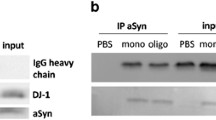
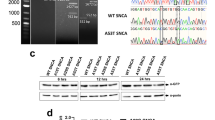
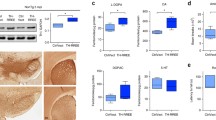
Abstract
Parkinson's disease (PD) is a common neurodegenerative disorder that results from the selective loss of midbrain dopaminergic neurons. Misfolding and aggregation of the protein α-synuclein, oxidative damage, and proteasomal impairment are all hypotheses for the molecular cause of this selective neurotoxicity. Here, we describe a Saccharomyces cerevisiae model to evaluate the misfolding, aggregation, and toxicity-inducing ability of wild-type α-synuclein and three mutants (A30P, A53T, and A30P/A53T), and we compare regulation of these properties by dysfunctional proteasomes and by oxidative stress. We found prominent localization of wild-type and A53T α-synuclein near the plasma membrane, supporting known in vitro lipid-binding ability. In contrast, A30P was mostly cytoplasmic, whereas A30P/A53T displayed both types of fluorescence. Surprisingly, α-synuclein was not toxic to several yeast strains tested. When yeast mutants for the proteasomal barrel (doa3-1) were evaluated, delayed α-synuclein synthesis and membrane association were observed; yeast mutant for the proteasomal cap (sen3-1) exhibited increased accumulation and aggregation of α-synuclein. Both sen3-1 and doa3-1 mutants exhibited synthetic lethality with α-synuclein. When yeasts were challenged with an oxidant (hydrogen peroxide), α-synuclein was extremely lethal to cells that lacked managanese superoxide dismutase Mn-SOD (sod2Δ) but not to cells that lacked copper, zinc superoxide dismutase Cu,Zn-SOD (sod1Δ). Despite the toxicity, sod2Δ cells never displayed intracellular aggregates of α-synuclein. We suggest that the toxic α-synuclein species in yeast are smaller than the visible aggregates, and toxicity might involve α-synuclein membrane association. Thus, yeasts have emerged effective organisms for characterizing factors and mechanisms that regulate α-synuclein toxicity.
Similar content being viewed by others
References
Abeliovich A., Schmitz Y., Farinas I., Choi-Lundber D., Wei-Hsien H., Castillo P. E., et al. (2000) Mice lacking α-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron 25, 239–252.
Alim M. A., Ma Q. L., Takeda K., Aizawa T., Matsubara M., Nakamura M., et al. (2004) Demonstration of a role for alpha-synuclein as a functional microtubule-associated protein. J. Alzheimer's Dis. 6, 435–449.
Andreassen O. A., Ferrante R. J., Dedeoglu A., Albers D. W., Klivenyi P., Carlson E. J., et al. (2001) Mice with a partial deficiency of manganese superoxide dismutase show increased vulnerability to the mitochondrial toxins malonate, 3-nitropropionic acid, and MPTP. Exp. Neurol. 167, 189–195.
Arendt C. C. and Hochstrasser M. (1999) Eukaryotic 20S proteasome catalytic subunit propeptides prevent active site inactivation by N-terminal acetylation and promote particle assembly. EMBO J. 18, 3575–3585.
Auluck P., Chan E., Trojanowski J., Lee V., and Bonini N. (2002) chaperone suppression of α-synuclein toxicity in a Drosophila model for Parkinson's disease. Science 295, 865–868.
Bonifati V., Rizzu P., van Baren M. J., Schaap O., Breedveld G. J., Krieger E., et al. (2003) Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science 299, 256–259.
Brandis K., Holmes I., and England S. (2006) α-Synuclein fission yeast model: concentration-dependent aggregation without membrane localization or toxicity. J. Mol. Neurosci., 28(6), 179–191.
Burke D., Dawson D., and Stearns T. (2000) Methods in Yeast Genetics, Cold Spring Harbor Laboratory Press, New York.
Caughey B. and Lansbury P. T. (2003) Protofibrils, pores, fibrils, and neurodegeneration: separating the responsible protein aggregates from the innocent bystanders. Annu. Rev. Neurosci. 26, 267–298.
Ciechanover A. and Brundin P. (2003) The ubiquitin-proteasome system in neurodegenerative diseases: some-times the chicken, sometimes the egg. Neuron 40, 427–446.
Clayton D. and George J. (1998) The synucleins: a family of proteins involved in synaptic function, plasticity, neurodegeneration and disease. Trends Neurosci. 21, 249–254.
