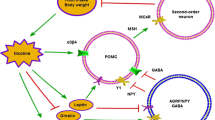
Abstract
Although numerous epidemiological studies have provided convincing evidence for the inverse association between tobacco smoking and body weight, the molecular mechanisms underlying this relationship are not well-understood. Nicotine, as a potent secretagogue, could be expected to influence the levels and expression of many classes of neurotransmitters, as well as of cell-membrane constituents linked to neurotransmission, including signal transducers and related effectors. A potentially major group of candidate molecules that could be involved in feeding-related actions of nicotine are the numerous neuropeptides and peptide hormones shown in the past two decades to regulate food intake and energy expenditure. These could include neuropeptide Y (NPY), orexins, leptins, and uncoupling proteins (UCPs). Some of these peptides were already shown to respond to nicotine treatment in terms of regulation of levels and of activity at the level of cell-membrane receptors. The primary objective of this review is to summarize our current understanding of the regulatory effects of nicotine on the food intake and energy expenditure as related to the expression levels of leptin, NPY, orexin, uncoupling proteins, and of NPY and orexin receptors.
Similar content being viewed by others
References
Benowitz N. L. (1997) The role of nicotine in smoking-related cardiovascular disease. Prev. Med. 26, 412–417.
Krupski W. C. (1991) The peripheral vascular consequences of smoking. Ann. Vasc. Surg. 5, 291–304.
Taylor B. V., Oudit G. Y., Kalman P. G., and Liu P. (1998) Clinical and pathophysiological effects of active and passive smoking on the cardiovascular system. Can. J. Cardiol. 14, 1129–1139.
Villablanca A. C., McDonald J. M., and Rutledge J. C. (2000) Smoking and cardiovascular disease. Clin. Chest. Med. 21, 159–172.
Birtwistle J. and Hall K. (1996) Does nicotine have beneficial effects in the treatment of certain diseases? Br. J. Nurs. 5, 1195–1202.
Lee P. N. (1994) Smoking and Alzheimer’s disease: a review of the epidemiological evidence. Neuroepidemiology 13, 131–144.
Lopez-Arrieta J. M., Rodriguez J. L., and Sanz F. (2000) Nicotine for Alzheimer’s disease. Cochrane Database Syst. Rev. 2, CD001749.
Balfour D. J. and Ridley D. L. (2000) The effects of nicotine on neural pathways implicated in depression: a factor in nicotine addiction? Pharmacol. Biochem. Behav. 66, 79–85.
Benowitz N. L. (1999) Nicotine addiction. Prim. Care 26, 611–631.
Batel P. (2000) Addiction and schizophrenia. Eur. Psychiatry 15, 115–122.
Covey L. S., Glassman A. H., and Stetner F. (1998) Cigarette smoking and major depression. J. Addict. Dis. 17, 35–46.
Dalack G. W. and Meador-Woodruff J. H. (1996) Smoking, smoking withdrawal and schizophrenia: case reports and a review of the literature. Schizophr. Res. 22, 133–141.
Lyon F. R. (1999) A review of the effects of nicotine on schizophrenia and antipsychotic medications. Psychiatr. Serv. 50, 1346–1350.
USDHHS (1988) The health consequences of smoking: nicotine addiction. A report of the Surgeon general. US Government Printing Office DHHS Publication No. (CDC):88-8406.
Pomerleau C. S. and Kurth C. L. (1996) Willingness of female smokers to tolerate postcessation weight gain. J. Subst. Abuse. 8, 371–378.
Ghosheh O., Dwoskin L. P., Li W. K., and Crooks P. A. (1999) Residence times and half-lives of nicotine metabolites in rat brain after acute peripheral administration of [2′-(14)C]nicotine. Drug. Metab. Dispos. 27, 1448–1455.
Anderson D. J. and Arneric S. P. (1994) Nicotinic receptor binding of [3H]cytisine, [3H]nicotine and [3H]methylcarbamylcholine in rat brain. Eur. J. Pharmacol. 253, 261–267.
Pool W. F. and Crooks P. A. (1985) Biotransformation of primary nicotine metabolites. I. In vivo metabolism of R-(+)-[14C-NCH3]N-methylnicotinium ion in the guinea pig. Drug. Metab. Dispos. 13, 578–581.
Siegel R. A., Andersson K., Fuxe K., Eneroth P., Lindbom L. O., and Agnati L. F. (1983) Rapid and discrete changes in hypothalamic catecholamine nerve terminal systems induced by audiogenic stress, and their modulation by nicotine-relationship to neuroendocrine function. Eur. J. Pharmacol. 91, 49–56.
Sakurai Y., Takano Y., Kohjimoto Y., Honda K., and Kamiya H. O. (1982) Enhancement of [3H]dopamine release and its [3H]metabolites in rat striatum by nicotinic drugs. Brain Res. 242, 99–106.
Li X., Rainnie D. G., McCarley R. W., and Greene R. W. (1998) Presynaptic nicotinic receptors facilitate monoaminergic transmission. J. Neurosci. 18, 1904–1912.
