Abstract
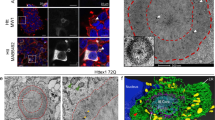
Although expansion of a polyglutamine tract in the huntingtin protein is known to cause Huntington’s disease (HD), there is considerable debate as to how this mutation leads to the selective neuronal loss that characterizes the disease. The observation that mutant huntingtin accumulates in neuronal nuclei has led to the hypothesis that the molecular mechanism may involve the disruption of specific nuclear activities. Recently, several nuclear interaction partners for huntingtin have been identified, including HypA, a splicing factor-like protein of unknown function. Using a yeast two-hybrid screen, we have identified the interaction of HypA with the nuclear scaffold protein NAKAP. Interaction of NAKAP with HypA is specific and occurs both in yeast and in vitro. Deletion-mapping studies indicate that binding occurs via a proline-rich domain in NAKAP with a WW domain of HypA. In cultured cells, NAKAP and HypA localize within the nucleus and copurify with the nuclear matrix. Furthermore, NAKAP associates with HypA from human brain and copurifies with huntingtin protein in brain tissue obtained from HD patients. In HD neurons, NAKAP and mutant huntingtin were colocalized to the nuclear matrix and were found to be components of nuclear aggregates. Hence, the NAKAP-HypA scaffold is a potential nuclear docking site for huntingtin protein and may contribute to the nuclear accumulation of huntingtin observed in HD.
Similar content being viewed by others
References
Akileswaran L., Taraska J. W., Sayer J. A., Gettemy J. M., and Coghlan V. M. (2001) A-kinase-anchoring protein AKAP95 is targeted to the nuclear matrix and associates with p68 RNA helicase. J. Biol. Chem. 276, 17,448–17,454.
Barrack E. R. and Coffey D. S. (1982) Biological properties of the nuclear matrix: steroid hormone binding. Recent Prog. Horm. Res. 38, 133–195.
Bates G. P. (2001) Huntington’s disease. Exploiting expression. Nature 413, 691–694.
Becker M. W., Kotzuk J. A., Sharp, A. H., et al. (1998) Intranuclear neuronal inclusions in Huntington’s disease and dentatorubral and pallidoluysian atrophy: correlation between the density of inclusions and IT15 CAG triplet repeat length. Neurobiol. Dis. 4, 387–397.
Berezney R. and Coffey D. S. (1974) Identification of a nuclear protein matrix. Biochem. Biophys. Res. Commun. 60, 1410–1417.
Berezney R., Mortillaro M., Ma H., Wei X., and Samarabandu J. (1995) The nuclear matrix: a structural milieu for genomic function, In: Berezney R. and Jeon K. W. (eds) International Reviews of Cytology, New York: Academic Press, Vol. 162A, pp. 1–65.
Boutell J. M., Thomas P., Neal J. W., et al. (1999) Aberrant interactions of transcriptional repressor proteins with the Huntington’s disease gene product, huntingtin. Hum. Mol. Genet. 8, 1647–1655.
Coghlan V. M. (1998) In: Hames B. D. (ed), Gel Electrophoresis of Proteins—A Practical Approach, Oxford: IRL Press, pp. 293–311.
Coghlan V. M., Langeberg L. K., Fernandez A., Lamb N. J. C., and Scott J. D. (1994) Cloning and characterization of AKAP95, a nuclear protein that associates with the regulatory subunit of type II cAMP-dependent protein kinase. J. Biol. Chem. 269, 7658–7665.
Coghlan V. M., Perrino B. A., Howard M., et al. (1995) Association of protein kinase A and protein phosphatase 2B with a common anchoring protein. Science 267, 108–111.
Criqui-Filipe P., Ducret C., Maira S. M., and Wasylyk B. (1999) Net, a negative Ras-switchable TCF, contains a second inhibition domain, the CID, that mediates repression through interactions with CtBP and de-acetylation. EMBO J. 18, 3392–3403.
Davie J. R., Samuel S. K., Spencer V. A., et al. (1999) Organization of chromatin in cancer cells: role of signalling pathways. Biochem. Cell Biol. 77, 265–275.
Davies S. W., Turmaine M., Cozens B. A., et al. (1997) Formation of neuronal intranuclear inclusions underlies the neurological dysfunction in mice transgenic for the HD mutation. Cell 90, 537–548.
DiFiglia M., Sapp E., Chase K., et al. (1995) Huntingtin is a cytoplasmic protein associated with vesicles in human and rat brain neurons. Neuron 14, 1075–1081.
DiFiglia M., Sapp E., Chase K. O., et al. (1997) Aggregation of huntingtin in neuronal intranuclear inclusions and dystrophic neurites in brain. Science 277, 1990–1993.
Duttweiler H. M. (1996) A highly sensitive and nonlethal beta-galactosidase plate assay for yeast. Trends Genet. 12, 340–341.
