Abstract
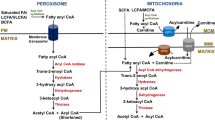
Peroxisomal fatty acid oxidation enzymes are summarized in comparison to their mitochondrial counterparts. The peroxisomal enzymes involved in the β-oxidation spiral are schematically classified into two groups. The first group consists of hitherto purified and characterized classical enzymes: palmitoyl-CoA oxidase, the L-bifunctional protein, and 3-ketoacyl-CoA. These enzymes are inducible and act on the straight chain substrates. The second group consists of recently identified enzymes, branched-chain oxidase, the d-bifunctional protein, and sterolcarrier protein x, which catalyze four reactions of β-oxidation cycle. These are noninducible and act on branched-chain substrates.
Similar content being viewed by others
References
Lazarow, P. B. and de Duve, C. (1976) A fatty acyl-CoA oxidizing system in rat liver peroxisomes: enhancement by clofibrate, a hypolipidemic drug. Proc. Natl. Acad. Sci. USA 73, 2043–2046.
Izai, K., Uchida, Y., Orii, T., Yamamoto, S., and Hashimoto, T. (1992) Novel fatty acid β-oxidation enzymes in rat liver mitochondria. I. Purification and properties of very-long-chain acyl-coenzyme A dehydrogenase. J. Biol. Chem. 267, 1027–1033.
Uchida, Y., Izai, K., Orii, T., and Hashimoto, T. (1992) Novel fatty acid β-oxidation enzymes in rat liver mitochondria. 2. Purification and properties of enoyl-coenzyme A (CoA) hydratase/3-hydroxyacyl-CoA dehydrogenase/3-ketoacyl-CoA thiolase trifunctional protein. J. Biol. Chem. 267, 1034–1041.
Shindo, Y. and Hashimoto, T. (1978) Acyl-coenzyme synthetase and fatty acid oxidation in rat liver peroxisomes. J. Biochem. 87, 1177–1181.
Miyazawa, S., Hashimoto, T., and Yokota, S. (1985) Identity of long-chain acyl-CoA synthetase of microsomes, mitochondria, and peroxisomes in rat liver. J. Biochem. 98, 723–733.
Miyazawa, S., Ozasa, H., Osumi T., and Hashimoto, T. (1983) Purification and properties of carnitine octanoyltransferase and carnitine palmitoyltransferase. J. Biochem. 94, 529–542
Hashimoto, T. (1982) Individual peroxisomal β-oxidation enzymes. Ann. NY Acad. Sci. 386, 5–12.
Hashimoto, T. (1996) Peroxisomal β-oxidation: enzymology and molecular biology. Ann. NY Acad. Sci. 804, 86–98.
Schepers, L., van Veldhoven, P. P., Casteels, M., Eyssen, H. J., and Mannaerts, G. P. (1990) Presence of three acyl-CoA oxidases in rat liver peroxisomes. An inducible fatty acyl-CoA oxidase, a non-inducible fatty acyl-CoA oxidase, and a non-inducible trihydroxycoprostanoyl-CoA oxidase. J. Biol. Chem. 265, 5242–5246.
Casteels, M., Schepers, L., Van Veldhoven, P. P., Eyssen, H. J., and Mannaerts, G. P. (1990) Separate peroxisomal oxidases for fatty acyl-CoAs and trihydroxycoprostanoyl-CoA in human liver. J. Lipid. Res. 31, 1865–1872.
Jiang, L. L., Miyazawa, S., and Hashimoto, T. (1996) Purification and properties of rat D-3-hydroxyacyl-CoA dehydratase/D-3-hydroxy-acyl-CoA dehydrogenase bifunctional protein. J. Biochem. 120, 633–641.
Seedorf, U., Brysch, P., Engel, T., Schrage, K., and Assmann, G. (1994) Sterol carrier protein x is proxisomal 3-oxoacyl coenzyme A thiolase with intrinsic sterol carrier and lipid transfer activity. J. Biol. Chem. 269, 21277–21283.
Uchida, Y., Kondo, N., Orii, T., and Hashimoto, T. (1996) Purification and properties of rat liver peroxisomal very-long-chain acyl-CoA synthetase. J. Biochem. 199, 565–571.
Uchiyama, A., Aoyama, T., Kamijo, K., Uchida, Y., Kondo, N., Orii, T., and Hashimoto, T. (1996) Molecular cloning of cDNA encoding rat very long-chain acyl-CoA synthetase. J. Biol. Chem. 271, 30,360–30,365.