Sorenson E. M., Shiroyama T., and Kitai S. T. (1998) Postsynaptic nicotinic receptors on dopaminergic neurons in the substantia nigra pars compacta of the rat. Neuroscience 87, 659–673.
Fu Y., Matta S. G., Valentine J. D., and Sharp B. M. (1998) Desensitization and resensitization of norepinephrine release in the rat hippocampus with repeated nicotine administration. Neurosci. Lett. 241, 147–150.
Kirch D. G., Gerhardt G. A., Shelton R. C., Freedman R., and Wyatt R. J. (1987) Effect of chronic nicotine administration on monoamine and monoamine metabolite concentrations in rat brain. Clin. Neuropharmacol. 10, 376–383.
Reuben M., Louis M., and Clarke P. B. (1998) Persistent nicotinic blockade by chlorisondamine of noradrenergic neurons in rat brain and cultured PC12 cells. Br. J. Pharmacol. 125, 1218–1227.
Iversen L. L., Lee C. M., Gilbert R. F., Hunt S., and Emson P. C. (1980) Regulation of neuropeptide release. Proc. R. Soc. Lond. B. Biol. Sci. 210, 91–111.
Iversen L. L. (1984) The Ferrier Lecture, (1983). Amino acids and peptides: fast and slow chemical signals in the nervous system? Proc. R. Soc. Lond. B. Biol. Sci. 221, 245–260.
Marty M. A., Erwin V. G., Cornell K., and Zgombick J. M. (1985) Effects of nicotine on beta-endorphin, alpha MSH, and ACTH secretion by isolated perfused mouse brains and pituitary glands, in vitro. Pharmacol. Biochem. Behav. 22, 317–325.
Matta S. G., Foster C. A., and Sharp B. M. (1993) Nicotine stimulates the expression of cFos protein in the parvocellular paraventricular nucleus and brainstem catecholaminergic regions. Endocrinology 132, 2149–2156.
Matta S. G., Foster C. A., and Sharp B. M. (1993) Selective administration of nicotine into catecholaminergic regions of rat brainstem stimulates adrenocorticotropin secretion. Endocrinology 133, 2935–2942.
Zaninetti M., Blanchet C., Tribollet E., Bertrand D., and Raggenbass M. (2000) Magnocellular neurons of the rat supraoptic nucleus are endowed with functional nicotinic acetylcholine receptors. Neuroscience 95, 319–323.
Kasting N. W. (1988) Simultaneous and independent release of vasopressin and oxytocin in the rat. Can. J. Physiol. Pharmacol. 66, 22–26.
Maitra S. C., Chakraverty K., Shipstone A. C., and Kar K. (1983) Ultrastructural changes in the neural lobe of the rat pituitary following nicotine pretreatment. Exp. Clin. Endocrinol. 82, 376–379.
Iselin C. E., Martin J. L., Magistretti P. J., and Ferrero J. D. (1988) Stimulation by nicotine of enteric inhibitory nerves and release of vasoactive intestinal peptide in the taenia of the guinea-pig caecum. Eur. J. Pharmacol. 148, 179–186.
Lapchak P. A. and Beaudet A. (1990) Cholinergic regulation of vasoactive intestinal peptide content and release in rat frontal cortex and hippocampus. J. Neurochem. 55, 1340–1345.
Karlsson S. and Ahren B. (1998) Insulin and glucagon secretion by ganglionic nicotinic activation in adrenalectomized mice. Eur. J. Pharmacol. 342, 291–295.
Andersson K., Fuxe K., Eneroth P., and Agnati L. F. (1982) Involvement of cholinergic nicotine-like receptors as modulators of amine turnover in various types of hypothalamic dopamine and noradrenaline nerve terminal systems and of prolactin, LH, FSH and TSH secretion in the castrated male rat. Acta. Physiol. Scand. 116, 41–50.
Andersson K., Siegel R., Fuxe K., and Eneroth P. (1983) Intravenous injections of nicotine induce very rapid and discrete reductions of hypothalamic catecholamine levels associated with increases of ACTH, vasopressin and prolactin secretion. Acta. Physiol. Scand. 118, 35–40.
Fuxe K., Andersson K., Eneroth P., Harfstrand A., and Agnati L. F. (1989) Neuroendocrine actions of nicotine and of exposure to cigarette smoke: medical implications. Psychoneuroen-docrinology 14, 19–41.
Karlin A. and Akabas M. H. (1995) Toward a structural basis for the function of nicotinic acetylcholine receptors and their cousins. Neuron 15, 1231–1244.
Kao P. N. and Karlin A. (1986) Acetylcholine receptor binding site contains a disulfide cross-link between adjacent half-cystinyl residues. J. Biol. Chem. 261, 8085–8088.
Changeux J. P., Bertrand D., Corringer P. J., Dehaene S., Edelstein S., Lena C., et al. (1998) Brain nicotinic receptors: structure and regulation, role in learning and reinforcement. Brain Res. Brain. Res. Rev. 26, 198–216.
Alkondon M. and Albuquerque E. X. (1993) Diversity of nicotinic acetylcholine receptors in rat hippocampal neurons. I. Pharmacological and functional evidence for distinct structural subtypes. J. Pharmacol. Exp. Ther. 265, 1455–1473.