Cole N. B., Murphy D. D., Grider T., Rueter S., Brasaemle D., and Nusbaum R. L. (2002) Lipid droplet binding and oligomerization properties of the Parkinson's disease protein α-synuclein. J. Biol. Chem. 277, 6344–6352.
Conway K., Harper J., and Lansbury P. (1998) Accelerated in vitro fibril formation by a mutant α-synuclein linked to early-onset Parkinson's disease. Nat. Med. 4, 1318–1320.
Conway K., Harper J., and Lansbury P. (2000) Fibrils formed in vitro from α-synuclein and two mutant forms linked to Parkinson's disease are typical amyloid. Biochemistry 39, 2552–2563.
Dauer W. and Przedborski S. (2003) Parkinson's disease: mechanisms and models. Neuron 39, 889–909.
Davidson W. S., Jonas A., Clayton D. F., and George J. M. (1998) Stabilization of α-synuclein secondary structure upon binding to synthetic membranes. J. Biol. Chem. 273, 9443–9449.
Dawson T. M. and Dawson V. L. (2003) Molecular pathways of neurodegeneration in Parkinson's disease. Science 302, 819–822.
DeMarini D. J., Papa F. R., Swaminathan S., Ursic D., Rassmussen, T. P., Culbertson M. R., and Hochstrasser M. (1995) The yeast sen3 gene encodes a regulatory subunit of the 26s proteasome complex required for ubiquitin-dependent protein degradation in vivo. Mol. Cell. Biol. 15, 6311–6321.
Dixon C., Mathias N., Zwieg R. M., Davis D. A., and Gross D. S. (2005) Alpha-Synuclein targets the plasma membrane via the secretory pathway and induces toxicity in yeast. Genetics 170, 47–59.
Eliezer D., Kutluay E., Bussell R. Jr., and Browne G. (2001) Conformational properties of α-synuclein in its free and lipid-associated states. J. Mol. Biol. 307, 1061–1073.
Engelender S., Kaminsky Z., Guo X., Sharp A. H., Amaravi R. K., Kleiderlein J. J., et al. (1999) Synphilin-1 associates with alpha-synuclein and promotes the formation of cytosolic inclusions. Nat. Genet. 22, 110–114.
Feany M. and Bender W. (2000) A Drosophila model of Parkinson's disease. Nature 23, 294–298.
Funayama M., Hasegawa K., Kowa H., Saito M., Tsuji S., and Obata F. (2002) A new locus for Parkinson's disease (PARK8) maps to chromosome 12p11.2-q13.1. Ann. Neurol. 51, 296–301.
George J. M., Jin H., Woods W. S., and Clayton D. F. (1995) Characterization of a novel protein regulated during the critical period for song learning in the zebra finch. Neuron 15, 361–372.
Giasson B., Duda J., Quinn S., Zhang B., Trojanowski J., and Lee V. (2002) Neuronal α-synucleinopathy with severe movement disorder in mice expressing A53T human α-synuclein. Neuron 34, 521–533.
Giasson B., Uryu K., Trojanowski J., and Lee V. (1999) Mutant and wild type human α-synucleins assemble into elongated filaments with distinct morphologies in vitro. J. Biol. Chem. 274, 7619–7622.
Hashimoto M., Takeda A., Hsu L. J., Takenouchi T., and Malsiah E. (1999) Role of cytrochrome c as a stimulator of α-synuclein aggregation in Lewy body disease. J. Biol. Chem. 274, 28,849–28,852.
Hinerfeld D., Traini M. D., Weinberger R. P., Cochran B., Doctrow S. R., Harry J., and Melov S. (2004) Endogenous mitochondrial oxidative stress: neurodegeneration, proteomic analysis, specific respiratory chain defects, and efficacious antioxidant therapy in superoxide dismutase 2 null mice. J. Neurochem. 88, 657–667.
Jenco J. M., Rawlingson A., Daniels B., and Morris A. J. (1998) Regulation of phospholipase D2: selective inhibition of mammalian phospholipase D isoenzymes by alpha- and beta-synucleins. Biochemistry 7, 4901–4909.
Jenner P. and Olanow C. W. (1996) Oxidative stress and the pathogenesis of Parkinson's disease. Neurology. 47, (6 Suppl. 3), S161-S170.
Jensen P. H., Nielsen M., Jakes R., Dottis C. G., and Goedert M. (1998) Binding of α-synuclein to brain vesicles is abolished by familial Parkinson's disease mutation. J. Biol. Chem. 273, 26,292–26,294.
