Abstract
Patients with chronic renal failure (CRF) usually have a lower than healthy level of selenium (Se) in whole blood and plasma. Plasma glutathione peroxidase (GSH-Px) is synthesized mostly in the kidney. In CRF patients, activity of this enzyme is significantly reduced and its reduction increases with the progress of the disease. The aim of the study was to evaluate the effect of Se supplementation to CRF patients at various stages of the disease on Se concentration in blood components and on plasma GSH-Px activity.
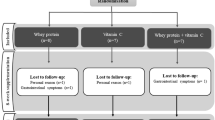
The study group comprised 53 CRF patients at various stages of the disease supplemented with Se (200 µg/d for 3 mo as Se-enriched yeast, containing about 70% l-selenomethionine [SeMet]). The control group consisted of 20 healthy subjects. The Se concentration in blood components was measured spectrofluorometrically with 2,3-diaminonaphthalene as a complexing reagent. GSH-Px activity in red cell hemolysates and plasma was assayed by the coupled method with tert-butyl hydroperoxide as a substrate.
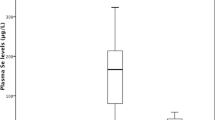
The Se concentration in whole blood and plasma of CRF patients is significantly lower as compared with healthy subjects, but similar at all stages of the disease. In the patients’ plasma, total protein and albumin levels are also significantly lower than in healthy subjects. Plasma GSH-Px activity in patients is extremely low, and contrary to Se concentration, it decreases linearly with the increasing stage of the illness. Se-supplied patients show an increased Se concentration in all blood components and at all disease stages, whereas plasma GSH-Px activity is enhanced only at the incipient stage of the disease. Se supply has no effect on plasma GSH-Px activity in uremic patients at the end stage of the disease. Total plasma protein and albumin levels did not change after Se supplementation. Our data seem to show that in patients with CRF lower total protein and albumin levels in plasma may be the chief cause of the low blood and plasma Se concentrations. GSH-Px activity decreases along with the kidney impairment. At the end stage of the disease, Se supplementation in the form of Se-enriched yeast has no effect on the increase in plasma GSH-Px activity.
Similar content being viewed by others
References
M. P. Rayman, Dietary selenium: time to act, Br. Med. J. 314, 387–388 (1997).
M. P. Rayman, The argument for increasing selenium intake, Proc. Nutr. Soc. 61, 203–215 (2002).
R. F. Burk and K. E. Hill, Orphan selenoproteins, BioEssays 21, 231–237 (1999).
J. R. Arthur and G. J. Beckett, Newer aspects of micronutrients in at risk groups. New metabolic roles for selenium, Proc. Nutr. Soc. 53, 615–624 (1994).
B. Dworkin, S. Weseley, W. S. Rosenthal, et al., Diminished blood selenium levels in renal failure patients on dialysis: correlations with nutritional status, Am. J. Med. Sci. 30, 6–12 (1987).
M. J. Richard, J. Arnaud, C. Jurkovitz, et al., Trace elements and lipid peroxidation abnormalities in patients with chronic renal failure, Nephron 57, 10–15 (1991).
J. W. Foote, L. J. Hinks, and B. Lloyd, Reduced plasma and white blood cell selenium levels in haemodialysis patients, Clin. Chim. Acta 164, 323–328 (1987).
B. A. Zachara, U. Trafikowska, A. Adamowicz, et al., Selenium, glutathione peroxidases, and some other parameters in blood of patients with chronic renal failure, J. Trace Elements Med. Biol. 15, 161–166 (2001).
S. Yoshimura, H. Suemizu, Y. Nomoto, et al., Plasma glutathione peroxidase deficiency caused by renal dysfunction, Nephron 73, 207–211 (1996).
H. E. Roxborough, C. Mercer, D. McMaster, et al., Plasma glutathione peroxidase activity is reduced in haemodialysis patients, Nephron 81, 278–283 (1999).
T. Zima, S. Stipek, J. Crkovska, et al., Antioxidant enzymes—superoxide dismutase and glutathione peroxidase—in haemodialyzed patients, Blood Purif. 14, 257–264 (1996).
M. C. Martin-Mateo, E. del Canto-Jafiez, and M. J. Barrero-Martinez, Oxidative stress and enzyme activity in ambulatory renal patients undergoing continuous peritoneal dialysis, Renal Failure 20, 117–124 (1998).
