-
PDF
- Split View
-
Views
-
Cite
Cite
James P Corsetti, David L Rainwater, Arthur J Moss, Wojciech Zareba, Charles E Sparks, High Lipoprotein-Associated Phospholipase A2 Is a Risk Factor for Recurrent Coronary Events in Postinfarction Patients, Clinical Chemistry, Volume 52, Issue 7, 1 July 2006, Pages 1331–1338, https://doi.org/10.1373/clinchem.2006.066845
Close - Share Icon Share
Abstract
Background: Recent studies demonstrate that lipoprotein-associated phospholipase A2 (Lp-PLA2) is a risk factor for cardiovascular disease presumably deriving from generation of proinflammatory and proatherogenic species through its hydrolytic activity on lipoprotein-associated phospholipids. The goal of this study was to assess the relationship of Lp-PLA2 with a set of thrombogenic, lipid, inflammatory, and metabolic blood markers and to determine whether plasma Lp-PLA2 is a risk factor for recurrent coronary events in postinfarction patients.
Methods: Factor analysis on the set of blood markers and Lp-PLA2 was performed for 766 patients of the Thrombogenic Factors and Recurrent Coronary Events (THROMBO) postinfarction study. Recurrent coronary event risk was assessed as a function of blood marker concentrations and Lp-PLA2 by Cox proportional hazards multivariable regression adjusted for significant clinical covariates.
Results: Factor analysis revealed that Lp-PLA2 was associated with one factor dominated by cholesterol and apolipoprotein B and another factor dominated by HDL-cholesterol and triglycerides, with little association with an inflammatory factor dominated by C-reactive protein. Multivariable analysis demonstrated as significant and independent predictors of risk of secondary coronary events only apolipoprotein B in a model without Lp-PLA2 (hazard ratio, 1.66; 95% confidence interval, 1.14–2.40) and only Lp-PLA2 in a model with Lp-PLA2 included [1.90 (1.31–2.75)].
Conclusions: Lp-PLA2 is a significant and independent predictor of risk for recurrent coronary events in postinfarction patients, and Lp-PLA2 is related to both hypercholesterolemia and high triglyceride–low HDL dyslipidemia in this study population.
Hydrolysis of phospholipids associated with lipoprotein particles by the phospholipase A2 family of enzymes leads to modulation of lipoprotein particle phospholipid content and size as well as production of both pro- and antiinflammatory intermediates, all of which may serve as a basis for involvement of these enzymes in the development of atherosclerosis (1)(2). Studies have shown that a member of this family, secretory phospholipase A2, is an independent risk factor for cardiovascular disease (CVD)1 (3)(4). Additionally, positive association of CVD with another member of the family, lipoprotein-associated phospholipase A2 (Lp-PLA2), has also been demonstrated, as reviewed recently(1)(2)(5)(6)(7). This is in contrast to initial impressions that this enzyme was protective in atherosclerosis because of its presumed ability to deactivate platelet-activating factor (PAF), leading to its initial designation as PAF acetylhydrolase.
We recently performed multivariable analysis on a set of thrombogenic, lipid, inflammatory, and metabolic blood markers in all patients of the Thrombogenic Factors and Recurrent Coronary Events (THROMBO) postinfarction study. Results showed that high concentrations of D-dimer and apolipoprotein B (apoB) and low concentrations of apolipoprotein A-I (apoAI) are significant and independent risk factors for recurrent coronary events (8). In a subsequent study, we demonstrated that only apoB was a significant predictor of risk for nondiabetic patients of the THROMBO study(9). It is thought that Lp-PLA2 is predominantly bound to LDL, where it is presumed to be largely proinflammatory and/or proatherogenic in contrast to HDL, where binding is less but where the Lp-PLA2 is thought to be antiinflammatory and/or antiatherogenic.
In view of the close association of Lp-PLA2 with CVD risk (1)(2)(5)(6)(7), we hypothesized that this tendency would also be present in myocardial infarction patients. We tested this hypothesis by assessing Lp-PLA2 as a predictor of risk of recurrent coronary events in nondiabetic patients of the THROMBO study, using multivariable modeling with adjustment for significant clinical covariates. We also examined the relationship of Lp-PLA2 with a set of thrombogenic, lipid, inflammatory, and metabolic blood markers, using factor analysis.
Materials and Methods
study population
The study population comprised 766 patients of the postinfarction THROMBO study who were nondiabetic and had complete laboratory data. Diabetic patients were excluded to give a more homogeneous study population, to minimize potential confounding of results for the majority of patients, who were nondiabetic. Details of the entire THROMBO study population (8) and of the present study population(9)(10)(11) have been reported previously; and as noted, the study was carried out with approval of and according to guidelines of the Research Subjects Review Boards. Recurrent coronary outcome events for this study were cardiac death, myocardial infarction (MI), or unstable angina [hospitalization during follow-up with an increase in either frequency or duration of angina symptoms or with development of new angina at rest, with both requiring ischemic electrocardiographic (ECG)] changes without enzyme increases), whichever occurred first. Mean patient follow-up was 26 months.
blood markers
Blood markers in fasting sera were determined 2 months after the index MI. Concentrations of the following markers were determined as described previously (8)(10): apoB, total cholesterol, apoAI, HDL, triglycerides, LDL peak particle diameter, glucose, insulin, body mass index (BMI), plasminogen activator inhibitor-1, lipoprotein(a), C-reactive protein (CRP), von Willebrand factor antigen, fibrinogen, D-dimer, factor VII, and factor VIIa. Gradient gel electrophoresis was used to determine LDL peak particle diameter and median HDL particle diameter as described previously(12)(13). Plasma Lp-PLA2 activity was determined by a commercial colorimetric assay (Cayman Chemical Co.) with 2-thio-PAF as substrate and according to the manufacturer’s directions; enzymatic hydrolysis of the acetyl thioester bond was detected with 5,5′-dithio-bis-(2-nitrobenzoic acid) at 405 nm. Two control products were run with each assay. Between-assay CVs were 1.6% for a low control and 5.6% for a control near the sample mean; all but 2 samples gave rates greater than the low control. Samples were run in duplicate, and the mean CV was 2.5%. Enzyme activity was expressed in units of μmol · min−1 · mL−1.
statistical analyses
Statistica 7.0 (StatSoft, Inc.) was used for all statistical and graphical analyses. Linear regression was used to adjust variables for age. Significant differences between populations were assessed by Mann–Whitney U-test.