Suzuki, H., Kawarabayashi, Y., Kondo, J., Abe, T., Nishikawa, K., Kimura, S., Hashimoto, T., and Yamamoto, T. (1990) Structure and regulation of rat long-chain acyl-CoA synthetase. J. Biol. Chem. 265, 8681–8685.
Schaffer, J. E. and Lodish, H. F. (1994) Expression cloning and characterization of novel adipocyte long chain fatty acid transport protein. Cell 79, 427–436.
Osumi, T., Hashimoto, T., and Ui, N. (1980) Purification and properties of acyl-CoA oxidase from rat liver. J. Biochem. 87, 1735–1746.
Miyazawa, S., Hayashi, H., Hijikata, M., Ishii, N., Furuta, S., Kagamiyama, H., Osumi, T., and Hashimoto, T. (1987) Complete nucleotide sequence of cDNA and predicted amino acid sequence of rat acyl-CoA oxidase. J. Biol. Chem. 262, 8131–8137.
Osumi, T., Ishii, N., Miyazawa, S., and Hashimoto, T. (1987) Isolation and structural characterization of the rat acyl-CoA oxidase gene. J. Biol. Chem. 262, 8138–8143.
Varanasi, U., Chu, R., Chu, S., Espinosa, R., LeBeau, M., and Reddy, J. K. (1994) Isolation of the human peroxisomal acyl-CoA oxidase gene: organization, promotor analysis, and chromosomal localization. Proc. Natl. Acad. Sci. USA 91, 3107–3111.
Fournier, B., Saudubray, J. M., Benichou, B., Lyonnet, S., Munnich, A., Clevers, H., and Poll-Thé, B. T. (1994) Large deletion of the peroxisomal acyl-CoA oxidase gene in pseudoneonatal adrenoleukodystrophy. J. Clin. Invest. 94, 526–531.
Aoyama, T., Tsushima, K., Souri, M., Kamijo, T., Suzuki, Y., Shimozawa, N., Orii, T., and Hashimoto, T. (1994) Molecular cloning and functional expression of human peroxisomal acyl-coenzyme A oxidase. Biochem. Biophys. Res. Commun. 198, 1113–1118.
Miyazawa, S., Osumi, T., Hashimoto, T., Ohno, K., Miura, S., and Fujiki, Y. (1989) Peroxisome targeting signal of rat liver acyl-coenzyme A oxidase resides at the carboxy terminus. Mol. Cell. Biol. 9, 83–91.
Van Veldhoven, P. P., Vanhove, G., Asselberghs, S., Eyssen, H. J., and Mannaerts, G. P. (1992) Substrate specificities of rat peroxisomal acyl-CoA oxidases: palmitoyl-CoA oxidase (inducible acyl-CoA oxidase), pristanoyl-CoA oxidase (non-inducible acyl-CoA oxidase) and trihydroxycoprostanoyl-CoA oxidase. J. Biol. Chem. 267, 20,065–20,074.
Vanhove, G. F., van Veldhoven, P. P., Fransen, M., Denis, S., Eyssen, H. J., Wanders, R. J. A., and Mannaerts, G. P. (1993) The CoA esters of 2-methyl-branched chain fatty acids and of the bile acid intermediates di- and trihydroxycoprostanoic acids are oxidized by one single peroxisomal branched chain acyl-CoA oxidase in human liver and kidney. J. Biol. Chem. 268, 10,335–10,344.
Matsubara, Y., Krauss, J., Ozasa, H., Glassberg, R., Finocchiaro, G., Ikeda, Y., et al. (1987) Molecular cloning and nucleotide sequence of cDNA encoding the entire precursor of rat liver medium chain acyl coenzyme A dehydrogenase. J. Biol. Chem. 262, 10,104–10,108.
Matsubara, Y., Indo, Y., Naito, E., Ozasa, H., Glassberg, R., Vockley, J. et al. (1989) Molecular cloning and nucleotide sequence of cDNAs encoding the precursors of rat long chain acyl-coenzyme A, short chain acyl-coenzyme A, and isovaleryl-coenzyme A dehydrogenases: Sequence homology of four enzymes of the acyl-CoA dehydrogenase family. J. Biol. Chem. 264, 16,321–16,331.
Aoyama, T., Ueno, I., Kamijo, T., and Hashimoto, T. (1994) Rat very-long-chain acyl-CoA dehydrogenase, a novel mitochondrial acyl-CoA dehydrogenase gene product, is rate-limiting enzyme in long-chain fatty acid β-oxidation system. J. Biol. Chem. 269, 19,088–19,094.