F. Bulucu, A. Vural, A. Aydin, et al., Oxidative stress status in adults with nephrotic syndrome, Clin. Nephrol. 53, 169–173 (2000).
I. Ceballos-Picot, V. Witko-Sarsat, M. Merad-Boudia, et al., Glutathione antioxidant system as a marker of oxidative stress in chronic renal failure, Free Radical Biol. Med. 21, 845–853 (1996).
N. Avissar, D. B. Ornt, Y. Yagil, et al., Human kidney proximal tubules are the main source of plasma glutathione peroxidase, Am. J. Physiol. 266 (Cell Physiol. 35), C367-C375 (1994).
B. A. Zachara, A. Trafikowska, J. Manitius, et al., Third International Meeting “Advances in Trace Elements, Minerals and Vitamins in Humans: Functional and Clinical Aspects,” Monastir, Tunisia 2002, Abstracts p. 49.
B. A. Zachara, A. Adamowicz, U. Trafikowska, et al., Decreased plasma glutathione peroxidase activity in uremic patients, Nephron 84, 278–279 (2000).
G. N. Schrauzer, Nutritional selenium supplementation: product types, quality, and safety, J. Am. Coll. Nutr. 20, 1–4 (2001).
M. A. Beilstein and P. D. Whanger, Metabolism of selenomethionine and effects of interacting compounds by mammalian cells in culture, J. Inorg. Biochem. 29, 137–152 (1987).
N. Esaki, T. Nakamura, H. Tanaka, et al., Selenocysteine lyase, a novel enzyme that specifically acts on selenocysteine, J. Biol. Chem. 257, 4386–4391 (1982).
C. Ip, Differential effects of dietary methionine on the biopotency of selenomethionine and selenite in cancer chemoprevention, J. Natl. Cancer Inst. 80, 258–262 (1988).
B. A. Zachara, D. Koterska, A. Trafikowska, et al., The effect of selenium supplementation on plasma glutathione peroxidase activity in patients with different stages of chronic renal failure, in Macro and Trace Elements; 21 Workshop, M. Anke, R. Muller, U. Schafer, and M Stoeppler, eds., 2002, pp. 945–951.
B. A. Zachara, M. Sklodowska, W. Wasowicz, et al., Selenium status, glutathione peroxidase activity and lipid peroxides concentration in blood of Polish population. III. Effect of age and sex in healthy adults, in Elements in Health and Disease, M. Said, M. A. Rahman, and L. A. D’Silva, eds., Hamdard University Press, Karachi, Pakistan, pp. 501–512 (1989).
J. H. Watkinson, Fluorometric determination of selenium in biological material with 2,3-diaminonaphthalene, Anal. Chem. 38, 92–97 (1966).
D. E. Paglia and W. H. Valentine, Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase, J. Lab. Clin. Med. 70, 158–169 (1967).
J. Ringstad, B. Jacobsen, S. Tretli, et al., Serum selenium concentration associated with risk of cancer, J. Clin. Pathol. 41, 454–457 (1988).
B. Tiran, Selen ein essentielles Spurenelement, Wien. Klin. Wochenschr. 109, 3–6 (1997).
W. Wasowicz, J. Gromadzinska, K. Rydzynski, et al., Selenium status of low-selenium area residents: Polish experience, Toxicol. Lett. 137, 95–101 (2003).
B. A. Zachara and W. Wasowicz, Selenium concentration in blood components of Polish sub-population and in some other countries, in Arsenium and Selenium in the Environment—Ecological and Methodological Problems, A. Kabata-Pendias, and B. Szteke, eds., Polish Academy of Sciences, Warsaw, pp. 157–166 (1994) (in Polish).
G.-Q. Yang, L.-Z. Zhu, S.-J. Liu, et al., Human selenium requirement in China, in Selenium in Biology and Medicine, Part B, G. F. Combs, Jr., J. E. Spallholz, O. A. Levander, and J. E. Oldfield, eds., Van Nostrand Reinhold, New York, pp. 589–607 (1987).
A. Madaric, J. Kadrabova, and E. Ginter, Selenium concentration in plasma and erythrocytes in healthy Slovak population, J. Trace Elements Electrolytes Health Dis. 8, 43–47 (1994).