Eide T., Coghlan V. M., Orstavik S., et al. (1998) Molecular cloning, chromosomal localization, and cell cycle-dependent subcellular distribution of the A-kinase anchoring protein, AKAP95. Exp. Cell Res. 238, 305–316.
Faber P. W., Barnes G. T., Srinidhi J., Chen J., Gusella J. F., and MacDonald M. E. (1998) Huntingtin interacts with a family of WW domain proteins. Hum. Mol. Genet. 7, 1463–1474.
Feuerstein N., Chan P. K., and Mond J. J. (1988) Identification of numatrin, the nuclear matrix protein associated with induction of mitogenesis, as the nucleolar protein B23. Implication for the role of the nucleolus in early transduction of mitogenic signals. J. Biol. Chem. 263, 10,608–10,612.
Fey E. G., Krochmalnic G., and Penman S. (1986) The nonchromatin substructures of the nucleus: the ribonucleoprotein (RNP)-containing and RNP-depleted matrices analyzed by sequential fractionation and resinless section electron microscopy. J. Cell Biol. 102, 1654–1665.
Folstein S. E. (1990) Huntington’s Disease. Baltimore: Johns Hopkins University Press.
Freiman R. N. and Tjian R. (2002) Neurodegeneration. A glutamine-rich trail leads to transcription factors. Science 296, 2149–2150.
Gutekunst C. A., Levey A. I., Heilman C. J., et al. (1995) Identification and localization of huntingtin in brain and human lymphoblastoid cell lines with anti-fusion protein antibodies. Proc. Natl. Acad. Sci. USA 92, 8710–8714.
Harjes P. and Wanker E. E. (2003) The hunt for huntingtin function: interaction of partners tell many different stories. Trends Biochem. Sci. 28, 425–433.
Hodgson J. G., Agopyan N., Gutekunst C-A., et al. (1999) A YAC mouse model for Huntington’s disease with full-length mutant huntingtin, cytoplasmic toxicity, and selective striatal neurodegeneration. Neuron 23, 181–192.
Huang C. C., Faber P. W., Persichetti F., et al. (1998) Amyloid formation by mutant huntingtin: threshold, progressivity and recruitment of normal polyglutamine proteins. Somat. Cell Mol. Genet. 24, 217–233.
Hughes J. H. and Cohen M. B. (1999) Nuclear matrix proteins and their potential applications to diagnostic pathology Am. J. Clin. Pathol. 111, 267–274.
Kegel K. B., Meloni A. R., Yi Y., et al. (2002) Huntingtin is present in the nucleus, interacts with the transcriptional corepressor C-terminal binding protein, and represses transcription. J. Biol. Chem. 277, 7466–7476.
Khoshnan A., Ko J., and Patterson P. H. (2002) Effects of intracellular expression of anti-huntingtin antibodies of various specificities on mutant huntingtin aggregation and toxicity. Proc. Natl. Acad. Sci. USA 99, 1002–1027.
Ko J., Ou S., and Patterson P. H. (2001) New anti-huntingtin monoclonal antibodies: implications for huntingtin conformation and its binding proteins. Brain Res. Bull. 56, 319–329.
Koipally J. and Georgopoulos K. (2000) Ikaros interactions with CtBP reveal a repression mechanism that is independent of histone deacetylase activity. J. Biol. Chem. 275, 19,594–19,602.
Laforet G. A., Sapp E., Chase K., et al. (2001) Changes in cortical and striatal neurons predict behavioral and electrophysiological abnormalities in a transgenic murine model of Huntington’s disease. J. Neurosci. 21, 9112–9123.
Li S. -H. and Li X. -J. (2004) Huntingtin-protein interactions and the pathogenesis of Huntington’s disease. Trends Genet. 20, 146–154.
Manczak M., Park B. S., Jung Y., and Reddy P. H. (2004) Differential expression of oxidative phosphorylation genes in patients with Alzheimer’s disease. Neuromol. Med. 5, 147–163.
Martins S. B., Eide T., Steen R. L., Jahnsen T., Skalhegg B. S., and Collas P. (2000) HA95 is a protein of the chromatin and nuclear matrix regulating nuclear envelope dynamics. J. Cell Sci. 113, 3703–3713.
Meloni A. R., Smith E. J., and Nevins J. R. (1999) A mechanism for Rb/p130-mediated transcription repression involving recruitment of the CtBP corepressor. Proc. Natl. Acad. Sci. USA 96, 9574–9579.
Nakajima T., Uchida C., Anderson S. F., et al. (1997) RNA Helicase A mediates association of CBP with RNA polymerase II. Cell 90, 1107–1112.
Nelson W. G., Pienta K. J., Barrack E. R., and Coffey D. S. (1986) The role of the nuclear matrix in the organization and function of DNA. Ann. Rev. Biophys. Biophys. Chem. 15, 457–475.
Neri C. (2001) New light on polyglutamine neurodegenerative disorders: interference with transcription. Trends Mol. Med. 7, 283–284.