Kahle P. J., Neumann M., Ozmen L., Müller V., Jacobsen H., Schindzielorz A., et al., (2000) Subcellular localization of wild-type and Parkinson's disease-associated mutant α-synuclein in human and transgenic mouse brain. J. Neurosci. 20, 6365–6373.
Kang J. H. and Kim K. S. (2003) Enhanced oligomerization of the a-Synuclein mutant by the Cu,Zn-superoxide dismutase and hydrogen peroxide system. Mol. Cells 15, 87–93.
Kim K. S., Choi S. Y., Kwon H. Y., Won M. H., Kang T., and Kang J. H. (2002) Aggregation of a-synuclein induced by the Cu,Zn-superoxide dismutase and hydrogen peroxide system. Free Radic. Biol. Med. 32, 544–550.
Kitada T., Asakawa S., Hattori N., Matsumine H., Yamamura Y., Yocochi M., et al. (1998) Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature 392, 605–606.
Krobitsch S. and Lindquist S. (2000) Aggregation of huntingtin in yeast varies with the length of the polyglutamine expansion and the expression of chaperone proteins. Proc. Natl. Acad. Sci. U. S. A. 97, 1589–1594.
Kruger R., Kuhn W., Muller T., Woitalla D., Graeber M., Kosel S., et al. (1998) Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson's disease. Nat. Genet. 18, 106–108.
Kunst C., Mezey E., Brownstein M., and Patterson D. (1997) Mutations in SOD1 associated with amyotrophic lateral sclerosis cause novel protein interactions. Nat. Genet. 15, 91–94.
Kweon G. R., Marks J. D., Krencik R., Leung E. H., Schumacker P. T., Hyland K., and Kang U. J. (2004) Distinct mechanisms of neurodegeneration induced by chronic complex I inhibition in dopaminergic and non-dopaminergic cells. J. Biol. Chem. 279, 51,783–51,792.
Lasko M., Vartiainen S., Moilanen A., Sirvio J., Thomas J. H., Nass R., et al. (2003) Dopaminergic neuronal loss and motor deficits in Caenorhabditis elegans overexpressing human α-synuclein. J. Neurochem. 86, 165–172.
Lebovitz R. M., Zhang H., Vogel H., Cartwight J. Jr., Dionne L., Lu N., et al. (1996) Neurodegeneration, myocardial injury, and perinatal death in mitochond rial superoxide dismutase-deficient mice. Proc. Natl. Acad. Sci. U. S. A. 93, 9782–9787.
Leroy E., Boyer R., Auburger G., Leube B., Ulm G., Mezzey E., et al. (1998) The ubiquitin pathway in Parkinson's disease. Nature 395, 451–452.
Liang L. P. and Patel M. (2004) Mitochondrial oxidative stress and increased seizure susceptibility in Sod2 (−/+) mice. Free Radic. Biol. Med. 36, 542–554.
Liu Y., Fallon L., Lashuel H. A., Liu Z., and Lansbury P. T. (2002) The UCHL-1 gene encodes two opposing enzymatic activities that affect α-synuclein degradation in Parkinson's disease susceptibility. Cell 111, 209–218.
Lynn S., Huang E. J., Elchuri S., Naeemuddin M., Nishinaka Y., Yodoi J., et al. (2005) Selective neuronal vulnerability and inadequate stress response in superoxide dismutase mutant mice. Free Rad. Biol. Med. 38, 817–828.
Ma J. and Lindquist S. (1999) Denovo generation of a PrPsc-like conformation in living cells. Nat. Cell Biol. 1, 358–361.
Maguire-Zeiss K. A., Short D. W., and Federoff H. J. (2005) Synuclein, dopamine and oxidative stress: co-conspirators in Parkinson's disease? Mol. Brain Res. 134, 18–23.
Masliah E., Rockenstein E., Veinbergs I., Mallory M., Hashimoto M., Takeda A., and Mucke L. (2000) Dopaminergic loss and inclusion body formation in α-Synuclein mice: implications for neurodegenerative disorders. Science 287, 1265–1269.
McDonough V. M. and Roth T. M. (2004) Growth temperature affects accumulation of exogenous fatty acids and fatty acid composition in Schizosaccharomyces pombe. Antonie Van Leeuwenhoek 86, 349–354.
McDonough and Roth (2004) not cited in text.
McNaught K. S. and Jenner P. (2001) Proteasomal function is impaired in substantia nigra in Parkinson's disease. Neurosci. Lett. 297, 191–194.
McNaught K. S., Belizaire R., Isacson O., Jenner P., and Olanow C. W. (2003) Altered proteasomal function in sporadic Parkinson's disease. Exp. Neurol. 179, 38–46.
