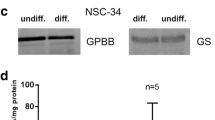
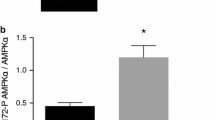
Abstract
Adenosine monophosphate-activated protein kinase (AMPK) is a member of metabolite-sensing kinase family that plays important roles in responses of muscle cells to metabolic stress. AMPK is a heterotrimer of a catalytic α subunit (α1 or α2), and β (β1 or β2) and γ (γ1 or γ2) subunits. Because the brain has a high metabolic rate and is sensitive to changes in the supply of glucose and oxygen, we investigated the expression of AMPK in rat embryonic and adult brain and its role in modifying neuronal survival under conditions of cellular stress. We report that catalytic (α1 and α2) and noncatalytic (β2 and γ1) subunits of AMPK are present at high levels in embryonic hippocampal neurons in vivo and in cell culture. In the adult rat brain, the catalytic subunits α1 and α2 are present in neurons throughout the brain. The AMPK-activating agent AICAR protected hippocampal neurons against death induced by glucose deprivation, chemical hypoxia, and exposure to glutamate and amyloid β-peptide. Suppression of levels of the AMPK α1 and α2 subunits using antisense technology resulted in enhanced neuronal death following glucose deprivation, and abolished the neuroprotective effect of AICAR. These findings suggest that AMPK can protect neurons against metabolic and excitotoxic insults relevant to the pathogenesis of several different neurodegenerative conditions.
Similar content being viewed by others
References
Bruce-Keller, A. J., Li, Y. J., Lovell, M. A., Kraemer, P. J., Gary, D. S., Brown, R. R., et al. (1998) 4-Hydroxynonenal, a product of lipid peroxidation, damages cholinergic neurons and impairs visuospatial memory in rats. J. Neuropathol. Exp. Neurol. 57, 257–267.
Carling, D. and Hardie, D. G. (1989) The substrate and sequence specificity of the AMP-activated protein kinase. Phosphorylation of glycogen synthase and phosphorylase kinase. Biochim. Biophys. Acta. 1012, 81–86.
Carling, D., Zammit, V. A., and Hardie, D. G. (1987) A common bicyclic protein kinase cascade inactivates the regulatory enzymes of fatty acid and cholesterol biosynthesis. FEBS Lett. 223, 217–222.
Cheng, B. and Mattson, M. P. (1991) NGF and bFGF protect rat and human central neurons against hypoglycemic damage by stabilizing calcium homeostasis. Neuron 7, 1031–1041.
Clough-Helfman, C. and Phillis, J. W. (1990) Brain 5-Aminoimidazole-4-carboxamide riboside (AICAR) administration reduces cerebral ischemic damage in the Mongolian gerbil. Brain Res. Bull. 25, 203–206.
Corton, J. M., Gillespie, J. G., and Hardie, D. G. (1994) Role of the AMP-activated protein kinase in the cellular stress response. Curr. Biol. 4, 315–324.
Corton, J. M., Gillespie, J. G., Hawley, S. A., and Hardie, D. G. (1995) 5-aminoimidazole-4-carboxamide ribonucleoside. A specific method for activating AMP-activated protein kinase in intact cells? Eur. J. Biochem. 229, 558–565.
Davies, S. P., Helps, N. R., Cohen, P. T., and Hardie, D. G. (1995) 5′-AMP inhibits dephosphorylation, as well as promoting phosphorylation, of the AMP-activated protein kinase. Studies using bacterially expressed human protein phosphatase-2C alpha and native bovine protein phosphatase-2AC. FEBS Lett. 377, 421–425.
Endres, M., Laufs, U., Huang, Z., Nakamura, T., Huang, P., Moskowitz, M. A., and Liao, J. K. (1998) Stroke protection by 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase inhibitors mediated by endothelial nitric oxide synthase. Proc. Natl. Acad. Sci. USA 95, 8880–8885.
Ferrer, A., Caelles, C., Massot, N., and Hegardt, F. G. (1985) Activation of rat liver cytosolic 3-hydroxy-3-methylglutaryl coenzyme A reductase kinase by adenosine 5′-monophosphate. Biochem. Biophys. Res. Commun. 132, 497–504.
Foretz, M., Carling, D., Guichard, C., Ferre, P., and Foufelle, F. (1998) AMP-activated protein kinase inhibits the glucose-activated expression of fatty acid synthase gene in rat hepatocytes. J. Biol. Chem. 273, 14,767–14,771.