Factor analysis was used to elucidate the relationship of Lp-PLA2 with other laboratory markers and pathophysiologic processes. Factor analysis was performed as described previously (9) with use of the normalized varimax rotation approach to aid in factor identification. A factor loading magnitude >0.4 was the criterion used for inclusion of variables in a factor. Factor analysis is an exploratory data analysis technique for simplification of multivariable data sets having significant intercorrelation among variables. The technique identifies subsets of variables that are thought to represent more basic underlying explanatory processes, in this case physiologic associations. Factor analysis simplifies multivariable data by generating a set of new variables, termed “factors”, that are fewer in number than the set of original variables while at the same time accounting for a significant proportion of variance in the original data. This enables factors to faithfully represent the original data set. Each factor is associated with a subset of the original variables according to a “factor loading” that represents the correlation of each variable with the particular factor and thus is a measure of the strength of the association of the variable with the factor.
Significant variables contributing to time to outcome event were determined by Kaplan–Meier analysis (log rank statistic, P <0.05) and the Cox multivariable proportional hazards regression model. Associations between variables were assessed by use of Spearman correlation coefficients. Multiple regression was performed by a forward stepwise approach with order of entry according to highest correlation. Only independent variables having correlation coefficients with the dependent variable significantly different from zero were included in the model (to minimize multicollinearity effects), and regression-associated contributions to variance of the dependent variable by independent variables were estimated as the square of multiple correlation coefficients derived from the regression models.
multivariable risk models
To assess risk of secondary coronary events as a function of blood marker variables, we used Cox regression analysis of the study population, using 17 laboratory markers and Lp-PLA2 as independent variables with adjustment of the clinical covariates—sex, race, smoking, previous MI (an MI before the entrance MI into the study), myocardial index [index infarct type by ECG (Q-wave vs non-Q-wave)], pulmonary congestion, ejection fraction during entrance MI [EF30 (>0.30 vs ≤0.30)], and claudication—found to be significant with single entry into the model at P <0.1. Blood markers were dichotomized as the highest risk quartile vs the 3 lower risk quartiles combined (for all markers except HDL and apoAI, dichotomization was according to quartile with the highest concentration vs a combination of the 3 quartiles with lower concentrations; for HDL and apoAI, dichotomization was according to the lowest concentration quartile vs a combination of the 3 quartiles with the highest concentrations). Separate univariate models were run for each laboratory variable dichotomized as described above. A multivariable model adjusted for significant clinical covariates was then run with simultaneous entry of all univariate significant laboratory values (P <0.05). Lastly, assessment of medication effects was performed by single entry into the resulting model of the following medications: statins, beta blockers, aspirin, calcium channel blockers, nitrates, angiotensin-converting enzyme (ACE) inhibitors, and oral anticoagulants (P <0.05). With the dichotomization scheme for biomarkers as described above and use of the overall raw recurrent coronary event rate of ∼16% for the entire study population as a base for the combined lower-risk 3 quartiles and with statistical significance set at α = 0.05, we obtained the following values for statistical power for detecting recurrent coronary event rates in the high-risk quartiles relative to base: 26% for an increase in recurrent coronary event rate from 16% to 20%, 69% for an increase from 16% to 24%, and 94% for an increase from 16% to 28%.
Results
The clinical and laboratory characteristics of the study population have been given previously (10). In summary, patients were 77% male, 79% white, had a mean age of 58 years, were overweight, and had increased triglyceride and low HDL-cholesterol concentrations. Additionally, 35.6% of patients had metabolic syndrome according to slightly modified criteria(11) of the Adult Treatment Panel III of the National Cholesterol Education Program.
relationship of Lp-PLA2 with other blood markers
Spearman correlation coefficients between Lp-PLA2 and the remaining blood markers (Table 11 ) were significantly different from zero (P <0.01) for apoB, cholesterol, HDL, triglycerides, LDL peak particle diameter, factor VIIa, and HDL median diameter. These markers were then used as independent variables in forward stepwise multiple regression with Lp-PLA2 as dependent variable. This indicated for Lp-PLA2 significant and independent contributions from cholesterol, HDL, apoB, and factor VIIa. Approximate contributions to Lp-PLA2 regression-associated variance were as follows: cholesterol, 20%; HDL, 17%; apoB, 1%; and factor VIIa, 1%.
Results of factor analysis of the 17 blood markers plus Lp-PLA2 (Table 22 ) were similar to corresponding factor analysis results for the set of blood markers without Lp-PLA2 as reported previously (10) in that 5 factors were generated that could be identified as associated with cholesterol-lipoprotein, dyslipidemia, glycemia, inflammation, and coagulation. Regarding Lp-PLA2, Table 22 shows a split in its factor association almost equal between the cholesterol-lipoprotein and dyslipidemia factors as manifested by its factor loadings. From the magnitudes and signs of the loadings, Lp-PLA2 in the dyslipidemia factor is directly correlated with triglycerides but inversely correlated with HDL, LDL peak particle diameter, and apoAI. We designated this factor as “dyslipidemia” because of its similarity to the small LDL, high triglycerides, and low HDL dyslipidemia associated with metabolic syndrome. For the cholesterol-lipoprotein factor, Lp-PLA2 was directly correlated with cholesterol and apoB, somewhat less correlated with apoAI, and even less correlated with triglycerides. This factor seemed to most closely represent hypercholesterolemia. We assessed the role of LDL-cholesterol in this factor with LDL estimates obtained with the Friedewald equation because direct LDL determinations were not available. LDL-cholesterol as calculated by the Friedewald equation for the 715 patients of the total population of 766 who had triglycerides <4.52 mmol/L was highly correlated with total cholesterol (Spearman correlation coefficient = 0.90; P <0.000001), suggesting the importance of LDL in this factor. Identification of the cholesterol-lipoprotein factor with LDL hypercholesterolemia was further supported by separate factor analysis results substituting LDL-cholesterol for total cholesterol. Results were essentially the same as with total cholesterol. Lastly, we found that Lp-PLA2 had no significant association with any of the 3 remaining factors, including inflammation.