Z. Maksimovic, V. Jovic, I. Djujic, et al., Selenium deficiency in Yugoslavia and possible effects on health, Environ. Geochem. Health 14, 107–111 (1992).
B. Tiran, A. Tiran, W. Petek, et al., Selenium status of healthy children and adults in Styria (Austria). Trace Elements Med. 9, 75–79 (1992).
J. Kalouskova, J. Dedina, L. Pavlik, et al., Selenium concentration in blood of the Czech Population, Cas. Lek. Ces. 127, 277–281 (1987).
G. Alfthan, G. Bogye, A. Aro, et al., The human selenium status in Hungary, J. Trace Elements Electrolytes Health Dis. 6, 233–238 (1992).
M. Bonomini, S. Forster, F. de Risio, et al., Effects on selenium supplementation on immune parameters in chronic uraemic patients on hemodialysis, Nephrol. Dial. Transplant. 10, 1654–1661 (1995).
K. Milly, L. Witt, C. Diskin, et al., Selenium in renal failure patients. Nephron 61, 139–144 (1992).
J. M. Marchante-Gayon, J. E. Sanchez-Uria, and A. Sanz-Medel, Serum and tissue selenium contents related to renal disease and colon cancer as determined by electrothermal atomic absorption spectrometry, J. Trace Elements Med. Biol. 10, 229–236 (1996).
J. T. Deagan, J. A. Butler, B. A. Zachara, et al., Determination of the distribution of selenium between glutathione peroxidase, selenoprotein P, and albumin in plasma, Anal. Biochem. 208, 176–181 (1993).
K. A. Temple, A. M. Smith, and D. B. Cockram, Selenate-supplemented nutritional formula increases plasma selenium in hemodialysis patients, J. Renal Nutr. 10, 16–23 (2000).
H. Koyama, K. Omura, A. Ejima, et al., Separation of selenium-containing proteins in human and mouse plasma using tandem high-performance liquid chromatography columns coupled with inductively coupled plasma-mass spectrometry, Anal. Biochem. 267, 84–91 (2000).
M. Persson Moschos, Selenoprotein P. Cell. Mol. Life Sci. 57, 1836–1845 (2000).
R. Read, T. Bellow, J.-G. Yang, et al., Selenium and amino acid composition of selenoprotein P, the major selenoprotein in rat serum, J. Biol. Chem. 265, 17,899–17,905 (1990).
M. J. Richard, J. Arnaud, K. Sirajeddine, et al., Zinc, selenium and lipid peroxides levels in hemodialyzed patients, in Trace Elements in Man and Animals 7 (TEMA-7), B. Momcilovic (ed.), University of Zagreb, Zagreb, pp. 12.7–12.9 (1991).
G. Bellisola, G. C. Guidi, G. Cinque, et al., Selenium status and plasma glutathione peroxidase in patients with IgA nephropathy, J. Trace Elements Med. Biol. 10, 189–196 (1996).
J. C. Whitin, D. M. Tham, S. Bhamre, et al., Plasma glutathione peroxidase and its relationship to renal proximal tubule function, Mol. Genet. Metab. 65, 238–245 (1998).
K. M. Brown and J. H. Arthur, Selenium, selenoproteins and human health: a review, Public Health Nutr. 4, 593–599 (2001).
J. R. Arthur, The glutathione peroxidases, Cell. Mol. Life Sci. 57, 1825–1835 (2002).
M. D. Saint-Georges, D. J. Bonnefont, B. A. Bourlery, et al., Correction of selenium deficiency in hemodialyzed patients, Kidney Int. 36 (Suppl. 27), S-274–S-277 (1989).
G. Bellisola, G. Perona, S. Galassini, et al., Plasma selenium and glutathione peroxidase activities in individuals living in the Veneto region of Italy, J. Trace Elements Electrolytes Health Dis. 7, 242–244 (1993).
J. S. Koenig, M. Fischer, E. Bulant, et al., Antioxidant status in patients on chronic hemodialysis therapy: impact of parenteral selenium supplementation, Wien. Klin. Wochenschr. 109, 13–19 (1997).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Zachara, B.A., Koterska, D., Manitius, J. et al. Selenium supplementation on plasma glutathione peroxidase activity in patients with end-stage chronic renal failure. Biol Trace Elem Res 97, 15–30 (2004). https://doi.org/10.1385/BTER:97:1:15
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1385/BTER:97:1:15