Galinanes, M., Bullough, D., Mullane, K. M., and Hearse, D. J. (1992a) Sustained protection by acadesine against ischemia- and reperfusion-induced injury. Studies in the transplanted rat heart. Circulation 86, 589–597.
Galinanes, M., Mullane, K. M., Bullough, D., and Hearse, D. J. (1992b) Acadesine and myocardial protection. Studies of time of administration and dose-response relations in the rat. Circulation 86, 598–608.
Galinanes, M., Zhai, X., Bullough, D., Mullane, K. M., and Hearse, D. J. (1995) Protection against injury during ischemia and reperfusion by acadesine derivatives GP-1-468 and GP-1-668. Studies in the transplanted rat heart. Thorac. Cardiovasc. Surg. 110, 752–761.
Gao, G., Fernandez, C. S., Stapleton, D., Auster, A. S., Widmer, J., Dyck, J. R., et al. (1996) Non-catalytic β-and γ-subunit isoforms of the 5′-AMP-activated protein kinase. J. Biol. Chem. 271, 8675–8681.
Gao, G., Widmer, J., Stapleton, D., Teh, T., Cox, T., Kemp, B. E., and Witters, L. A. (1995) Catalytic subunits of the porcine and rat 5′-AMP-activated protein kinase are members of the SNF1 protein kinase family. Biochim. Biophys. Acta 1266, 73–82.
Garton, A. J., Campbell, D. G., Carling, D., Hardie, D. G., Colbran, R. J., and Yeaman, S. J. (1989) Phosphorylation of bovine hormone-sensitive lipase by the AMP-activated protein kinase. A possible antilipolytic mechanism. Eur. J. Biochem. 179, 249–254.
Goodman, Y. and Mattson, M. P. (1994) Secreted forms of β-amyloid precursor protein protect hippocampal neurons against amyloid β-peptide-induced oxidative injury. Exp. Neurol. 128, 1–12.
Guo, Q., Fu, W., Xie, J., Luo, H., Sells, S. F., Geddes, J. W., Bondada, V., Rangnekar, V. M., and Mattson, M. P. (1998) Par-4 is a mediator of neuronal degeneration associated with the pathogenesis of Alzheimer disease. Nat. Med. 4, 957–962.
Hardie, D. G. and Carling, D. (1997) The AMP-activated protein kinase: fuel gauge of the mammalian cell? Eur. J. Biochem. 246, 259–273.
Hawley, S. A., Davison, M., Woods, A., Davies, S. P., Beri, R. K., Carling, D., and Hardie, D. G. (1996) Characterization of the AMP-activated protein kinase kinase from rat liver and identification of threonine 172 as the major site at which it phosphorylates AMP-activated protein kinase. J. Biol. Chem. 271, 27,879–27,887.
Henin, N., Vincent, M. F., Gruber, H. E., and Van den Berghe, G. (1995) Inhibition of fatty acid and cholesterol synthesis by stimulation of AMP-activated protein kinase. FASEB J. 9, 541–546.
Kemp, B. E., Mitchelhill, K. I., Stapleton, D., Michell, B. J., Chen, Z. P., and Witters, L. A. (1999) Dealing with energy demand: the AMP-activated protein kinase. Trends Biochem. Sci. 24, 22–25.
Kingma, J. G. Jr., Simard, D., and Rouleau, J. R. (1994) Timely administration of AICA riboside reduces reperfusion injury in rabbits. Cardiovasc. Res. 28, 1003–1007.
Kruman, I., Bruce-Keller, A. J., Bredesen, D. E., Waeg, G., and Mattson, M. P. (1997) Evidence that 4-hydroxynonenal mediates oxidative stress-induced neuronal apoptosis. J. Neurosci. 17, 5089–5100.
Kudo, N., Gillespie, J. G., Kung, L., Witters, L. A., Schulz, R., Clanachan, A. S., and Lopaschuk, G. D. (1996) Characterization of 5′AMP-activated protein kinase activity in the heart and its role in inhibiting acetyl-CoA carboxylase during reperfusion following ischemia. Biochim. Biophys. Acta 1301, 67–75.
Kuo, W. L., Abe, M., Rhee, J., Eves, E. M., McCarthy, S. A., Yan, M., et al. (1996) Raf, but not MEK or ERK, is sufficient for differentiation of hippocampal neuronal cells. Mol. Cell. Biol. 16, 1458–1470.