Zoli M., Lena C., Picciotto M. R., and Changeux J. P. (1998) Identification of four classes of brain nicotinic receptors using beta2 mutant mice. J. Neurosci. 18, 4461–4472.
Drisdel R. C. and Green W. N. (2000) Neuronal alpha-bungarotoxin receptors are alpha7 subunit homomers. J. Neurosci. 20, 133–139.
Broide R. S. and Leslie F. M. (1999) The alpha7 nicotinic acetylcholine receptor in neuronal plasticity. Mol. Neurobiol. 20, 1–16.
Galzi J. L. and Changeux J. P. (1995) Neuronal nicotinic receptors: molecular organization and regulations. Neuropharmacology 34, 563–582.
Vidal C. (1996) Nicotinic receptors in the brain. Molecular biology, function, and therapeutics. Mol. Chem. Neuropathol. 28, 3–11.
Green M. S. and Harari G. (1995) A prospective study of the effects of changes in smoking habits on blood count, serum lipids and lipoproteins, body weight and blood pressure in occupationally active men. The Israeli CORDIS Study [see comments]. J. Clin. Epidemiol. 48, 1159–1166.
Grinker J. A., Tucker K., Vokonas P. S., and Rush D. (1995) Body habitus changes among adult males from the normative aging study: relations to aging, smoking history and alcohol intake. Obes. Res. 3, 435–446.
Williamson D. F., Madans J., Anda R. F., Kleinman J. C., Giovino G. A., and Byers T. (1991) Smoking cessation and severity of weight gain in a national cohort [see comments]. N. Engl. J. Med. 324, 739–745.
Richmond R. L., Kehoe L., and Webster I. W. (1993) Weght change after smoking cessation in general practice. Med. J. Aust. 158, 821–822.
Caan B., Coates A., Schaefer C., Finkler L., Sternfeld B., and Corbett K. (1996) Women gain weight 1 year after smoking cessation while dietary intake temporarily increases. J. Am. Diet. Assoc. 96, 1150–1155.
Kawachi I., Troisi R. J., Rotnitzky A. G., Coakley E. H., and Colditz G. A. (1996) Can physical activity minimize weight gain in women after smoking cessation? Am. J. Public Health 86, 999–1004.
Swan G. E. and Carmelli D. (1995) Characteristics associated with excessive weight gain after smoking cessation in men. Am. J. Public Health 85, 73–77.
Grunberg N. E. (1991) Smoking cessation and weight gain [editorial; comment]. N. Engl. J. Med. 324, 768–769.
Li M. D., Kane J. K., Parker S. L., McAllen K., Matta S. G., and Sharp B. M. (2000) Nicotine administration enhances NPY expression in the rat hypothalamus. Brain Res. 867, 157–164.
Bowen D. J., Eury S. E., and Grunberg N. E. (1986) Nicotine’s effects on female rats’ body weight: caloric intake and physical activity. Pharmacol. Biochem. Behav. 25, 1131–1136.
Frankish H. M., Dryden S., Wang Q., Bing C., MacFarlane I. A., and Williams G. (1995) Nicotine administration reduces neuropeptide Y and neuropeptide Y mRNA concentrations in the rat hypothalamus: NPY may mediate nicotine’s effects on energy balance. Brain Res. 694, 139–146.
Grunberg N. E., Bowen D. J., and Winders S. E. (1986) Effects of nicotine on body weight and food consumption in female rats. Psychopharmacology 90, 101–105.
Hofstetter A., Schutz Y., Jequier E., and Wahren J. (1986) Increased 24-hour energy expenditure in cigarette smokers. N. Engl. J. Med. 314, 79–82.
Perkins K. A., Epstein L. H., Marks B. L., Stiller R. L., and Jacob R. G. (1989) The effect of nicotine on energy expenditure during light physical activity. N. Engl. J. Med. 320, 898–903.
Sztalryd C., Hamilton J., Horwitz B. A., Johnson P., and Kraemer F. B. (1996) Alterations of lipolysis and lipoprotein lipase in chronically nicotine-treated rats. Am. J. Physiol. 270, E215–223.
Zhang Y., Proenca R., Maffei M., Barone M., Leopold L., and Friedman J. M. (1994) Positional cloning of the mouse obese gene and its human homologue. Nature 372, 425–432.
Tatemoto K. (1982) Isolation and characterization of peptide YY (PYY), a candidate gut hormone that inhibits pancreatic exocrine secretion. Proc. Natl. Acad. Sci. USA 79, 2514–2518.
Sakurai T., Amemiya A., Ishii M., Matsuzaki I., Chemelli R. M., Tanaka H., et al. (1998) Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior [see comments]. Cell 92, 573–585.
de Lecea L., Kilduff T. S., Peyron C., Gao X., Foye P. E., Danielson P. E., et al. (1998) The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc. Natl. Acad. Sci. USA 95, 322–327.