Lp-PLA2 and risk of recurrent coronary events
Results (Table 33 ) of Cox multivariable regression models, using blood markers without and with Lp-PLA2 and dichotomized as highest risk quartile vs the 3 lower risk quartiles combined, showed clinical covariates requiring adjustment, significant univariate markers along with corresponding dichotomization cut points, and hazard ratios (HRs) and 95% confidence intervals (CIs) for the 2 final multivariable models. Shown in Fig. 11 are Kaplan–Meier plots for the markers found to be significant in the univariate models (Lp-PLA2, apoB, cholesterol, and BMI, respectively) for comparisons. As shown in Table 33 , apoB was the only independent and significant risk factor for recurrent coronary events in the study population when Lp-PLA2 is not included [HR (95% CI) = 1.66 (1.14–2.40); P = 0.0005]; however, when Lp-PLA2 was included, apoB lost significance whereas Lp-PLA2 became the sole independent and significant risk factor in the population [HR (95% CI) = 1.90 (1.31–2.75)]. Furthermore, the P values for overall model fit suggested better fit for the model with Lp-PLA2 (P = 0.00008) compared with the model without Lp-PLA2 (P = 0.0005). Calcium channel blockers successfully entered both multivariable models but did not change the independent contribution of either apoB or Lp-PLA2. To further explore the association of apoB and Lp-PLA2 with recurrent risk with regard to cutpoint values, we ran proportional hazards models with apoB and Lp-PLA2 quartile stratification, with Q1 the lowest concentration quartile and Q4 the highest concentration quartile. For both apoB and Lp-PLA2, the Q2/Q1 and Q3/Q1 comparisons did not attain significance, whereas the Q4/Q1 comparison did for both [HR (95% CI) = 1.61 (0.099–2.61) and 1.68 (1.04–2.73), respectively]. This suggests that recurrent risk in each case is associated with the highest concentrations for both markers.
To address the concern that risk dependence associated with Lp-PLA2 is often confounded by that of LDL-cholesterol, we entered into the model non–HDL-cholesterol (total cholesterol minus HDL-cholesterol) as a substitute for LDL-cholesterol because LDL-cholesterol estimates from the Friedewald equation were not available for all patients and because non–HDL-cholesterol correlated well with available LDL-cholesterol estimates (Spearman correlation coefficient = 0.90; P <0.000001). Non–HDL-cholesterol was dichotomized as the highest quartile of concentration vs the lower 3 quartiles combined. Although non–HDL-cholesterol was univariately significant in the model, it did not achieve significance in the multivariable model, which still demonstrated Lp-PLA2 as the only significant and independent predictor of risk.
characterization of patients in high-risk Lp-PLA2 quartile
The raw recurrent coronary event rate in the entire study population was 15.9%. Comparison of patients in the Lp-PLA2 high-risk quartile vs the combined 3 lower risk quartiles (Table 44 ) showed higher raw recurrent coronary event rate (24.1% vs 13.2%). Additionally, in the high-risk quartile, there were more males (86.9% vs 73.7%) and whites (85.3% vs 76.2%), and generally similar proportions of patients taking each medication except for statins, for which the high-risk group had a lower value (29.3% vs 43.8%). Several lines of evidence make unlikely that lower statin use in patients in the high Lp-PLA2 quartile played a significant role in development of high-risk in this subgroup, including that statins failed to enter the multivariable model and that, when the multivariable model was run for patients not on statins, Lp-PLA2 remained a significant predictor of risk. For blood markers, patients in the Lp-PLA2 high-risk quartile had higher concentrations of apoB [1.41 (0.28) vs 1.17 (0.25) mmol/L], cholesterol [5.82 (1.19) vs 4.89 (1.03) mmol/L], and triglycerides [2.82 (1.50) vs 2.05 (1.16) mmol/L]; smaller LDL peak particle diameter [25.99 (0.69) vs 26.36 (0.84) nm]; and lower concentrations of HDL-cholesterol [0.93 (0.21) vs 1.03 (0.28) mmol/L] and activated factor VII [2.34 (1.63) vs 2.58 (1.71) μg/L].
Discussion
Results of this study in nondiabetic postinfarction patients demonstrated that high Lp-PLA2 is a significant and independent predictor of risk for recurrent coronary events and that it replaces apoB as the only such predictor from a set of thrombogenic, lipid, inflammatory, and metabolic blood markers when Lp-PLA2 is included in the model. Furthermore, the study demonstrated that Lp-PLA2 is not correlated to CRP and several other inflammatory markers, but is related to both hypercholesterolemia and high triglyceride–low HDL dyslipidemia.
Identification of Lp-PLA2 as an independent predictor of risk of recurrent coronary events in our study population is consistent with findings of prospective studies as reviewed recently (6)(7), with most showing significant and independent Lp-PLA2–associated risk as determined from multivariable models even after adjustment of other risk factors. Additionally, in a more recent study of patients undergoing coronary angiography, high Lp-PLA2 was found to be a significant and independent risk factor for major adverse events (death, MI, coronary revascularization, and stroke)(14). Our results agree well qualitatively with earlier studies, and additionally, Lp-PLA2 HR (1.90) agrees well with the range of values reported in previous studies (1.18–2.08)(7). Furthermore, our results also extend earlier reports, given that our study was on predictors of recurrent coronary events in a study population of patients with previous MI.
