of dementia in adult Down syndrome patients. PLoS One 2019;14, e0211575.
Committee Report: Glycemic targets for elderly patients with diabetes: Japan Diabetes Society (JDS)/Japan Geriatrics Society (JGS) Joint Committee on Improving Care for Elderly Patients with Diabetes. J Diabetes Investig 2017;8, 126–128.
Umemura S, Arima H, Arima S, Asayama K, Dohi Y, Hirooka Y, Horio T, Hoshide S, Ikeda S, Ishimitsu T, Ito M, Ito S, Iwashima Y, Kai H, Kamide K, Kanno Y, Kashihara N, Kawano Y, Kikuchi T, Kitamura K, Kitazono T, Kohara K, Kudo M, Kumagai H, Matsumura K, Matsuura H, Miura K, Mukoyama M, Nakamura S, Ohkubo T, Ohya Y, Okura T, Rakugi H, Saitoh S, Shibata H, Shimosawa T, Suzuki H, Takahashi S, Tamura K, Tomiyama H, Tsuchihashi T, Ueda S, Uehara Y, Urata H, Hirawa N. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2019). Hypertens Res 2019;42, 1235–1481.
Kinoshita M, Yokote K, Arai H, Iida M, Ishigaki Y, Ishibashi S, Umemoto S, Egusa G, Ohmura H, Okamura T, Kihara S, Koba S, Saito I, Shoji T, Daida H, Tsukamoto K, Deguchi J, Dohi S, Dobashi K, Hamaguchi H, Hara M, Hiro T, Biro S, Fujioka Y, Maruyama C, Miyamoto Y, Murakami Y, Yokode M, Yoshida H, Rakugi H, Wakatsuki A, Yamashita S; Committee for Epidemiology and Clinical Management of Atherosclerosis (2018) Japan Atherosclerosis Society (JAS) Guidelines for Prevention of Atherosclerotic Cardiovascular Diseases. J Atheroscler Thromb 2017;25, 846–984.
Shimada H, Makizako H, Doi T, Park H, Tsutsumimoto K, Verghese J, Suzuki T. Effects of Combined Physical and Cognitive Exercises on Cognition and Mobility in Patients With Mild Cognitive Impairment: A Randomized Clinical Trial. J Am Med Dir Assoc 2018;19, 584–591.
Bull FC, Maslin TS, Armstrong T. Global physical activity questionnaire (GPAQ): nine country reliability and validity study. J Phys Act Health. 2009;6, 790–804.
Sechrist KR, Walker SN, Pender NJ. Development and psychometric evaluation of the exercise benefits/barriers scale. Res Nurs Health 1987;10, 357–365.
Attkisson CC, Zwick R. The client satisfaction questionnaire. Psychometric properties and correlations with service utilization and psychotherapy outcome. Eval Program Plann 1982;5, 233–237.
Ministry of Health, Labour and Welfare of Japan, 2020. The dietary reference intakes for Japanese, 2020. https://www.mhlw.go.jp/stf/newpage_08517.html, Last updated December 24, 2019, Accessed on November 29, 2020.
Yokoyama Y, Nishi M, Murayama H, Amano H, Taniguchi Y, Nofuji Y, Narita M, Matsuo E, Seino S, Kawano Y, Shinkai S. Association of Dietary Variety with Body Composition and Physical Function in Community-dwelling Elderly Japanese. J Nutr Health Aging 2016;20, 691–696.
Otsuka R, Nishita Y, Tange C, Tomida M, Kato Y, Nakamoto M, Imai T, Ando F, Shimokata H. Dietary diversity decreases the risk of cognitive decline among Japanese older adults. Geriatr Gerontol Int. 2017;17, 937–944.
Scarmeas N, Anastasiou CA, Yannakoulia M. Nutrition and prevention of cognitive impairment. Lancet Neurol 2018;17, 1006–1015.
Otsuka R, Tange C, Nishita Y, Kato Y, Imai T, Ando F, Shimokata H. Serum docosahexaenoic and eicosapentaenoic acid and risk of cognitive decline over 10 years among elderly Japanese. Eur J Clin Nutr 2014;68, 503–509.
Ozawa M, Ohara T, Ninomiya T, Hata J, Yoshida D, Mukai N, Nagata M, Uchida K, Shirota T, Kitazono T, Kiyohara Y. Milk and dairy consumption and risk of dementia in an elderly Japanese population: the Hisayama Study. J Am Geriatr Soc. 2014;62, 1224–1230.
Otsuka R, Kato Y, Nishita Y, Tange C, Nakamoto M, Tomida M, Imai T, Ando F, Shimokata H. Cereal Intake Increases and Dairy Products Decrease Risk of Cognitive Decline among Elderly Female Japanese. J Prev Alzheimers Dis. 2014;1, 160–167.
Tomata Y, Sugiyama K, Kaiho Y, Honkura K, Watanabe T, Zhang S, Sugawara Y, Tsuji I. Green Tea Consumption and the Risk of Incident Dementia in Elderly Japanese: The Ohsaki Cohort 2006 Study. Am J Geriatr Psychiatry 2016;24, 881–889.
Smith GE, Housen P, Yaffe K, Ruff R, Kennison RF, Mahncke HW, Zelinski EM. A cognitive training program based on principles of brain plasticity: results from the Improvement in Memory with Plasticity-based Adaptive Cognitive Training (IMPACT) study. J Am Geriatr Soc 2009;57, 594–603.
Coley N, Ngandu T, Lehtisalo J, Soininen H, Vellas B, Richard E, Kivipelto M, Andrieu S; HATICE, FINGER, and MAPT/DSA groups. Adherence to multidomain interventions for dementia prevention: Data from the FINGER and MAPT trials. Alzheimers Dement 2019;15, 729–741.
Acknowledgments
We thank all the patients for their participation in the study and all members of the J-MINT study group (see Appendix 2) and on-site study staff for their efforts in the conduct of assessments and interventions.
Funding
Funding: This work was financially supported by Japan Agency for Medical Research and Development (AMED) under Grant Number JP20de0107002. The sponsors had no role in the design and conduct of the study, in the collection, analysis, and interpretation of data, in the preparation of the manuscript or in the review or approval of the manuscript.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflicts of interest: The authors have no conflict of interest to declare.
Additional information
Trial registration: UMIN000038671; Registered on the University Hospital Medical Information Network Clinical Trials Registry (UMIN-CTR) 24 November 2019. (https://upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000044075).
How to cite this article: T. Sugimoto, T. Sakurai, H. Akatsu, et al. The Japan-Multimodal Intervention Trial for Prevention of Dementia (J-MINT): The Study Protocol for an 18-Month, Multicenter, Randomized, Controlled Trial. J Prev Alz Dis 2021;https://doi.org/10.14283/jpad.2021.29
Electronic Supplementary Material
Appendix 1
. SPIRIT 2013 Checklist: Recommended items to address in a clinical trial protocol and related documents
Appendix 2
. The members of the J-MINT study group.
Rights and permissions
About this article
Cite this article
Sugimoto, T., Sakurai, T., Akatsu, H. et al. The Japan-Multimodal Intervention Trial for Prevention of Dementia (J-MINT): The Study Protocol for an 18-Month, Multicenter, Randomized, Controlled Trial. J Prev Alzheimers Dis 8, 465–476 (2021). https://doi.org/10.14283/jpad.2021.29
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.14283/jpad.2021.29