McNaught K. S., Bjorklund L. M., Belizaire R., Isacson O., Jenner P., and Olanowski C. W. (2002a) Proteasomal inhibition causes nigral degeneration with inclusion bodies in rats. Neuroreport 13, 1437–1441.
McNaught K. S., Mytilineou C., Jnobaptiste R., Yabut J., Shashidharan P., Jennert P., and Olanow C. W. (2002b) Impairment of the ubiquitin-proteasome system causes dopaminergic cell death and inclusion body formation in ventral mesencephalic cultures. J. Neurochem. 81, 301–306.
Mizuno Y., Ohta S., Tanaka M., Takamiya S., Suzuki K., Sato T., et al. (1989) Deficiencies in complex I subunits of the respiratory chain in Parkinson's disease. Biochem. Biophys. Res. Commun. 163, 1450–1455.
Muchowski P., Schaffar G., Sittler A., Wanker E., Hayer-Hartl M., and Hartyl F. (2000) Hsp70 and Hsp40 chaperones can inhibit self-assembly of polyglutamine proteins into amyloid-like fibrils. Proc. Natl. Acad. Sci. U. S. A. 97, 7841–7846.
Murphy D. D., Rueter S. M., Trojanowski J. Q., and Lee V. M. (2000) Synucleins are developmentally expressed, and α-synuclein regulates the size of the presynaptic vesicular pool in primary hippocampal neurons. J. Neurosci. 20, 3214–3220.
Narhi L., Wood S. J., Steavenson S., Jiang Y., Wu G. M., Anafi D., et al. (1999) Both familial Parkinson's disease mutations accelerate α-synuclein aggregation. J. Biol. Chem. 274, 9843–9846.
Olanow C. W. and Tatton W. G. (1999) Etiology and pathogenesis of Parkinson's disease. Annu. Rev. Neurosci. 22, 123–144.
Ostrerova N., Petrucellil L., Farrer M., Mehta N., Choil P., Hardy J., and Wolozin B. (1999) α-Synuclein shares physical and functional homology with 14-3-3-proteins. J. Neurosci. 19, 5782–5791.
Outeiro T. F. and Lindquist S. (2003) Yeast cells provide insight into alpha-synuclein biology and pathobiology. Science 203, 1772–1775.
Outeiro T. F. and Muchowski P. J. (2004) Molecular genetics approaches in yeast to study amyloid diseases. J. Mol. Neurosci. 23, 49–60.
Paisan-Ruiz C., Jain S., Evans E. W., Gilks W. P., Simon J., van der Brug M., et al. (2004) Cloning of the gene containing mutations that cause PARK8-linked Parkinson's disease. Neuron 44, 595–600.
Perrin R. J., Woods W. S., Clayton D. F., and George J. M. (2000) Interaction of human α-synuclein and Parkinson's disease variants with phospholipids. J. Biol. Chem. 275, 34,393–34,398.
Pias E. K., Ekshyyan O. Y., Rhoads C. A., Fuseler J., Harrison L., and Aw T. Y. (2003) Differential effects of superoxide dismutase isoform expression on hydroperoxide-induced apoptosis in PC-12 cells. J. Biol. Chem. 278, 13,294–13,301.
Petrucelli L., O'Farell C., Lockhart P. J., Baptisa M., Kehoe K., Vink L., et al. (2002) Parkin protects against the toxicity associated with mutant α-synuclein: proteasome dysfunction selectively affects catecholaminergic neurons. Neuron 36, 1007–1019.
Polymeropoulos M. H., Lavedan C., Leroy E., Ide S. E., Dehejia A., Dutra A., et al. (1997) Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science 276, 2045–2047.
Poon H. F., Frasier M., Shreve N., Calabrese V., Wolozin B., and Butterfield D. (2005) Mitochondrial associated metabolic proteins are selectively oxidized in A30P alpha-synuclein transgenic mice—a model of familial Parkinson's disease. Neurobiol. Dis. 18, 492–498.
Rochet J. C., Outeiro T. F., Conway K. A., Ding T. T., Volles M. J., Lashuel H. A., et al. (2004) Interactions among alpha-synuclein, dopamine, and biomembranes: some clues for understanding neurodegeneration in Parkinson's disease. J. Mol. Neurosci. 23, 23–34.
Sharon R., Goldberg M., Bar I., Betensky R., Shen J., and Selkoe D. (2001) α-Synuclein occurs in lipid-rich high molecular weight complexes, binds fatty acids, and shows homology to the fatty-acid binding proteins. Proc. Natl. Acad. Sci. U. S. A. 98, 9110–9115.