Fan W., Boston B. A., Kesterson R. A., Hruby V. J., and Cone R. D. (1997) Role of melanocortinergic neurons in feeding and the agouti obesity syndrome [see comments]. Nature 385, 165–168.
Huszar D., Lynch C. A., Fairchild-Huntress V., Dunmore J. H., Fang Q., Berkemeier L. R., et al. (1997) Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell 88, 131–141.
Douglass J., McKinzie A. A., and Couceyro P. (1995) PCR differential display identifies a rat brain mRNA that is transcriptionally regulated by cocaine and amphetamine. J. Neurosci. 15, 2471–2481.
Kristensen P., Judge M. E., Thim L., Ribel U., Christjansen K. N., Wulff B. S., et al. (1998) Hypothalamic CART is a new anorectic peptide regulated by leptin. Nature 393, 72–76.
Barrachina M. D., Martinez V., Wang L., Wei J. Y., and Tache Y. (1997) Synergistic interaction between leptin and cholecystokinin to reduce short-term food intake in lean mice. Proc. Natl. Acad. Sci. USA 94, 10,455–10,460.
Morley J. E. (1987) Neuropeptide regulation of appetite and weight. Endocr. Rev. 8, 256–287.
Scarpace P. J., Matheny M., Pollock B. H., and Tumer N. (1997) Leptin increases uncoupling protein expression and energy expenditure. Am. J. Physiol. 273, E226-E230.
Scarpace P. J., Nicolson M., and Matheny M. (1998) UCP2, UCP3 and leptin gene expression: modulation by food restriction and leptin. J. Endocrinol. 159, 349–357.
Elmquist J. K., Maratos-Flier E., Saper C. B., and Flier J. S. (1998) Unraveling the central nervous system pathways underlying responses to leptin. Nat. Neurosci. 1, 445–450.
Flier J. and Maratos-Flier E. (2000) Energy homeostasis and body weight. Curr. Biol. 10, R215-R217.
Tartaglia L. A., Dembski M., Weng X., Deng N., Culpepper J., Devos R., et al. (1995) Identification and expression cloning of a leptin receptor, OB-R. Cell 83, 1263–1271.
Burguera B., Couce M. E., Long J., Lamsam J., Laakso K., Jensen M. D., et al. (2000) The long form of the leptin receptor (OB-Rb) is widely expressed in the human brain. Neuroendocrinology 71, 187–195.
Ahima R. S., Prabakaran D., Mantzoros C., Qu D., Lowell B., Martos-Flier E., and Flier J. S. (1996) Role of leptin in the neuroendocrine response to fasting. Nature 382, 250–252.
Schwartz M. W., Seeley R. J., Campfield L. A., Burn P., and Baskin D. G. (1996) Identification of targets of leptin action in rat hypothalamus. J. Clin. Invest. 98, 1101–1106.
Stephens T. W., Basinski M., Bristow P. K., Bue-Valleskey J. M., Burgett S. G., Craft L., et al. (1995) The role of neuropeptide Y in the antiobesity action of the obese gene product. Nature 377, 530–532.
Fei H., Okano H. J., Li C., Lee G. H., Zhao C., Darnell R., and Friedman J. M. (1997) Anatomic localization of alternatively spliced leptin receptors (Ob-R) in mouse brain and other tissues. Proc. Natl. Acad. Sci. USA 94, 7001–7005.
Halaas J. L., Gajiwala K. S., Maffei M., Cohen S. L., Chait B. T., Rabinowitz D., et al. (1995) Weight-reducing effects of the plasma protein encoded by the obese gene [see comments]. Science 269, 543–546.
Levin N., Nelson C., Gurney A., Vandlen R., and de Sauvage F. (1996) Decreased food intake does not completely account for adiposity reduction after ob protein infusion. Proc. Natl. Acad. Sci. USA 93, 1726–1730.
Pelleymounter M. A., Cullen M. J., Baker M. B., Hecht R., Winters D., Boone T., and Collins F. (1995) Effects of the obese gene product on body weight regulation in ob/ob mice [see comments]. Science 269, 540–543.
Collins S., Kuhn C. M., Petro A. E., Swick A. G., Chrunyk B. A., and Surwit R. S. (1996) Role of leptin in fat regulation [letter]. Nature 380, 677.
Hodge A. M., Westerman R. A., de Courten M. P., Collier G. R., Zimmet P. Z., and Alberti K. G. (1997) Is leptin sensitivity the link between smoking cessation and weight gain? Int. J. Obes. Relat. Metab. Disord. 21, 50–53.
Wei M., Stern M. P., and Haffner S. M. (1997) Serum leptin levels in Mexican Americans and non-Hispanic whites: association with body mass index and cigarette smoking [see coments]. Ann. Epidemiol. 7, 81–86.
Mantzoros C. S., Varvarigou A., Kaklamani V. G., Beratis N. G., and Flier J. S. (1997) Effect of birth weight and maternal smoking on cord blood leptin concentrations of full-term and preterm newborns. J. Clin. Endocrinol. Metab. 82, 2856–2861.
Eliasson B. and Smith U. (1999) Leptin levels in smokers and long-term users of nicotine gum. Eur. J. Clin. Invest. 29, 145–152.
