Abstract
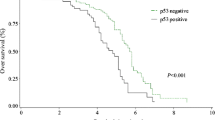
Colorectal cancer (CRC) is one of the most frequent and aggressive types of cancer. Several clinicopathologic features have been studied to identify the prognostic factors that can provide information concerning the favorable or the poor outcome of colorectal cancer. In the present study, the relationship between serum CEA, p53 expression, and DNA index to the different clinicopathological characteristics of colorectal cancer patients was sought. Fifty patients with CRC were included in this study, p53 protein was detected immunohistochemically using specific monoclonal antibodies. Samples were investigated for DNA index using flow cytometry. In addition, the serum CEA was determined using ELISA. The results showed that 27/50 (54%) were positive for p53. Concerning CEA reactivity, it was found that 35/50 (70%) were reactive for CEA. These results indicate that CEA is more sensitive than p53 to detect colorectal cancer. There was a statistically significant difference between the recurrent and nonrecurrent groups in the CRC Duke's stages, survival time, serum CEA (p=0.001, 0.016, <0.001, respectively). Kaplan-Meier method and log-rank test showed that the mean survival time for cases positive for both p53 and CEA is significantly different from cases positive for CEA only, positive for p53 only, and negative for both p53 and CEA (p=0.0002). Survival time was statistically significant with respect to sex, p53, CEA, and Duke's stages (p=0.006, 0.024, 0.001, 0.017, respectively). Cox regression model showed that the prognosis of colorectal cancer is influenced by sex, p53, CEA reactivity, and CRC Duke's stages (p=0.014, 0.006, 0.019, 0.014, respectively). In conclusion, the use of more than one tumor marker may successfully aid in the prediction of colorectal cancer prognosis.
Similar content being viewed by others
References
American Cancer Society. Cancer facts and figures. Available at: http://www.cancer.org/downloads/STT/CAFF_finalPWSecured.pdf. Accessed August 4, 2004.
Hollstein M, et al. Database of p53 gene somatic mutations in human tumors and cell lines. Nucleic Acids Res 1994; 22: 3551–3555.
Prives C, Hall PA: The p53 pathway (review). J Pathol 1999; 187: 112–126.
Levine AJ. p53, the cellular gatekeeper for growth and division. Cell 1997; 88: 323–331.
Maki CG, Howley PM. Ubiquitination of p53 and p21 is differentially affected by ionizing and UV radiation. Mol Cell Biol 1997: 17: 355–363.
Maltzman W, Czyzyk L UV irradiation stimulates levels of p53 cellular tumor antigen in nontransformed mouse cells. Mol Cell Biol 1984; 4: 1689–1694.
Fritsche M, Haessler C, Brandner G. Induction of nuclear accumulation of the tumor-suppressor protein p53 by DNA-damaging agents. Oncogene 1993; 8: 307–318.
Kastan MB, et al. A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in ataxiatelangiectasia. Cell 1992; 71: 587–597.
el-Deiry WS, et al. WAF1, a potential mediator of p53 tumor suppression. Cell 1993: 75: 817–825.
Harper JW, Adami GR, Wei N, Keyomarsi K, Elledge SJ, The p21 Cdk-interacting protein Cip1 is a potent inhibitor of Glcyclin-dependent kinases. Cell 1993; 75: 805–816.
Han J, Sabbatini P, Perez D, Rao L, Modha D, White E. The E1B 19K protein blocks apoptosis by interacting with and inhibiting the p53-inducible and death-promoting Bax protein. Genes Dev 1996; 10: 461–477.
Gold P, Freedman SO. Demonstration of tumor specific antigens in human colonic carcinomata by immunological tolerance and absorption techniques. J Exp Med 1965; 121: 439–462.
Paxton RJ, Mooser G, Pande H, Lee TD, Shively JE. Sequence analysis of carcinoembryonic antigen: identification of glycosylation sites and homology with the immunoglobulin supergene family. Proc Natl Acad Sci USA 1987; 84: 920–924.
Benchimol S, Fuks A, Jothy S, Beauchemin N, Shirota K, Stanners CP. Carcinoembryonic antigen, a human tumor marker, functions as an intercellular adhesion molecule. Cell 1989; 57: 327–334.
Kornek GV, Depisch D, Rosen HR, Temsch EM, Scheithauer W. Comparative analysis of CA72-4, CA195 and carcinoembryonic antigen in patients with gastrointestinal malignancies. J Cancer Res Clin Oncol 1992; 118: 318–320.
Posner MR, Mayer RJ. The use of serologic tumor markers in gastrointestinal malignancies. Hematol Oncol Clin North Am 1994; 8: 533–553.
Zhao JZ, Wu BH. Clinical significance of CA19-9 in diagnosis of digestive tract tumors. China Natl J New Gastroenterol 1997; 3: 253–254.
Kouri M, Pyrhnen S, Kuusela P. Elevated CA19-9 as the most significant prognostic factor in advanced colorectal carcinoma. J Surg Oncol 1992; 49: 78–85.
Zhao XY, et al. A clinical evaluation of serological diagnosis for pancreatic cancer. World J Gastroenterol 1998; 4: 147–149.