Leclerc, I., Kahn, A., and Doiron, B. (1998) The 5′-AMP-activated protein kinase inhibits the transcriptional stimulation by glucose in liver cells, acting through the glucose response complex. FEBS Lett. 431, 180–184.
Mark, R. J., Hensley, K., Butterfield, D. A., and Mattson, M. P. (1995) Amyloid β-peptide impairs ion-motive ATPase activities: evidence for a role in loss of neuronal Ca2+ homeostasis and cell death. J. Neurosci. 15, 6239–6249.
Martin, R. L., Lloyd, H. G., and Cowan, A. I. (1994) The early events of oxygen and glucose deprivation: setting the scene for neuronal death? Trends Neurosci. 17, 251–257.
Mattson, M. P., Guthrie, P. B., Hayes, B. C., and Kater, S. B. (1989) Roles for mitotic history in the generation and degeneration of hippocampal neuroarchitecture. J. Neurosci. 9, 1223–1232.
Mattson, M. P., Cheng, B., Davis, D., Bryant, K., Lieberburg, I., and Rydel, R. E. (1992) β-amyloid peptides destabilize calcium homeostasis and render human cortical neurons vulnerable to excitotoxicity. J. Neurosci. 12, 376–389.
Mattson, M. P., Zhang, Y., and Bose, S. (1993) Growth factors prevent mitochondrial dysfunction, loss of calcium homeostasis and cell injury, but not ATP depletion in hippocampal neurons deprived of glucose. Exp. Neurol. 121, 1–13.
Mattson, M. P., Lovell, M. A., Furukawa, K., and Markesbery, W. R. (1995) Neurotrophic factors attenuate glutamate-induced accumulation of peroxides, elevation of intracellular Ca2+ concentration, and neurotoxicity and increase antioxidant enzyme activities in hippocampal neurons. J. Neurochem. 65, 1740–1751.
Mattson, M. P., Goodman, Y., Luo, H., Fu, W., and Furukawa, K. (1997) Activation of NF-κB protects hippocampal neurons against oxidative stress-induced apoptosis: evidence for induction of manganese superoxide dismutase and suppression of peroxynitrite production and protein tyrosine nitration. J. Neurosci. Res. 49, 681–697.
Michell, B. J., Stapleton, D., Mitchellhill, K. I., House, C. M., Katsis, F., Witters, L. A., and Kemp, B. E. (1996) Isoform-specific purification and substrate specificity of the 5′-AMP-activated protein kinase. J. Biol. Chem. 271, 28,445–28,450.
Michikawa, M. and Yanagisawa, K. (1999) Inhibition of cholesterol production but not of nonsterol isoprenoid products induces neuronal cell death. J. Neurochem. 72, 2278–2285.
Mitchelhill, K. I., Stapleton, D., Gao, G., House, C., Michell, B., Katsis, F., et al. (1994) Mammalian AMP-activated protein kinase shares structural and functional homology with the catalytic domain of yeast Snf1 protein kinase. J. Biol. Chem. 269, 2361–2364.
Moore, F., Weekes, J., and Hardie, D. G. (1991) Evidence that AMP triggers phosphorylation as well as direct allosteric activation of rat liver AMP-activated protein kinase. A sensitive mechanism to protect the cell against ATP depletion. Eur. J. Biochem. 199, 691–697.
Ponticos, M., Lu, Q. L., Morgan, J. E., Hardie, D. G., Partridge, T. A., and Carling, D. (1998) Dual regulation of the AMP-activated protein kinase provides a novel mechanism for the control of creatine kinase in skeletal muscle. EMBO J. 17, 1688–1699.
Rami, A. and Krieglstein, J. (1993) Brain damage caused by ischemia: pathophysiological and pharmacological aspects. Dementia 4, 21–31.
Rasmussen, B. B. and Winder, W. W. (1997) Effect of exercise intensity on skeletal muscle malonyl-CoA and acetyl-CoA carboxylase. J. Appl. Physiol. 83, 1104–1109.
Sabina, R. L., Patterson, D., and Holmes, E. W. (1985) 5-Amino-4-imidazolecarboxamide riboside (Z-riboside) metabolism in eukaryotic cells. J. Biol. Chem. 260, 6107–6114.
Salt, I. P., Johnson, G., Ashcroft, S. J., and Hardie, D. G. (1998) AMP-activated protein kinase is activated by low glucose in cell lines derived from pancreatic beta cells, and may regulate insulin release. Biochem. J. 335, 533–539.