Oeser A., Goffaux J., Snead W., and Carlson M. G. (1999) Plasma leptin concentrations and lipid profiles during nicotine abstinence. Am. J. Med. Sci. 318, 152–157.
Li M. D., Kane J. K., Matta S. G., Huang W., Fu Y., McAllen K., and Sharp B. M. (2000) Effects of nicotine on the expression of plasma leptin and its receptor mRNA in rat. 30th Annual Meeting of the Society for Neuroscience. Abstract.
Grassi G., Seravalle G., Calhoun D. A., Bolla G. B., Giannattasio C., Marabini M., et al. (1994) Mechanisms responsible for sympathetic activation by cigarette smoking in humans. Circulation 90, 248–253.
Neese R. A., Benowitz N. L., Hoh R., Faix D., LaBua A., Pun K., and Hellerstein M. K. (1994) Metabolic interactions between surplus dietary energy intake and cigarette smoking or its cessation. Am. J. Physiol. 267, E1023–1034.
Skulachev V. P. (1991) Fatty acid circuit as a physiological mechanism of uncoupling of oxidative phosphorylation. FEBS Lett. 294, 158–162.
Garlid K. D., Orosz D. E., Modriansky M., Vassanelli S., and Jezek P. (1996) On the mechanism of fatty acid-induced proton transport by mitochondrial uncoupling protein. J. Biol. Chem. 271, 2615–2620.
Jezek P., Hanus J., Semrad C., and Garlid K. D. (1996) Photoactivated azido fatty acid irreversibly inhibits anion and proton transport through the mitochondrial uncoupling protein. J. Biol. Chem. 271, 6199–6205.
Bouillaud F., Ricquier D., Thibault J., and Weissenbach J. (1985) Molecular approach to thermogenesis in brown adipose tissue: cDNA cloning of the mitochondrial uncoupling protein. Proc. Natl. Acad. Sci. USA 82, 445–448.
Jacobsson A., Stadler U., Glotzer M. A., and Kozak L. P. (1985) Mitochondrial uncoupling protein from mouse brown fat. Molecular cloning, genetic mapping, and mRNA expression. J. Biol. Chem. 260, 16,250–16,254.
Silva J. E. and Rabelo R. (1997) Regulation of the uncoupling protein gene expression. Eur. J. Endocrinol. 136, 251–264.
Boss O., Samec S., Paoloni-Giacobino A., Rossier C., Dulloo A., Seydoux J., et al. (1997) Uncoupling protein-3: a new member of the mitochondrial carrier family with tissue-specific expression. FEBS Lett. 408, 39–42.
Fleury C., Neverova M., Collins S., Raimbault S., Champigny O., Levi-Meyrueis C., et al. (1997) Uncoupling protein-2: a novel gene linked to obesity and hyperinsulinemia [see comments]. Nat. Genet. 15, 269–272.
Vidal-Puig A., Solanes G., Grujic D., Flier J. S., and Lowell B. B. (1997) UCP3: an uncoupling protein homologue expressed preferentially and abundantly in skeletal muscle and brown adipose tissue. Biochem. Biophys. Res. Comm. 235, 79–82.
Lee G. H., Proenca R., Montez J. M., Carroll K. M., Darvishzadeh J. G., Lee J. I., and Friedman J. M. (1996) Abnormal splicing of the leptin receptor in diabetic mice. Nature 379, 632–635.
Lupien J. R. and Bray G. A. (1988) Nicotine increases thermogenesis in brown adipose tissue in rats. Pharmacol. Biochem. Behav. 29, 33–37.
Yoshida T., Sakane N., Umekawa T., Kogure A., Kondo M., Kumamoto K., et al. (1999) Nicotine induces uncoupling protein 1 in white adipose tissue of obese mice. Int. J. Obes. Relat. Metab. Disord. 23, 570–575.
Allen Y. S., Adrian T. E., Allen J. M., Tatemoto K., Crow T. J., Bloom S. R., and Polak J. M. (1983) Neuropeptide Y distribution in the rat brain. Science 221, 877–879.
Wahlestedt C. and Reis D. J. (1993) Neuropeptide Y-related peptides and their receptor — are the receptors potential therapeutic drug targets? Annu. Rev. Pharmacol. Toxicol. 32, 309–352.
Gehlert D. R. (1998) Multiple receptors for the pancreatic polypeptide (PP-fold) family: physiological implications. Proc. Soc. Exp. Biol. Med. 218, 7–22.
Higuchi H., Yang H. Y., and Costa E. (1988) Age-related bidirectional changes in neuropeptide Y peptides in rat adrenal glands, brain, and blood. J. Neurochem. 50, 1879–1886.
Kastin A. J. and Akerstrom V. (1999) Nonsaturable entry of neuropeptide Y into brain. Am. J. Physiol. 276, E479-E482.
Burnstock G. (1987) Mechanisms of interaction of peptide and nonpeptide vascular neurotransmitter systems. J. Cardiovasc. Pharmacol. 10, S74-S81.
