Summary
Background. Resected pancreatic cancer has a high risk of recurrence and mortality despite the the use of chemoradiotherapy. Because pancreatic cancers express tumor antigens such as carcinoembryonic antigen (CEA), it may be possible to immunize patients to induce tumor antigen-specific immune responses. We hypothesize that high-frequency tumor antigen-specific immune responses will reduce recurrence and increase survival. Autologous dendritic cells (DCs) loaded with tumor antigens are particularly potent at inducing tumor antigen-specific immune responses.
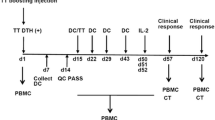
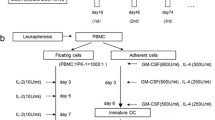
Methods. Three patients with resected pancreatic adenocarcinoma following neoadjuvant chemoradiotherapy received autologous, monocyte-derived DCs loaded with the mRNA encoding CEA monthly for 6 mo.
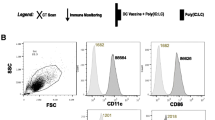
Results. It was feasible to generate an adequate number of DC from these patients and to cryopreserve them for repeated use. The DC demonstrated the typical immature phenotype. The immunizations were well-tolerated without evidence of adverse events. All three developed injection site reactivity. All three are alive without evidence of disease at more than 21/2 yr from the original diagnosis.
Conclusion. The postoperative period following neoadjuvant chemoradiotherapy and pancreaticoduo-denectomy for pancreatic cancer is an ideal environment to test novel immune-based therapies. DC-based immunotherapy in this setting is safe and feasible and may lead to prolonged survival.
Similar content being viewed by others
References
Ghaneh P, Slavin J, Sutton R, Hartley M, Neoptolemos JP. Adjuvant therapy in pancreatic cancer. World J Gastroenterol 2001;7:482–489.
Ahmad NA, Lewis JD, Ginsberg GG, Haller DG, Morris JB, Williams NN, et al. Long term survival after pancreatic resection for pancreatic adenocarcinoma. Am J Gastroenterol 2001;96:2609–2615.
Lieberman SM, Horig H, Kaufman HL. Innovative treatments for pancreatic cancer. Surg Clin North Am 2001;81:715–739.
Gjertsen MK, Buanes T, Rosseland AR, Bakka A, Gladhaug I, Soreide O, et al. Intradermal ras peptide vaccination with granulocyte-macrophage colony-stimulating factors as adjuvant: clinical and immunological responses in patients with pancreatic adenocarcinoma. Int J Cancer 2001;92:441–450.
Staib L, Birebent B, Somasundaram R, Purev E, Braumuller H, Leeser C, et al. Immunogenicity of recombinant GA733-2E antigen (CO17-1A, EGP, KS1-4, KSA, Ep-CAM) in gastro-intestinal carcinoma patients. Int J Cancer 2001;92:79–87.
Jaffee EM, Hruban RH, Biedrzycki B, Laheru D, Schepers K, Sauter PR, et al. Novel allogeneic granulocyte-macrophage colony-stimulating factor-secreting tumor vaccine for pancreatic cancer: a phase I trial of safety and immune activation. J Clin Oncol 2001;19:145–156.
Nestle FO, Banchereau J, Hart D. Dendritic cells: on the move from bench to bedside. Nat Med 2001;7:761–765.
Piemonti L, Monti P, Zerbi A, Balzano G, Allavena P, Di Carlo V. Generation and functional characterisation of dendritic cells from patients with pancreatic carcinoma with special regard to clinical applicability. Cancer Immunol Immunother 2000;49:544–550.
Nair SK, Hull S, Coleman D, Gilboa E, Lyerly HK, Morse MA. Induction of carcinoembryonic antigen (CEA)-specific cytotoxic T-lymphocyte responses in vitro using autologous dendritic cells loaded with CEA peptide or CEA RNA in patients with metastatic malignancies expressing CEA. Int J Cancer 1999;82:121–124.
Morse MA, Deng Y, Hull S, Coleman D, Nair S, Schlom J, et al. A phase I study of active immunotherapy with carcinoembryonic antigen peptide (CAP-1)-pulsed, autologous human cultured dendritic cells in patients with metastatic malignancies expressing carcinoembryonic antigen. Clin Cancer Res 1999;5:1331–1338.
Zamora C, Sahel J, Cantu DG, Heyries L, Bernard JP, Bastid C, et al. Intraductal papillary or mucinous tumors (IPMT) of the pancreas: report of a case series and review of the literature. Am J Gastroenterol 2001;96:1441–1447.
White RR, Hurwitz HI, Morse MA, Lee C, Anscher MS, Paulson EK, et al. Neoadjuvant chemoradiation for localized adenocarcinoma of the pancreas. Ann Surg Oncol 2001;8:758–765.
Coulie PG, Karanikas V, Colau D, Lurquin C, Landry C, Marchand M, et al. A monoclonal cytolytic T-lymphocyte response observed in a melanoma patient vaccinated with a tumor-specific antigenic peptide encoded by gene MAGE-3. Proc Natl Acad Sci USA 2001;98:10290–10295.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Morse, M.A., Nair, S.K., Boczkowski, D. et al. The feasibility and safety of immunotherapy with dendritic cells loaded with CEA mRNA following neoadjuvant chemoradiotherapy and resection of pancreatic cancer. Int J Gastrointest Canc 32, 1–6 (2002). https://doi.org/10.1385/IJGC:32:1:1
Issue Date:
DOI: https://doi.org/10.1385/IJGC:32:1:1