In the present study, we showed that adding Lp-PLA2 to the multivariable model that previously demonstrated only apoB as an independent predictor of risk in the same study population (9) led to replacement of apoB by Lp-PLA2. This is similar to results of a study by Caslake et al.(15) showing in patients with coronary artery disease, in comparison with age-matched healthy controls, that Lp-PLA2 was a stronger predictor of disease risk than was apoB.
CRP in our study did not achieve significance as a risk marker, not even in univariate analysis. This is in contrast to most other studies involving both CRP and Lp-PLA2, which indicated that CRP is a significant risk factor in multivariable models. These include the Women’s Health Study (16), the Atherosclerosis Risk in Communities study(17), and the MONItoring of trends and determinants in CArdiovascular disease (MONICA) protocol(18). On the other hand, CRP was not a significant predictor of risk in a multivariable model for men of the West of Scotland Coronary Prevention Study (WOSCOPS) who had coronary events (nonfatal MI, death from coronary heart disease, or coronary revascularization) vs controls(19). This study population is similar to that of the current study, and interestingly, it gives similar results regarding CRP. Additionally, the mean CRP concentration in our study population (4.36 mg/L) was higher than in the previously cited studies, a finding consistent with the presence of higher extent of inflammatory processes in most patients of our study population, which could lead to less discrimination of risk based on high CRP concentrations.
In addition to assessing the role of Lp-PLA2 as a risk factor in our study population, we also elucidated the relationships of Lp-PLA2 with other blood markers, using correlation analysis, multiple regression, and factor analysis. Correlation analysis in our study population revealed no association of Lp-PLA2 with CRP consistent with multiple previous studies (14)(17)(18)(19)(20). However, in our study we also used factor analysis to investigate relationships among variables. Results revealed no association of Lp-PLA2 with CRP and, furthermore, that there were no associations of Lp-PLA2 with several other inflammatory markers. This lack of association signifies disparate pathways of involvement of Lp-PLA2 and inflammatory markers in the development of atherosclerosis(6). In our study, correlation analysis revealed associations of Lp-PLA2 with multiple markers that, when entered into a multiple regression model, led to regression-associated variance in Lp-PLA2 mostly accounted for by positive correlation of total cholesterol (20%) and inverse correlation of HDL-cholesterol (17%). That only ∼40% of Lp-PLA2 variance was accounted for by the regression may indicate that additional sources with significant pathophysiologic import contribute to Lp-PLA2 variance, enabling Lp-PLA2 to overcome apoB as the single significant and independent predictor of risk in the study population. The multiple regression results are also consistent with corresponding factor analysis results showing an almost even split in association of Lp-PLA2 with a cholesterol-lipoprotein factor largely representative of total and LDL hypercholesterolemia and a dyslipidemia factor characterized by high triglycerides and low HDL. We speculate that Lp-PLA2 in association with the hypercholesterolemia factor represents LDL-bound Lp-PLA2 and that Lp-PLA2 in association with the dyslipidemia factor represents HDL-bound Lp-PLA2. These findings are consistent with characteristics of patients in the high-risk upper quartile of Lp-PLA2 concentration who, compared with patients in the combined lower 3 quartiles, had higher concentrations of total cholesterol and apoB and higher triglyceride concentrations, lower HDL-cholesterol, and smaller LDL particles.
The limitation of the present study in terms of failing to demonstrate significance regarding several traditional risk markers may relate to a lack of sufficient statistical power. Another concern centers on possible confounding effects of LDL-cholesterol regarding Lp-PLA2–associated risk for recurrent coronary events. Direct LDL determinations were not available for patients in the study to allow direct assessment of this concern. However, it seems unlikely that LDL-cholesterol would have a significant effect in this regard in view of a lack of such an effect in our study population of non–HDL-cholesterol in multivariable analysis and similar results in factor analysis when LDL-cholesterol calculated by the Friedewald equation was substituted for total cholesterol. Additionally, to confirm identification of the split association of Lp-PLA2 with LDL-cholesterol (hypercholesterolemia) and HDL-cholesterol (dyslipidemia), determination of Lp-PLA2–associated mass or activity in LDL and HDL fractions would be helpful.
In conclusion, we demonstrated in this study that circulating Lp-PLA2 activity is a significant and independent risk factor for recurrent coronary events in nondiabetic postinfarction patients and that it replaces apoB as the sole predictor of risk when included in multivariable models. We also showed relationships of Lp-PLA2 with both hypercholesterolemia and high triglycerides–low HDL dyslipidemia. These findings extend the role of increased Lp-PLA2 as an emerging risk factor of CVD for primary events to recurrent coronary events in patients with previous MI.
Spearman correlation coefficients between Lp-PLA2 and blood markers in the study population (n = 766).
| Marker . | Correlation coefficient . |
|---|---|
| CRP | −0.05 |
| ApoB1 | 0.39 |
| Cholesterol1 | 0.43 |
| ApoAI | 0.02 |
| HDL1 | −0.30 |
| Triglycerides1 | 0.35 |
| LDL peak diameter1 | −0.30 |
| Glucose | 0.04 |
| Insulin | 0.02 |
| BMI | 0.01 |
| PAI-12 | 0.07 |
| Lp(a) | −0.04 |
| von Willebrand factor | −0.01 |
| Fibrinogen | −0.01 |
| D-Dimer | 0.04 |
| Factor VII | 0.03 |
| Factor VIIaa | −0.12 |
| HDL median diameter1 | −0.27 |
| Marker . | Correlation coefficient . |
|---|---|
| CRP | −0.05 |
| ApoB1 | 0.39 |
| Cholesterol1 | 0.43 |
| ApoAI | 0.02 |
| HDL1 | −0.30 |
| Triglycerides1 | 0.35 |
| LDL peak diameter1 | −0.30 |
| Glucose | 0.04 |
| Insulin | 0.02 |
| BMI | 0.01 |
| PAI-12 | 0.07 |
| Lp(a) | −0.04 |
| von Willebrand factor | −0.01 |
| Fibrinogen | −0.01 |
| D-Dimer | 0.04 |
| Factor VII | 0.03 |
| Factor VIIaa | −0.12 |
| HDL median diameter1 | −0.27 |
Significantly different from zero (P <0.01).