Josselyn S. A. and Beninger R. J. (1993) Neuropeptide Y: intraaccumbens injections produce a place preference that is blocked by cis-flupenthixol. Pharmacol. Biochem. Behav. 46, 543–552.
Boehm S. and Huck S. (1997) Receptors controlling transmitter release from sympathetic neurons in vitro. Prog. Neurobiol. 51, 225–242.
Harfstrand A., Fredholm B., and Fuxe K. (1987) Inhibitory effects of neuropeptide Y on cyclic AMP accumulation in slices of the nucleus tractus solitarius region of the rat. Neurosci. Lett. 76, 185–190.
Westlind-Danielsson A., Unden A., Abens J., Andell S., and Bartfai T. (1987) Neuropeptide Y receptors and the inhibition of adenylate cyclase in the human frontal and temporal cortex. Neurosci. Lett. 74, 237–242.
Parker S. L., Parker M. S., and Crowley W. R. (1998) Characterization of Y1, Y2 and Y5 subtypes of neuropeptide Y (NPY) receptor in rabbit kidney. Regul. Pept. 75/76, 127–143.
Parker S. L., Parker M. S., and Crowley W. R. (1999) Characterization of rabbit kidney and brain pancreatic polypeptide- binding neuropeptide Y receptors: differences with Y1 and Y2 sites in sensitivity to amiloride derivatives affecting sodium transport. Regul. Pept. 82, 91–102.
Brady L. S., Smith M. A., Gold P. W., and Herkenham M. (1990) Altered expression of hypothalamic neuropeptide mRNAs in food-restricted and food-deprived rats. Neuroendocrinology 52, 441–447.
Hiremagalur B. and Sabban E. L. (1995) Nicotine elicits changes in expression of adrenal catecholamine biosynthetic enzymes, neuropeptide Y and immediate early genes by injection but not continuous administration. Brain Res. Mol. Brain Res. 32, 109–115.
Kalra S. P., Dube M. G., Fournier A., and Kalra P. S. (1991) Structure-function analysis of stimulation of food intake by neuropeptide Y: effects of receptor agonists. Physiol. Behav. 50, 5–9.
Stanley B. G., Magdalin W., Seirafi A., Nguyen M. M., and Leibowitz S. F. (1992) Evidence for neuropeptide Y mediation of eating produced by food deprivation and for a variant of the Y1 receptor mediating this peptide’s effect. Peptides 13, 581–587.
Gerald C., Walker M. W., Criscione L., Gustafson E. L., Batzl-Hartmann C., Smith K. E., et al. (1996) A receptor subtype involved in neuropeptide-Y-induced food intake [see comments]. Nature 382, 168–171.
Criscione L., Rigollier P., Batzl-Hartmann C., Rueger H., Stricker-Krongrad A., Wyss P., et al. (1998) Food intake in free-feeding and energydeprived lean rats is mediated by the neuropeptide Y5 receptor. J. Clin. Invest. 102, 2136–2145.
Yokosuka M., Kalra P. S., and Kalra S. P. (1999) Inhibition of neuropeptide Y (NPY)-induced feeding and c-Fos response in magnocellular paraventricular nucleus by a NPY receptor antagonist: a site of NPY action. Endocrinology 140, 4494–4500.
Kanatani A., Mashiko S., Murai N., Sugimoto N., Ito J., Fukuroda T., et al. (2000) Role of the Y1 receptor in the regulation of neuropeptide Y-mediated feeding: comparison of wild-type, Y1 receptor-deficient, and Y5 receptor-deficient mice. Endocrinology 141, 1011–1016.
Wyss P., Stricker-Krongrad A., Brunner L., Miller J., Crossthwaite A., Whitebread S., and Criscione L. (1998) The pharmacology of neuropeptide Y (NPY) receptor-mediated feeding in rats characterizes better Y5 than Y1, but not Y2 or Y4 subtypes. Regul. Pept. 75–76, 363–371.
Statnick M. A., Schober D. A., Gackenheimer S., Johnson D., Beavers L., Mayne N. G., et al. (1998) Characterization of the neuropeptide Y5 receptor in the human hypothalamus: a lack of correlation between Y5 mRNA levels and binding sites. Brain Res. 810, 16–26.
Adie E. J. and Milligan G. (1994) Agonist regulation of cellular Gs alpha-subunit levels in neuroblastoma × glioma hybrid NG108-15 cells transfected to express different levels of the human beta 2 adrenoceptor. Biochem. J. 300, 709–715.
Jewell-Motz E. A., Donnelly E. T., Eason M. G., and Liggett S. B. (1998) Agonist-mediated downregulation of G alpha i via the alpha 2-adrenergic receptor is targeted by receptor-Gi interaction and is independent of receptor signaling and regulation. Biochemistry 37, 15,720–15,725.
Mixon M. B., Lee E., Coleman D. E., Berghuis A. M., Gilman A. G., and Sprang S. R. (1995) Tertiary and quaternary structural changes in Gi alpha 1 induced by GTP hydrolysis. Science 270, 954–960.
Calderwood S. K., Stevenson M. A., and Price B. D. (1993) Activation of phospholipase C by heat shock requires GTP analogs and is resistant to pertussis toxin. J. Cell Physiol. 156, 153–159.