Marrelli D, et al. Prognostic significance of CEA, CA19-9 and CA72-4 preoperative serum levels in gastric carcinoma. Oncology 1999; 57: 55–62.
Ohuchi N, et al. Comparison of serum assays for TAG-72, CA19-9, and CEA in gastrointestinal carcinoma patients. Jpn J Clin Oncol 1989; 19: 242–248.
Fucini C, et al. Follow-up of colorectal cancer resected for cure, An experience with CEA, TPA, Cal9-9 analysis and second look surgery. Dis Colon Rectum 1987; 30: 273–277.
Guadagni F, et al. CA72-4 serum marker-a new tool in the management of carcinoma patients. Cancer Invest 1995; 13: 227–238.
Hedley DW. Flow cytometry using paraffin-embedded tissue: five years on. Cytometry 1989; 10: 229–241.
Zhang YL, Zhang ZS, Wu BP, Zhou DY. Early diagnosis for colorectal cancer in China. World J Gastroenterol 2002: 8: 21–25.
Li S, et al. Colorectal cancer screening for the natural population of Beijing with sequential fecal occult blood test: A multicenter study. Chin Med J 2003; 116: 200–202.
Thiis-Evensen E, Hoff GS, Sauar J, Majak BM, Vatn MH. Flexible sigmoidoscopy or colonoscopy as a screening modality for colorectal adenomas in older age groups? Findings in a cohort of the normal population aged 63–72 years. Gut 1999; 45: 834–839.
Repetto L, et al. Geriatric oncology: a clinical approach to the older patient with cancer. Eur J Cancer 2003; 39: 870–880.
Gatta G, Faivre J. Capocaccia R, Ponz de Leon M. Survival of colorectal cancer patients in Europe during the period 1978–1989. Eur J Cancer 1998; 34: 2176–2183.
Fletcher RH. Carcinoembryonic antigen. Ann Intern Med 1996; 104: 66–73.
Ballesta AM, Molina R, Filella X, Jo J, Gimenez N. Carcinoembryonic antigen in staging and follow-up of patients with solid tumors. Tumour Biol 1995; 16: 32–41.
Gebauer G, Müller-Ruchholtz W. CEA concentration in colon mucosa correlates with prognosis in colorectal cancer patients. Cancer Detection and Prevention 1998; 22 (Supl).
Clinical practice guidelines for the use of tumor markers in breast and colorectal cancer. Adopted on May 17, 1996, by the American Society of Clinical Oncology. J Clin Oncol 1996: 14: 2843–2877.
Bast RC Jr., et al. Update of recommendations for the use of tumor markers in breast and colorectal cancer: clinical practice guidelines of the American Society of Clinical Oncology. J Clin Oncol 2001: 19: 1865–1878.
Bruinvels DJ, Stiggelbout AM, Kievit J, van Houwelingen HC, Habbema JD, van de Velde CJ. Follow-up of patients with colorectal cancer. A meta-analysis. Ann Surg 1994; 219: 174–182.
Perkins GL, Slater ED, Sanders GK, Prichard JG. Serum tumor markers. Am Fam Physician 2003: 68: 1075–1082.
Nan K-J, Qin H-X, Yang G. Prognostic factors in 165 elderly colorectal cancer patients. World J Gastroenterol 2003; 9 (10): 2207–2210.
Wang JP, et al. Multivariate regression analysis of clinicopathological characteristics and prognosis of colorectal cancer. Zhonghua Zhongliu Zazhi 2003; 25: 59–61.
Walker J, Quirke P. Prognosis and response to therapy in colorectal cancer. Eur J. Cancer 2002; 38: 880–886.
Gu J, Ma ZL, Li Y, Li M, Xu GW. Angiography for diagnosis and treatment of colorectal cancer. World J Gastroenterol 2003; 9: 288–290.
Kanthan R, Radhi JM, Kanthan SC. Gallbladder carcinomas: an immunoprognostic evaluation of P53, Bcl-2, CEA and alpha-fetoprotein. Canad J Gastroenterol 2000; 14 (3): 181–184.
Kim DY, Kim HR, Shim JH, Park CS, Kim SK, Kim YJ. Significance of serum and tissue carcinoem bryonic antigen for the prognosis of gastric carcinoma patients J Surg Oncol 2000; 74: 185–192.
Zeng ZS, et al. p53 nuclear overexpression: an independent predictor of survival in lymph node—positive colorectal cancer patients. J Clin Oncol 1994; 12: 2043–2050.
Tortola S, et al. p53 and K-ras gene mutations correlate with tumor aggressiveness but are not of routine prognostic value in colorectal cancer. J Clin Oncol 1999; 17 (5): 1375–1381.
Shiota G, et al. Circulating p53 antibody in patients with colorectal cancer. Dig Dis Sci 2000; 45 (1): 122–128.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nasif, W.A., Lotfy, M., El-Sayed, I.H. et al. Implications of CEA and p53 overexpression in the poor prognosis of colorectal cancer. Med Oncol 23, 237–244 (2006). https://doi.org/10.1385/MO:23:2:237
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1385/MO:23:2:237