Neuwald A. F. and Hirano T. (2000) HEAT repeats associated with condensins, cohesins, and other complexes involved in chromosome-related functions. Genome Res. 10, 1445–1452.
Nucifora F. C., Sasaki M., Peters M. F., et al. (2001) Interference by huntingtin and atrophin-1 with cbp-mediated transcription leading to cellular toxicity. Science 291, 2423–2428.
Orstavik S., Eide T., Collas P., et al. (2000) Identification, cloning and characterization of a novel nuclear protein, HA95, homologous to A-kinase anchoring protein 95. Biol. Cell 92, 27–37.
Passani L. A., Bedford M. T., Faber P. W., et al. (2000) Huntingtin’s WW domain partners in Huntington’s disease post-mortem brain fulfill genetic criteria for direct involvement in Huntington’s disease pathogenesis. Hum. Mol. Genet. 9, 2175–2182.
Perez M. K., Paulson H. L., Pendse S. J., Saionz S. J., Bonini N. M., and Pittman R. N. (1998) Recruitment and the role of nuclear localization in polyglutamine-mediated aggregation. J. Cell Biol. 143, 1457–1470.
Pienta K. J., Partin A. W., and Coffey D. S. (1989) Cancer as a disease of DNA organization and dynamic cell structure Cancer Res. 49, 2525–2532.
Pujol M. J., Bosse R., Vendrell M., Serratosa J., and Bachs O. (1993) Nuclear calmodulin-binding proteins in rat neurons J. Neurochem. 60, 1422–1428.
Reddy P. H., Williams M., and Tagle D. A. (1999) Recent advances in understanding the pathogenesis of Huntington’s disease. Trends Neurosci. 22, 248–255.
Reddy P.H., Williams M., Charles V., et al. (1998) Behavioural abnormalities and selective neuronal loss in HD transgenic mice expressing mutated full-length HD cDNA. Nat. Genet. 20, 198–202.
Replogle-Schwab R., Pienta K. J., and Getzenberg R. H. (1996) The utilization of nuclear matrix proteins for cancer diagnosis. Crit. Rev. Euk. Gene Expr. 6, 103–113.
Rossow K. L. and Janknecht R. (2003) Synergism between p68 RNA helicase and the transcriptional coactivators CBP and p300. Oncogene 22, 151–156.
Schilling G., Becher M. W., Sharp A. H., et al. (1999) Intranuclear inclusions and neuritic aggregates in transgenic mice expressing a mutant N-terminal fragment of huntingtin. Hum. Mol. Genet. 8, 397–407.
Seki N., Ueki N., Yano K., Saito T., Masuho Y., and Muramatsu M. (2000) cDNA cloning of a new member of the Ras superfamily, RAB9-like, on the human chromosome Xq22.1-q22.3 region. J. Hum. Genet. 45, 31–37.
Sharp A. H., Loev S. J., Schilling G., et al. (1995) Widespread expression of Huntington’s disease gene (IT15) protein product. Neuron 14, 1064–1075.
Shimohata T., Nakajima T., Yamada M., et al. (2000) Expanded polyglutamine stretches interact with TAFII130, interfering with CREB-dependent transcription. Nat. Genet. 26, 29–36.
Skinner P. J., Koshy B. T., Cummings C. J., et al. (1997) Ataxin-1 with an expanded glutamine tract alters nuclear matrix-associated structures. Nature 389, 971–974.
Staufenbiel M. and Deppert W. (1983) Different structural systems of the nucleus are targets for SV40 large T antigen. Cell 33, 173–181.
Steffan J. S., Kazantsev A., Spasic-Boskovic O., et al. (2000) The Huntington’s disease protein interacts with p53 and CREB-binding protein and represses transcription. Proc. Natl. Acad. Sci. USA 97, 6763–6768.
Vonsattel J. P., Myers R. H., Stevens T. J., Ferrante R. J., Bird E. D., and Richardson E. P., Jr. (1985) Neuropathological classification of Huntington’s disease. J. Neuropathol. Exp. Neurol. 44, 559–577.
Yang J. P., Tang H., Reddy T. R., and Wong-Staal F. (2001) Mapping the functional domains of HAP95, a protein that binds RNA helicase A and activates the constitutive transport element of type D retroviruses. J. Biol. Chem. 276, 30,694–30,700.
Zini N., Martelli A. M., Neri L. M., et al. (1995) Immunocytochemical evaluation of protein kinase C translocation to the inner nuclear matrix in 3T3 mouse fibroblasts after IGF-I treatment. Histochem. Cell Biol. 103, 447–457.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sayer, J.A., Manczak, M., Akileswaran, L. et al. Interaction of the nuclear matrix protein NAKAP with HypA and huntingtin. Neuromol Med 7, 297–310 (2005). https://doi.org/10.1385/NMM:7:4:297
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1385/NMM:7:4:297