PAI-1, plasminogen activator inhibitor-1; Lp(a), lipoprotein(a).
Spearman correlation coefficients between Lp-PLA2 and blood markers in the study population (n = 766).
| Marker . | Correlation coefficient . |
|---|---|
| CRP | −0.05 |
| ApoB1 | 0.39 |
| Cholesterol1 | 0.43 |
| ApoAI | 0.02 |
| HDL1 | −0.30 |
| Triglycerides1 | 0.35 |
| LDL peak diameter1 | −0.30 |
| Glucose | 0.04 |
| Insulin | 0.02 |
| BMI | 0.01 |
| PAI-12 | 0.07 |
| Lp(a) | −0.04 |
| von Willebrand factor | −0.01 |
| Fibrinogen | −0.01 |
| D-Dimer | 0.04 |
| Factor VII | 0.03 |
| Factor VIIaa | −0.12 |
| HDL median diameter1 | −0.27 |
| Marker . | Correlation coefficient . |
|---|---|
| CRP | −0.05 |
| ApoB1 | 0.39 |
| Cholesterol1 | 0.43 |
| ApoAI | 0.02 |
| HDL1 | −0.30 |
| Triglycerides1 | 0.35 |
| LDL peak diameter1 | −0.30 |
| Glucose | 0.04 |
| Insulin | 0.02 |
| BMI | 0.01 |
| PAI-12 | 0.07 |
| Lp(a) | −0.04 |
| von Willebrand factor | −0.01 |
| Fibrinogen | −0.01 |
| D-Dimer | 0.04 |
| Factor VII | 0.03 |
| Factor VIIaa | −0.12 |
| HDL median diameter1 | −0.27 |
Significantly different from zero (P <0.01).
PAI-1, plasminogen activator inhibitor-1; Lp(a), lipoprotein(a).
Factor analysis results for the set of 17 laboratory variables plus Lp-PLA2.1
| Factor (% contribution to variance) . | Factor components . | Factor loadings . |
|---|---|---|
| Cholesterol-lipoprotein (13.7%) | Cholesterol | 0.91 |
| ApoB | 0.79 | |
| Lp-PLA2 | 0.60 | |
| ApoAI | 0.49 | |
| Triglycerides | 0.43 | |
| Dyslipidemia (11.7%) | HDL | 0.80 |
| LDL peak diameter | 0.73 | |
| Triglycerides | −0.57 | |
| Lp-PLA2 | −0.49 | |
| ApoAI | 0.48 | |
| Inflammation (11.1%) | CRP | 0.80 |
| Fibrinogen | 0.73 | |
| D-Dimer | 0.60 | |
| von Willebrand Factor | 0.57 | |
| Glycemia (9.4%) | Glucose | 0.72 |
| Insulin | 0.72 | |
| PAI-12 | 0.42 | |
| Coagulation (9.3%) | Factor VIIa | 0.87 |
| Factor VII | 0.83 |
| Factor (% contribution to variance) . | Factor components . | Factor loadings . |
|---|---|---|
| Cholesterol-lipoprotein (13.7%) | Cholesterol | 0.91 |
| ApoB | 0.79 | |
| Lp-PLA2 | 0.60 | |
| ApoAI | 0.49 | |
| Triglycerides | 0.43 | |
| Dyslipidemia (11.7%) | HDL | 0.80 |
| LDL peak diameter | 0.73 | |
| Triglycerides | −0.57 | |
| Lp-PLA2 | −0.49 | |
| ApoAI | 0.48 | |
| Inflammation (11.1%) | CRP | 0.80 |
| Fibrinogen | 0.73 | |
| D-Dimer | 0.60 | |
| von Willebrand Factor | 0.57 | |
| Glycemia (9.4%) | Glucose | 0.72 |
| Insulin | 0.72 | |
| PAI-12 | 0.42 | |
| Coagulation (9.3%) | Factor VIIa | 0.87 |
| Factor VII | 0.83 |
Results give factor identifications with pathophysiologic processes, percentage factor contributions to total variance, factor components (variables with loadings >0.4), and factor loadings in decreasing order.
PAI-1, plasminogen activator inhibitor-1.
Factor analysis results for the set of 17 laboratory variables plus Lp-PLA2.1
| Factor (% contribution to variance) . | Factor components . | Factor loadings . |
|---|---|---|
| Cholesterol-lipoprotein (13.7%) | Cholesterol | 0.91 |
| ApoB | 0.79 | |
| Lp-PLA2 | 0.60 | |
| ApoAI | 0.49 | |
| Triglycerides | 0.43 | |
| Dyslipidemia (11.7%) | HDL | 0.80 |
| LDL peak diameter | 0.73 | |
| Triglycerides | −0.57 | |
| Lp-PLA2 | −0.49 | |
| ApoAI | 0.48 | |
| Inflammation (11.1%) | CRP | 0.80 |
| Fibrinogen | 0.73 | |
| D-Dimer | 0.60 | |
| von Willebrand Factor | 0.57 | |
| Glycemia (9.4%) | Glucose | 0.72 |
| Insulin | 0.72 | |
| PAI-12 | 0.42 | |
| Coagulation (9.3%) | Factor VIIa | 0.87 |
| Factor VII | 0.83 |
| Factor (% contribution to variance) . | Factor components . | Factor loadings . |
|---|---|---|
| Cholesterol-lipoprotein (13.7%) | Cholesterol | 0.91 |
| ApoB | 0.79 | |
| Lp-PLA2 | 0.60 | |
| ApoAI | 0.49 | |
| Triglycerides | 0.43 | |
| Dyslipidemia (11.7%) | HDL | 0.80 |
| LDL peak diameter | 0.73 | |
| Triglycerides | −0.57 | |
| Lp-PLA2 | −0.49 | |
| ApoAI | 0.48 | |
| Inflammation (11.1%) | CRP | 0.80 |
| Fibrinogen | 0.73 | |
| D-Dimer | 0.60 | |
| von Willebrand Factor | 0.57 | |
| Glycemia (9.4%) | Glucose | 0.72 |
| Insulin | 0.72 | |
| PAI-12 | 0.42 | |
| Coagulation (9.3%) | Factor VIIa | 0.87 |
| Factor VII | 0.83 |
Results give factor identifications with pathophysiologic processes, percentage factor contributions to total variance, factor components (variables with loadings >0.4), and factor loadings in decreasing order.