Baumgart, E., Vanhooren, J. C., Fransen, M., Marynen, P., Puype, M., Vandekerckhove, J. et al. (1996) Molecular characterization of the human peroxisomal branched-chain acyl-CoA oxidase: cDNA cloning, chromosomal assignment, tissue distribution, and evidence for the absence of the protein in Zellweger syndrome. Proc. Natl. Acad. Sci. USA 93, 13,748–13,753.
Baumgart, E., Vanhooren, J. C., Fransen, M., Van Leuven, F., Fahimi, H. D., van Veldhoven, P. P., and Mannaerts, G. P. (1996) Molecular cloning and further characterization of rat peroxisomal trihydroxycoprostanoyl-CoA oxidase. Biochem. J. 320, 115–121.
Vanhooren, J. C. T. T., Marynen, P., Mannaerts, G. P., and van Veldhoven, P. P. (1997) Evidende for the existence of a pristanoyl-CoA oxidase gene in man. Biochem. J. 325, 593–599.
Pawar, S. and Schulz, H. (1981) The structure of the multienzyme complex of fatty acid oxidation from Escherichia coli. J. Biol. Chem. 256, 3894–3899.
Imamura, S., Ueda, S., Mizugaki, M., and Kawaguchi, A. (1990) Purification of the multienzyme complex for fatty acid oxidation from Pseudomonas fragi and reconstitution of fatty acid oxidation system. J. Biochem. 107, 184–189.
Reddy, M. K., Usuda, N., Reddy, M. N., Kuczmarski, E. R., Rao, M. S., and Reddy, J. K. (1987) Purification, properties, and immunocytochemical localization of human liver peroxisomal enoyl-CoA hydratase/3-hydroxyacyl-CoA dehydrogenase. Proc. Natl. Acad. Sci. USA 84, 3214–3218.
Jiang, L. L., Kobayashi, A., Matsuura, H., Fukushima, H., and Hashimoto, T. (1996) Purification and Properties of human D-3-hydroxyacyl-CoA dehydratase: medium-chain enoyl-CoA hydratase is D-3-hydroxy-acyl-CoA dehydratase. J. Biochem. 120, 624–632.
Qin, Y. M., Poutanen, M. H., Helander, H. M., Kvist, A. P., Siivari, K. M., Schmitz, W., et al. (1997) Peroxisomal multifunctional enzyme of beta-oxidation metabolizing D-3-hydroxyacyl-CoA esters in rat liver: molecular cloning, expression and characterization. Biochem. J. 321, 21–28.
Dieuaide-Noubhani, M., Novikov, D., Baumgart, E., Vanhooren, J. C. T., Fransen, M., Goethals, M., et al. (1996) Further characterization of the peroxisomal 3-hydroxyacyl-CoA dehydrogenases from rat liver. Relationship between the different dehydrogenases and evidence that fatty acids and the C27-bile acids di- and trihydroxycoprostanoic acids are metabolized by separate multifunctional proteins. Eur. J. Biochem. 240, 660–666.
Jiang, L. L., Miyazawa, S., Souri, M., and Hashimoto, T. (1997) Structure of D-3-hydroxyacyl-CoA dehydratase/D-3-hydroxyacyl-CoA dehydrogenase bifunctional protein. J. Biochem. 121, 364–369.
Leenders, F., Tesdorpf, J. G., Markus, M., Engel, T., Seedorf, U., and Adamski, J. (1996) Porcine 80-kDa protein reveals intrinsic 17β-hydroxysteroid dehydrogenase, fatty acyl-CoA-hydratase/dehydrogenase, and sterol transfer activities. J. Biol. Chem. 271, 5438–5442.
Jiang, L. L., Miyazawa, S., and Hashimoto, T. (1996) Purification and properties of rat D-3-hydroxyacyl-CoA dehydratase: D-3-hydroxyacyl-CoA dehydratase/D-3-hydroxyacyl-CoA dehydrogenase bifunctional protein. J. Biochem. 120, 633–641.
Jiang, L. L., Kurosawa, T., Sato, M., Suzuki, Y., and Hashimoto, T. (1997) Physiological role of D-3-hydroxyacyl-CoA dehydratase/d-3-hydroxyacyl-CoA dehydrogenase bifunctional protein. J. Biochem. 12, 506–513.