Smith-Swintosky, V. L., Pettigrew, L. C., Sapolsky, R. M., Phares, C., Craddock, S. D., Brooke, S. M., and Mattson, M. P. (1996) Metyrapone, an inhibitor of glucocorticoid production, reduces brain injury induced by focal and global ischemia and seizures. J. Cereb. Blood Flow Metab. 16, 585–598.
Sprenkle, A. B., Davies, S. P., Carling, D., Hardie, D. G., and Sturgill, T. W. (1997) Identification of Raf-1 Ser621 kinase activity from NIH 3T3 cells as AMP-activated protein kinase. FEBS Lett. 403, 254–258.
Stapleton, D., Gao, G., Michell, B. J., Widmer, J., Mitchelhill, K., Teh, T., et al. (1994) Mammalian 5′-AMP-activated protein kinase non-catalytic subunits are homologs of proteins that interact with yeast Snf1 protein kinase. J. Biol. Chem. 269, 29,343–29,346.
Stapleton, D., Mitchelhill, K. I., Gao, G., Widmer, J., Michell, B. J., Teh, T., et al. (1996) Mammalian AMP-activated protein kinase subfamily. J. Biol. Chem. 271, 611–614.
Stapleton, D., Woollatt, E., Mitchelhill, K. I., Nicholl, J. K., Fernandez, C. S., Michell, B. J., et al. (1997) AMP-activated protein kinase isoenzyme family: subunit structure and chromosomal location. FEBS Lett. 409, 452–456.
Stefanelli, C., Stanic, I., Bonavita, F., Flamigni, F., Pignatti, C., Guarnieri, C., and Caldarera, C. M. (1998) Inhibition of glucocorticoid-induced apoptosis with 5-aminoimidazole-4-carboxamide ribonucleoside, a cell-permeable activator of AMP-activated protein kinase. Biochem. Biophys. Res. Commun. 243, 821–826.
Sullivan, J. E., Brocklehurst, K. J., Marley, A. E., Carey, F., Carling, D., and Beri, R. K. (1994) Inhibition of lipolysis and lipogenesis in isolated rat adipocytes with AICAR, a cell-permeable activator of AMP-activated protein kinase. FEBS Lett. 353, 33–36.
Tsuchida, A., Yang, X. M., Burckhartt, B., Mullane, K. M., Cohen, M. V., and Downey, J. M. (1994) Acadesine extends the window of protection afforded by ischaemic preconditioning. Cardiovasc. Res. 28, 379–383.
Turnley, A. M., Stapleton, D., Mann, R. J., Witters, L. A., Kemp, B. E., and Bartlett, P. F. (1999) Cellular distribution and developmental expression of AMP-activated protein kinase isoforms in mouse central nervous system. J. Neurochem. 72, 1707–1716.
Vavvas, D., Apazidis, A., Saha, A. K., Gamble, J., Patel, A., Kemp, B. E., et al. (1997) Contraction-induced changes in acetyl-CoA carboxylase and 5′-AMP-activated kinase in skeletal muscle. J. Biol. Chem. 272, 13,255–13,261.
Velasco, G., Geelen, M. J., and Guzman, M. (1997) Control of hepatic fatty acid oxidation by 5′-AMP-activated protein kinase involves a malonyl-CoA-dependent and a malonyl-CoA-independent mechanism. Arch. Biochem. Biophys. 337, 169–175.
Wang, H. G., Rapp, U. R., and Reed, J. C. (1996) Bcl-2 targets the protein kinase Raf-1 to mitochondria. Cell 87, 629–638.
Wang, H. G. and Reed, J. C. (1998) Bc1-2, Raf-1 and mitochondrial regulation of apoptosis. Biofactors 8, 13–16.
Weekes, J., Ball, K. L., Caudwell, F. B., and Hardie, D. G. (1993) Specificity determinants for the AMP-activated protein kinase and its plant homologue analysed using synthetic peptides. FEBS Lett. 334, 335–339.
Yu, Z., Bruce-Keller, A. J., Goodman, Y., and Mattson, M. P. (1998) Uric acid protects neurons against excitotoxic and metabolic insults in cell culture, and against focal ischemic brain injury in vivo. J. Neurosci. Res. 53, 613–625.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Culmsee, C., Monnig, J., Kemp, B.E. et al. AMP-activated protein kinase is highly expressed in neurons in the developing rat brain and promotes neuronal survival following glucose deprivation. J Mol Neurosci 17, 45–58 (2001). https://doi.org/10.1385/JMN:17:1:45
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1385/JMN:17:1:45