PAI-1, plasminogen activator inhibitor-1.
Cox proportional hazards models for study population giving clinical covariates requiring adjustment (P <0.10), significant laboratory variables (P <0.05) along with dichotomization cutpoints, and multivariable results of simultaneous entrance of significant laboratory variables with adjustment for relevant clinical covariates.
| Clinical covariates . | Significant variables . | Dichotomization cutpoints . | Multivariable model1 . | . | |
|---|---|---|---|---|---|
| . | . | . | Without Lp-PLA2 . | With Lp-PLA2 . | |
| Previous MI2 | ApoB | 1.39 g/L | ApoB | Lp-PLA2 | |
| EF303 | Cholesterol | 5.74 mmol/L | 1.66 (1.14–2.40) | 1.90 (1.31–2.75) | |
| Myocardial index4 | BMI | 29.64 kg/m2 | |||
| Factor VII | 76% | ||||
| Lp-PLA2 | 30.2 μmol · min−1 · mL−1 | ||||
| Clinical covariates . | Significant variables . | Dichotomization cutpoints . | Multivariable model1 . | . | |
|---|---|---|---|---|---|
| . | . | . | Without Lp-PLA2 . | With Lp-PLA2 . | |
| Previous MI2 | ApoB | 1.39 g/L | ApoB | Lp-PLA2 | |
| EF303 | Cholesterol | 5.74 mmol/L | 1.66 (1.14–2.40) | 1.90 (1.31–2.75) | |
| Myocardial index4 | BMI | 29.64 kg/m2 | |||
| Factor VII | 76% | ||||
| Lp-PLA2 | 30.2 μmol · min−1 · mL−1 | ||||
Results of multivariable models including HRs along with 95% CIs are given for models without and with Lp-PLA2.
MI before the index MI signaling entrance into the study.
Ejection fraction (>0.30 vs ≤0.30).
Index infarct type by ECG (Q-wave vs non-Q-wave).
Cox proportional hazards models for study population giving clinical covariates requiring adjustment (P <0.10), significant laboratory variables (P <0.05) along with dichotomization cutpoints, and multivariable results of simultaneous entrance of significant laboratory variables with adjustment for relevant clinical covariates.
| Clinical covariates . | Significant variables . | Dichotomization cutpoints . | Multivariable model1 . | . | |
|---|---|---|---|---|---|
| . | . | . | Without Lp-PLA2 . | With Lp-PLA2 . | |
| Previous MI2 | ApoB | 1.39 g/L | ApoB | Lp-PLA2 | |
| EF303 | Cholesterol | 5.74 mmol/L | 1.66 (1.14–2.40) | 1.90 (1.31–2.75) | |
| Myocardial index4 | BMI | 29.64 kg/m2 | |||
| Factor VII | 76% | ||||
| Lp-PLA2 | 30.2 μmol · min−1 · mL−1 | ||||
| Clinical covariates . | Significant variables . | Dichotomization cutpoints . | Multivariable model1 . | . | |
|---|---|---|---|---|---|
| . | . | . | Without Lp-PLA2 . | With Lp-PLA2 . | |
| Previous MI2 | ApoB | 1.39 g/L | ApoB | Lp-PLA2 | |
| EF303 | Cholesterol | 5.74 mmol/L | 1.66 (1.14–2.40) | 1.90 (1.31–2.75) | |
| Myocardial index4 | BMI | 29.64 kg/m2 | |||
| Factor VII | 76% | ||||
| Lp-PLA2 | 30.2 μmol · min−1 · mL−1 | ||||
Results of multivariable models including HRs along with 95% CIs are given for models without and with Lp-PLA2.
MI before the index MI signaling entrance into the study.
Ejection fraction (>0.30 vs ≤0.30).
Index infarct type by ECG (Q-wave vs non-Q-wave).
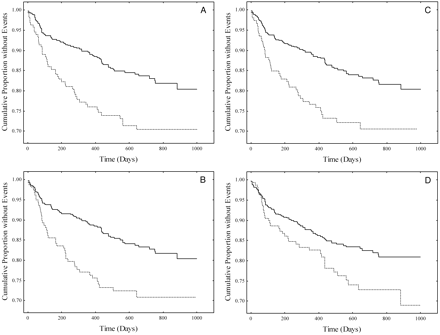
Kaplan–Meier curves for patients in the high-risk quartile (dotted line) vs the combined 3 lower risk quartiles (solid line) for Lp-PLA2 (A), apoB (B), cholesterol (C), and BMI (D).
Log-rank statistics: (A), P = 0.00082; (B), P = 0.00544; (C), P = 0.01181; (D), P = 0.0651.