Chen C. T., Dun S. L., Kwok E. H., Dun N. J., and Chang J. K. (1999) Orexin A-like immunoreactivity in the rat brain. Neurosci. Lett. 260, 161–164.
Cutler D. J., Morris R., Sheridhar V., Wattam T. A., Holmes S., Patel S., et al. (1999) Differential distribution of orexin-A and orexin-B immunoreactivity in the rat brain and spinal cord. Peptides 20, 1455–1470.
Date Y., Mondal M. S., Matsukura S., and Nakazato M. (2000) Distribution of orexin-A and orexin-B (hypocretins) in the rat spinal cord. Neurosci. Lett. 288, 87–90.
Date Y., Mondal M. S., Matsukura S., Ueta Y., Yamashita H., Kaiya H., et al. (2000) Distribution of orexin/hypocretin in the rat median eminence and pituitary. Brain Res. Mol. Brain Res. 76, 1–6.
Mondal M. S., Nakazato M., Date Y., Murakami N., Yanagisawa M., and Matsukura S. (1999) Widespread distribution of orexin in rat brain and its regulation upon fasting. Biochem. Biophys. Res. Comm. 256, 495–499.
Nambu T., Sakurai T., Mizukami K., Hosoya Y., Yanagisawa M., and Goto K. (1999) Distribution of orexin neurons in the adult rat brain. Brain Res. 827, 243–260.
Peyron C., Tighe D. K., van den Pol A. N., de Lecea L., Heller H. C., Sutcliffe J. G., and Kilduff T. S. (1998) Neurons containing hypocretin (orexin) project to multiple neuronal systems. J. Neurosci. 18, 9996–10,015.
Taheri S., Mahmoodi M., Opacka-Juffry J., Ghatei M. A., and Bloom S. R. (1999) Distribution and quantification of immunoreactive orexin A in rat tissues. FEBS Lett. 457, 157–161.
Taheri S., Sunter D., Dakin C., Moyes S., Seal L., Gardiner J., et al. (2000) Diurnal variation in orexin A immunoreactivity and preproorexin mRNA in the rat central nervous system. Neurosci. Lett. 279, 109–112.
Dube M. G., Kalra S. P., and Kalra P. S. (1999) Food intake elicited by central administration of orexins/hypocretins: identification of hypothalamic sites of action. Brain Res. 842, 473–477.
Lubkin M. and Stricker-Krongrad A. (1998) Independent feeding and metabolic actions of orexins in mice. Biochem. Biophys. Res. Comm. 253, 241–245.
Chemelli R. M., Willie J. T., Sinton C. M., Elmquist J. K., Scammell T., Lee C., et al. (1999) Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation [see comments]. Cell 98, 437–451.
Lin L., Faraco J., Li R., Kadotani H., Rogers W., Lin X., et al. (1999) The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene [see comments]. Cell 98, 365–376.
Kilduff T. S. and Peyron C. (2000) The hypocretin/orexin ligand-receptor system: implications for sleep and sleep disorders. Trends Neurosci. 23, 359–365.
Taheri S., Ward H., Ghatei M., and Bloom S. (2000) Role of orexins in sleep and arousal mechanisms [letter]. Lancet 355, 847.
van den Pol A. N. (1999) Hypothalamic hypocretin (orexin): robust innervation of the spinal cord. J. Neurosci. 19, 3171–3182.
Chen C. T., Hwang L. L., Chang J. K., and Dun N. J. (2000) Pressor effects of orexins injected intracisternally and to rostral ventrolateral medulla of anesthetized rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 278, R692-R697.
Shirasaka T., Nakazato M., Matsukura S., Takasaki M., and Kannan H. (1999) Sympathetic and cardiovascular actions of orexins in conscious rats. Am. J. Physiol. 277, R1780-R1785.
Ida T., Nakhara K., Murakami T., Hanada R., Nakazato M., and Murakami N. (2000) Possible involvement of orexin in the stress reaction in rats. Biochem. Biophys. Res. Comm. 270, 318–323.
Kuru M., Ueta Y., Serino R., Nakazato M., Yamamoto Y., Shibuya I., and Yamashita H. (2000) Centrally administered orexin/hypocretin activates HPA axis in rats. Neuroreport 11, 1977–1980.
Sakurai T. (1999) Orexins and orexin receptors: implication in feeding behavior. Regul. Pept. 85, 25–30.
Kane J. K., Parker S. L., Matta S. G., Fu Y., Sharp B. M., and Li M. D. (2000) Nicotine upregulates expression of orexin and its receptors in rat brain. Endocrinology 141, 3623–3629.
Yu Z. J. and Wecker L. (1994) Chronic nicotine administration differentially affects neurotransmitter release from rat striatal slices. J. Neurochem. 63, 186–194.
Takahashi H., Takada Y., Nagai N., Urano T., and Takada A. (1998) Nicotine increases stressinduced serotonin release by stimulating nicotinic acetylcholine receptor in rat striatum. Synapse 28, 212–219.