Dieuaide-Noubhani, M., Novikov, D., Vandekerckhove, J., Veldhoven, P. P., and Mannaerts, J. P. (1997) Identification and characterization of the 2-enoyl-CoA hydratases involved in peroxisomal beta-oxidation in rat liver. Biochem. J. 321, 253–259.
Dieuaide-Noubhani, M., Asselberghs, S., Mannaerts, J. P., and Veldhoven, P. P. (1997) Evidence that multifunctional protein 2, and not multifunctional protein 1, is involved in the peroxisomal beta-oxidation of pristanic acid. Biochem. J. 325, 367–373.
Novikov, D., Dieuaide-Noubhani, M., Vermeesch, R., Fournier, B., Mannaerts, J. P., and Veldhoven, P. P. (1997) The human peroxisomal multifunctional protein involved in bile acid synthesis: activity measurement, deficiency in Zellweger syndrome and chromosome mapping. Biochim. Biophys. Acta 1360, 229–240.
Watkins, P. A., Chen, W. W., Harris, C. J., Hoefler, G., Hoefler, S., Blake, D. C., Jr., et al. (1989) Peroxisomal bifunctional enzyme deficiency. J. Clin. Invest. 83, 771–777.
Suzuki, Y, Jiang, L. L., Souri, M., Miyazawa, S., Fukuda, S., Zhang, Z., et al. (1997) D-3-hydroxyacyl-CoA dehydratase /D-3-hydroxyacly-CoA dehydrogenase bifunctional protein deficiency: a newly identified peroxisomal disorder. Am. J. Hum. Genet. 61, 1153–1162.
Natowicz M. R., Evans J. E., Kelley, R. I., Moser, A. B., Watkins, P. A., and Moser, H. W. (1996) Urinary bile acids and peroxisomal bifunctional enzyme deficiency. Am. J. Med. Genet. 63, 356–362.
Osumi, T., Ishii, N., Hijikata, M., Kamijo, K., Ozasa, H., Furuta, S., et al. (1985) Molecular cloning and nucleotide sequence of the cDNA for rat peroxisomal enoyl-CoA: hydratase-3-hydroxyacyl-CoA dehydrogenase bifunctional enzyme. J. Biol. Chem. 260, 8905–8910.
Ishii, N., Hijikata, M., Osumi, T., and Hashimoto, T. (1987) Structural organization of gene for rat enoyl-CoA hydratase:3-hydroxyacyl-CoA dehydrogenase bifunction enzyme. J. Biol. Chem. 262, 8144–8150.
Minami, I. N., Taketani, S., Osumi, T., and Hashimoto, T. (1989) Molecular cloning and sequence analysis of the cDNA for art mitochondrial enoyl-CoA hydratase. Structural and evolutionary relationships linked to the bifunctional enzyme of the peroxisomal β-oxidation system. Eur. J. Biochem. 185, 73–78.
Bitar, K. G., Perez-Aranda, A., and Bradshaw, R. A., (1980). Amino acid sequence of L-3-hydroxyacyl-CoA dehydrogenase from pig heart muscle. FEBS Lett. 116, 196–198.
Kamijo, T., Aoyama, T., Miyazaki, J., and Hashimoto, T. (1993) Molecular cloning of the cDNAs for the subunits of rat mitochondrial fatty acid β-oxidation multienzyme complex. Structural and functional relationships to other mitochondrial and peroxisomal β-oxidation enzymes. J. Biol. Chem. 268, 26,452–26,460.
Kamijo, T., Aoyama, T., Komiyama, A., and Hashimoto, T. (1994) Structural analysis of cDNAs for subunits of human mitochondrial fatty acid β-oxidation trifunctional protein. Biochem. Biophys. Res. Commun. 199, 818–825.
Yang, X.-Y. H., Schulz, H., Elzinga, M., and Yang, S.-Y. (1991) Nucleotide sequence of the promoter and fadB gene of the, fadAB operon and primary structure of the multifunctional fatty acid oxidation protein from Escherichia coli. Biochemistry 30, 6788–6795.
Sato, S., Hayashi, M., Imamura, O., Ozeki, Y., and Kawaguchi, A. (1992) Primary structures of the genes, faoA and faoB, from Pseudomonas fragi B-0771 which encode the two subunits of the HDT multienzyme complex involved in fatty acid β-oxidation. J. Biochem., 111, 8–15.