Clinical characteristics and analyte means (SD) for the low-risk subgroup (combined lower 3 Lp-PLA2 quartiles) vs the high-risk subgroup (highest Lp-PLA2quartile).
| Characteristic . | Lower 3 quartiles . | Highest quartile . |
|---|---|---|
| Outcome rate, % | 13.2 | 24.1 |
| Males, % | 73.7 | 86.9 |
| Age, years | 59.1 | 56.1 |
| Race, % white | 76.2 | 85.3 |
| Previous MI, % | 17.2 | 15.7 |
| Medications, % | ||
| Statins | 43.8 | 29.3 |
| Beta blockers | 76.7 | 80.6 |
| Aspirin (%) | 82.8 | 79.6 |
| Calcium channel blockers | 20.9 | 16.8 |
| Nitrates | 34.1 | 32.5 |
| ACE2 inhibitors | 35.3 | 27.7 |
| Oral anticoagulants, % | 17.6 | 18.3 |
| Analytes [mean (SD)] | ||
| Lp-PLA2, μmol · min−1 · mL−1 | 23.67 (4.06) | 34.08 (3.91) |
| ApoB,1 g/L | 1.17 (0.25) | 1.41 (0.28) |
| Cholesterol,1 mmol/L | 4.89 (1.03) | 5.82 (1.19) |
| ApoAI, g/L | 1.18 (0.26) | 1.18 (0.21) |
| HDL-cholesterol,1 mmol/L | 1.03 (0.28) | 0.93 (0.21) |
| Triglycerides,1 mmol/L | 2.05 (1.16) | 2.82 (1.50) |
| LDL peak diameter,1 nm | 26.36 (0.84) | 25.99 (0.69) |
| Glucose, mmol/L | 4.94 (1.09) | 5.16 (1.40) |
| Insulin, pmol/L | 118 (180) | 127 (128) |
| BMI, kg/m2 | 27.49 (5.02) | 27.80 (4.48) |
| PAI-1, μg/L | 25.8 (23.3) | 29.2 (28.3) |
| Lp(a), mmol/L | 0.62 (0.57) | 0.67 (0.67) |
| CRP, mg/L | 4.5 (6.4) | 3.9 (8.2) |
| von Willebrand factor, % | 142 (62.5) | 143 (59.9) |
| Fibrinogen, g/L | 3.46 (0.79) | 3.49 (0.89) |
| D-Dimer, μg/L | 475 (438) | 465 (338) |
| Factor VII, % | 102 (44) | 104 (42) |
| Factor VIIa,1 μg/L | 2.58 (1.71) | 2.34 (1.63) |
| Characteristic . | Lower 3 quartiles . | Highest quartile . |
|---|---|---|
| Outcome rate, % | 13.2 | 24.1 |
| Males, % | 73.7 | 86.9 |
| Age, years | 59.1 | 56.1 |
| Race, % white | 76.2 | 85.3 |
| Previous MI, % | 17.2 | 15.7 |
| Medications, % | ||
| Statins | 43.8 | 29.3 |
| Beta blockers | 76.7 | 80.6 |
| Aspirin (%) | 82.8 | 79.6 |
| Calcium channel blockers | 20.9 | 16.8 |
| Nitrates | 34.1 | 32.5 |
| ACE2 inhibitors | 35.3 | 27.7 |
| Oral anticoagulants, % | 17.6 | 18.3 |
| Analytes [mean (SD)] | ||
| Lp-PLA2, μmol · min−1 · mL−1 | 23.67 (4.06) | 34.08 (3.91) |
| ApoB,1 g/L | 1.17 (0.25) | 1.41 (0.28) |
| Cholesterol,1 mmol/L | 4.89 (1.03) | 5.82 (1.19) |
| ApoAI, g/L | 1.18 (0.26) | 1.18 (0.21) |
| HDL-cholesterol,1 mmol/L | 1.03 (0.28) | 0.93 (0.21) |
| Triglycerides,1 mmol/L | 2.05 (1.16) | 2.82 (1.50) |
| LDL peak diameter,1 nm | 26.36 (0.84) | 25.99 (0.69) |
| Glucose, mmol/L | 4.94 (1.09) | 5.16 (1.40) |
| Insulin, pmol/L | 118 (180) | 127 (128) |
| BMI, kg/m2 | 27.49 (5.02) | 27.80 (4.48) |
| PAI-1, μg/L | 25.8 (23.3) | 29.2 (28.3) |
| Lp(a), mmol/L | 0.62 (0.57) | 0.67 (0.67) |
| CRP, mg/L | 4.5 (6.4) | 3.9 (8.2) |
| von Willebrand factor, % | 142 (62.5) | 143 (59.9) |
| Fibrinogen, g/L | 3.46 (0.79) | 3.49 (0.89) |
| D-Dimer, μg/L | 475 (438) | 465 (338) |
| Factor VII, % | 102 (44) | 104 (42) |
| Factor VIIa,1 μg/L | 2.58 (1.71) | 2.34 (1.63) |
Significant differences between analytes as determined by Mann–Whitney U-test (Lp-PLA2 not tested).
ACE, angiotensin-converting enzyme; PAI-1, plasminogen activator inhibitor-1; Lp(a), lipoprotein(a).