Kane J. K., Tanaka H., Parker S. L., Yanagisawa M., and Li M. D. (2000) Sensitivity of orexin-A binding to phospholipase C inhibitors, neuropeptide Y, and secretin. Biochem. Biophys. Res. Comm. 272, 959–965.
Clayton R. N., Solano A. R., Garcia-Vela A., Dufau M. L., and Catt K. J. (1980) Regulation of pituitary receptors for gonadotropin-releasing hormone during the rat estrous cycle. Endocrinology 107, 699–706.
Faussner A., Proud D., Towns M., and Bathon J. M. (1998) Influence of the cytosolic carboxyl termini of human B1 and B2 kinin receptors on receptor sequestration, ligand internalization, and signal transduction. J. Biol. Chem. 273, 2617–2623.
Hunyady L., Bor M., Balla T., and Catt K. J. (1994) Identification of a cytoplasmic Ser-Thr-Leu motif that determines agonist-induced internalization of the AT1 angiotensin receptor. J. Biol. Chem. 269, 31378–31382.
Kastin A. J. and Akerstrom V. (1999) Orexin A but not orexin B rapidly enters brain from blood by simple diffusion. J. Pharmacol. Exp. Ther. 289, 219–223.
Hoebel B. G., Hernandez L., Schwartz D. H., Mark G. P., and Hunter G. A. (1989) Microdialysis studies of brain norepinephrine, serotonin, and dopamine release during ingestive behavior. Theoretical and clinical implications. Ann. NY Acad. Sci. 575, 171–191.
Leibowitz S. F. (1986) Brain monoamines and peptides: role in the control of eating behavior. Fed. Proc. 45, 1396–1403.
Leibowitz S. F. and Shor-Posner G. (1986) Brain serotonin and eating behavior. Appetite 7, 1–14.
Leibowitz S. F. and Rossakis C. (1978) Analysis of feeding suppression produced by perifornical hypothalamic injection of catecholamines, amphetamines and mazindol. Eur. J. Pharmacol. 53, 69–81.
Waldbillig R. J., Bartness T. J., and Stanley B. G. (1981) Increased food intake, body weight, and adiposity in rats after regional neurochemical depletion of serotonin. J. Comp. Physiol. Phychol. 95, 391–405.
Leibowitz S. F. (1975) Catecholaminergic mechanisms of the lateral hypothalamus: their role in the mediation of amphetamine anorexia. Brain Res. 98, 529–545.
Leibowitz S. F., Shor-Posner G., Maclow C., and Grinker J. A. (1986) Amphetamine: effects on meal patterns and macronutrient selection. Brain Res. Bull. 17, 681–689.
Hernandez L. and Hoebel B. G. (1988) Food reward and cocaine increase extracellular dopamine in the nucleus accumbens as measured by microdialysis. Life Sci. 42, 1705–1712.
Hernandez L. and Hoebel B. G. (1988) Feeding and hypothalamic stimulation increase dopamine turnover in the accumbens. Physiol. Behav. 44, 599–606.
Schwartz D. H., McClane S., Hernandez L., and Hoebel B. G. (1989) Feeding increases extracellular serotonin in the lateral hypothalamus of the rat as measured by microdialysis. Brain Res. 479, 349–354.
Schwartz D. H., Hernandez L., and Hoebel B. G. (1990) Serotonin release in lateral and medial hypothalamus during feeding and its anticipation. Brain Res. Bull. 25, 797–802.
Aberman J. E. and Salamone J. D. (1999) Nucleus accumbens dopamine depletions make rats more sensitive to high ratio requirements but do not impair primary food reinforcement. Neuroscience 92, 545–552.
Radhakishun F. S., van Ree J. M., and Westerink B. H. (1988) Scheduled eating increases dopamine release in the nucleus accumbens of food-deprived rats as assessed with on-line brain dialysis. Neurosci. Lett. 85, 351–356.
Salamone J. D., Mahan K., and Rogers S. (1993) Ventrolateral striatal dopamine depletions impair feeding and food handling in rats. Pharmacol. Biochem. Behav. 44, 605–610.
Salamone J. D., Cousins M. S., McCullough L. D., Carriero D. L., and Berkowitz R. J. (1994) Nucleus accumbens dopamine release increases during instrumental level pressing for food but not free food consumption. Pharmacol. Biochem. Behav. 49, 25–31.
Miyata G., Meguid M. M., Fetissov S. O., Torelli G. F., and Kim H. J. (1999) Nicotine’s effect on hypothalamic neurotransmitters and appetite regulation. Surgery 126, 255–263.
Yang Z. J., Blaha V., Meguid M. M., Oler A., and Miyata G. (1999) Infusion of nicotine into the LHA enhances dopamine and 5-HT release and suppresses food intake. Pharmacol. Biochem. Behav. 64, 155–159.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, M.D., Parker, S.L. & Kane, J.K. Regulation of feeding-associated peptides and receptors by nicotine. Mol Neurobiol 22, 143–165 (2000). https://doi.org/10.1385/MN:22:1-3:143
Issue Date:
DOI: https://doi.org/10.1385/MN:22:1-3:143