Hiltunen, J. K., Wenzel, B., Beyer, A., Erdmann, R., Fosså, A., and Kunau, W.-H. (1992) Peroxisomal multifunctional protein of Saccharomyces cerevisiae. Molecular analysis of the FOX2 gene and gene product. J. Biol. Chem. 267, 6646–6653.
Miyazawa, S., Osumi, T., and Hashimoto, T. (1980) The presence of a new oxoacyl-CoA thiolase in rat liver peroxisomes. Eur. J. Biochem. 103, 589–596.
Hijikata, M., Wen, J.-K., Osumi, T., and Hashimoto, T. (1990) Rat peroxisomal 3-ketoacyl-CoA thiolase gene. Occurrence of two closely related but differentially regulated gene. J. Biol. Chem. 265, 4600–4606.
Arakawa, M., Takiguchi, M., Amaya, Y., Nagata, S., Hayashi, H., and Mori, M. (1987) cDNA-derived amino acid sequence of rat mitochondrial 3-oxoacyl-CoA thiolase with no transient presenquence: structural relationship with peroxisomal isozyme. EMBO J. 6, 1361–1366.
Fukao, T., Kamijo, K., Osumi, T., Fujiki, Y., Yamaguchi, S., Orii, T., and Hashimoto, T. (1989) Molecular cloning and nucleotide sequence of the complementary DNA encoding the entire precursor of rat mitochondrial acetoacetyl-CoA thiolase. J. Biochem. 106, 197–204.
Song, X.-Q., Fukao, T., Yamaguchi, S., Miyazawa, S., Hashimoto, T., and Orii, T. (1994) Molecular cloning and nucleotide sequence of complementary DNA for human hepatic cytosolic acetoacetyl-coenzyme A thiolase. Biochem. Biophys. Res. Commun. 201, 478–485.
Yang, S.-Y., Yang, X.-Y. H., Healy-Louie, G., Schulz, H., and Elzinga, M. (1990) Nucleotide sequence of the fadA gene. Primary structure of 3-ketoacyl-coenzyme A thiolase from Escherichia coli and the structural organization of the fadAb operon. J. Biol. Chem. 265, 10,424–10,429.
Pedersen, J. I. (1993) Peroxisomal oxidation of the steroid side chain in bile acid formation. Biochimie 75, 159–165.
Mori, T., Tsukamoto, T., Mori, H., Tashiro, Y., and Fujiki, Y. (1991) Molecular cloning and deduced amino acid sequence of nonspecific lipid transfer protein (sterol carrier protein 2) of rat liver: a higher molecular mass (60 kDa) protein contains primary sequence of nonspecific lipid transfer protein as its C-terminal part. Proc. Natl. Acad. Sci. USA 88, 4338–4342.
Antonenkov, V. D., Van Veldhoven, P. P., Waelkens, E., and Mannaerts, G. P. (1997) Substrate specificities of 3-oxoacyl-CoA thiolase A and sterol carrier protein 2/3-oxoacyl-CoA thiolase purified from normal rat liver peroxisomes. Sterol carrier protein 2/3-oxoacyl-CoA thiolase is involved in the metabolism of 2-methyl-branched fatty acids and bile acid intermediates. J. Biol. Chem. 272, 26,023–26,031.
Wanders, R. J. A., Denis, S., Wouters, F., Wirtz, K. W. A., and Seedorf, U. (1997) Sterol carrier protein x (SCPx) is a peroxisomal branchedchain β-ketothiolase specifically reacting with 3-oxo-pristanoyl-CoA: A new, unique role for SCPx in branched-chain fatty acid metabolism in peroxisomes. Biochem. Biophys. Res. Commun. 236, 565–569.
Bun-ya, M., Maebuchi, M., Kamiryo, T., Kurosawa, T., Sato, M., Tohma, M., Jiang, L. L., and Hashimoto, T. (1998) Thiolase involved in bile acid formation. J. Biochem. 123, 347–352.
Bun-ya, M., Maebuchi, M., Hashimoto, T., Yokota, S., and Kamiryo, T. (1997) A second isoform of 3-ketoacyl-CoA thiolase found in Caenorhabditis elegans, which is similar to sterol carrier protein x but lacks the sequence of sterol carrier protein 2. Eur. J. Biochem. 245, 252–259.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hashimoto, T. Peroxisomal β-oxidation enzymes. Cell Biochem Biophys 32, 63–72 (2000). https://doi.org/10.1385/CBB:32:1-3:63
Published:
Issue Date:
DOI: https://doi.org/10.1385/CBB:32:1-3:63