Shendelman S., Jonason A., Martinat C., Leete T., and Abeliovich A. (2004) DJ-1 is a redox-dependent molecular chaperone that inhibits alpha-synuclein aggregate formation. PLoS Biol. 2, e362.
Sherer T. B., Betarbet R., Stout A. K., Lund S., Baptista M., Panov A. V., et al. (2002) An in vitro model of Parkinson's disease: linking mitochondrial impairment to altered alpha-synuclein metabolism and oxidative damage. J. Neurosci. 22, 7006–7015.
Shimura H., Schlossmacher M., Hattori N., Frosch M., Trockenbacher A., Schneider R., et al. (2001) Ubiquitination of a new form of α-synuclein by parkin from human brain: implications for Parkinson's disease. Science 293, 263–269.
Sisodia S. S. (1998) Nuclear inclusions in glutamine repeat disorders: are they pernicious, coincidental, or beneficial? Cell 95, 1–4.
Snyder H., Mensh K., Theisler C., Lee J. L., Matouschek A., and Wolozin B. (2003) Aggregated and monomeric forms of α-synuclein bind to the S6′ proteasomal protein and inhibit proteasomal function. J. Biol. Chem. 278, 11,753–11,759.
Song D. D., Shults C. W., Sisk A., Rockenstein E., and Masliah E. (2004) Enhanced substantia nigra mitochondrial pathology in human alpha-synuclein transgenic mice after treatment with MPTP. Exp. Neurol. 186, 158–172.
Spillantini M., Schmidt M., Lee V., Trojanowski J., Lakes R., and Goedert M. (1998) α-Synuclein in filamentous inclusions of Lewy bodies from Parkinson's disease. Proc. Natl. Acad. Sci. U. S. A. 95, 6469–6473.
Taylor J. P., Hardy J., and Fishbeck K. H. (2002) Toxic proteins in neurodegenerative disease. Science 296, 1991–1995.
Testa C. M., Sherer T. B., and Greenamyre J. T. (2005) Rotenone induces oxidative stress and dopaminergic neuron damage in organotypic substantia nigra cultures. Mol. Brain Res. 134, 109–118.
Thiruchelvam M., Prokopenk O., Cory-Slechta D. A., Richfield E. K., Buckley B., and Mirochnitchenko O. (2005) Overexpression of superoxide dismutase or glutathione peroxidase protects against the paraquat+ maneb-induced Parkinson's disease phenotype. J. Biol. Chem. 280, 22,530–22,539.
Valente E. M., Abou-Sleiman P. M., Caputo V., Muqit M. M., Harvey K., Gispert S., et al. (2004) Hereditary earlyonset Parkinson's disease caused by mutations in PINK1. Science 304, 1158–1160.
Wersinger C., Prou D., Vernier P., Niznik H. B., and Sidhu A. (2003) Mutations in the lipid-binding domain of alpha-synuclein confer overlapping yet distinct, functional properties in the regulation of dopamine transporter activity. Mol. Cell. Neurosci. 24, 91–105.
Willingham S., Outeiro T. F., DeVit M. J., Lindquist S., and Muchowski P. J. (2003) Yeast genes that enhance the toxicity of a mutant huntingtin or α-synuclein. Science 302, 1769–1772.
Zabrocki P., Pellens K., Vanhelmont T., Vandebroek T., Griffioen G., Wera S., et al. (2005) Characterization of α-synuclein aggregation and synergistic toxicity of protein tau in yeast. FEBS J. 272, 1386–1400.
Zarranz J. J., Alegre J., Gomez-Esteban J. C., Lezcano E., Ros R., Ampuero I. et al. (2004) The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann. Neurol. 55, 164–173.
Zhou Y., Gu G., Goodlett D. R., Zhang T., Pan C., Montine T. J., e al. (2004) Analysis of alpha-synuclein-associated proteins by quantitative proteomics. J. Biol. Chem. 279, 39,155–39,164.
Zimprich A., Biskup S., Leitner P., Lichtner P., Farrer M., Lincoln S., et al. (2004) Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron 44, 601–607.
Author information
Authors and Affiliations
Corresponding author
Additional information
Author to whom all correspondence and requests should be addressed.
Rights and permissions
About this article
Cite this article
Sharma, N., Brandis, K.A., Herrera, S.K. et al. α-synuclein budding yeast model. J Mol Neurosci 28, 161–178 (2006). https://doi.org/10.1385/JMN:28:2:161
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1385/JMN:28:2:161