Clinical characteristics and analyte means (SD) for the low-risk subgroup (combined lower 3 Lp-PLA2 quartiles) vs the high-risk subgroup (highest Lp-PLA2quartile).
| Characteristic . | Lower 3 quartiles . | Highest quartile . |
|---|---|---|
| Outcome rate, % | 13.2 | 24.1 |
| Males, % | 73.7 | 86.9 |
| Age, years | 59.1 | 56.1 |
| Race, % white | 76.2 | 85.3 |
| Previous MI, % | 17.2 | 15.7 |
| Medications, % | ||
| Statins | 43.8 | 29.3 |
| Beta blockers | 76.7 | 80.6 |
| Aspirin (%) | 82.8 | 79.6 |
| Calcium channel blockers | 20.9 | 16.8 |
| Nitrates | 34.1 | 32.5 |
| ACE2 inhibitors | 35.3 | 27.7 |
| Oral anticoagulants, % | 17.6 | 18.3 |
| Analytes [mean (SD)] | ||
| Lp-PLA2, μmol · min−1 · mL−1 | 23.67 (4.06) | 34.08 (3.91) |
| ApoB,1 g/L | 1.17 (0.25) | 1.41 (0.28) |
| Cholesterol,1 mmol/L | 4.89 (1.03) | 5.82 (1.19) |
| ApoAI, g/L | 1.18 (0.26) | 1.18 (0.21) |
| HDL-cholesterol,1 mmol/L | 1.03 (0.28) | 0.93 (0.21) |
| Triglycerides,1 mmol/L | 2.05 (1.16) | 2.82 (1.50) |
| LDL peak diameter,1 nm | 26.36 (0.84) | 25.99 (0.69) |
| Glucose, mmol/L | 4.94 (1.09) | 5.16 (1.40) |
| Insulin, pmol/L | 118 (180) | 127 (128) |
| BMI, kg/m2 | 27.49 (5.02) | 27.80 (4.48) |
| PAI-1, μg/L | 25.8 (23.3) | 29.2 (28.3) |
| Lp(a), mmol/L | 0.62 (0.57) | 0.67 (0.67) |
| CRP, mg/L | 4.5 (6.4) | 3.9 (8.2) |
| von Willebrand factor, % | 142 (62.5) | 143 (59.9) |
| Fibrinogen, g/L | 3.46 (0.79) | 3.49 (0.89) |
| D-Dimer, μg/L | 475 (438) | 465 (338) |
| Factor VII, % | 102 (44) | 104 (42) |
| Factor VIIa,1 μg/L | 2.58 (1.71) | 2.34 (1.63) |
| Characteristic . | Lower 3 quartiles . | Highest quartile . |
|---|---|---|
| Outcome rate, % | 13.2 | 24.1 |
| Males, % | 73.7 | 86.9 |
| Age, years | 59.1 | 56.1 |
| Race, % white | 76.2 | 85.3 |
| Previous MI, % | 17.2 | 15.7 |
| Medications, % | ||
| Statins | 43.8 | 29.3 |
| Beta blockers | 76.7 | 80.6 |
| Aspirin (%) | 82.8 | 79.6 |
| Calcium channel blockers | 20.9 | 16.8 |
| Nitrates | 34.1 | 32.5 |
| ACE2 inhibitors | 35.3 | 27.7 |
| Oral anticoagulants, % | 17.6 | 18.3 |
| Analytes [mean (SD)] | ||
| Lp-PLA2, μmol · min−1 · mL−1 | 23.67 (4.06) | 34.08 (3.91) |
| ApoB,1 g/L | 1.17 (0.25) | 1.41 (0.28) |
| Cholesterol,1 mmol/L | 4.89 (1.03) | 5.82 (1.19) |
| ApoAI, g/L | 1.18 (0.26) | 1.18 (0.21) |
| HDL-cholesterol,1 mmol/L | 1.03 (0.28) | 0.93 (0.21) |
| Triglycerides,1 mmol/L | 2.05 (1.16) | 2.82 (1.50) |
| LDL peak diameter,1 nm | 26.36 (0.84) | 25.99 (0.69) |
| Glucose, mmol/L | 4.94 (1.09) | 5.16 (1.40) |
| Insulin, pmol/L | 118 (180) | 127 (128) |
| BMI, kg/m2 | 27.49 (5.02) | 27.80 (4.48) |
| PAI-1, μg/L | 25.8 (23.3) | 29.2 (28.3) |
| Lp(a), mmol/L | 0.62 (0.57) | 0.67 (0.67) |
| CRP, mg/L | 4.5 (6.4) | 3.9 (8.2) |
| von Willebrand factor, % | 142 (62.5) | 143 (59.9) |
| Fibrinogen, g/L | 3.46 (0.79) | 3.49 (0.89) |
| D-Dimer, μg/L | 475 (438) | 465 (338) |
| Factor VII, % | 102 (44) | 104 (42) |
| Factor VIIa,1 μg/L | 2.58 (1.71) | 2.34 (1.63) |
Significant differences between analytes as determined by Mann–Whitney U-test (Lp-PLA2 not tested).
ACE, angiotensin-converting enzyme; PAI-1, plasminogen activator inhibitor-1; Lp(a), lipoprotein(a).
Nonstandard abbreviations: CVD, cardiovascular disease; Lp-PLA2, lipoprotein-associated phospholipase A2; PAF, platelet-activating factor; apo, apolipoprotein; MI, myocardial infarction; ECG, electrocardiography; BMI, body mass index; CRP, C-reactive protein; ACE, angiotensin-converting enzyme; HR, hazard ratio; and CI, confidence interval.
This study was supported by Grant HL-48259 from the National Institutes of Health. We are indebted to the Study Coordinators who enrolled and followed up the patients from the 13 participating centers.
Tselepis AD, Chapman MJ. Inflammation, bioactive lipids and atherosclerosis: potential roles of a lipoprotein-associated phospholipase A2, platelet activating factor-acetylhydrolase.
Caslake MJ, Packard CJ. Lipoprotein-associated phospholipase A2 (platelet-activating factor acetylhydrolase) and cardiovascular disease.
Hurt-Camejo E, Camejo G, Peilot H, Oorni K, Kovanen P. Phospholipase A2 in vascular disease.
Jaross W, Eckey R, Menschikowski M. Biological effects of secretory phospholipase A2 group IIA on lipoproteins and in atherogenesis.
Macphee CH. Lipoprotein-associated phospholipase A2: a potential new risk factor for coronary artery disease and a therapeutic target.
Zalewski A, Macphee C. Role of lipoprotein-associated phospholipase A2 in atherosclerosis.
Sudhir K. Clinical review: lipoprotein-associated phospholipase A2, a novel inflammatory biomarker and independent risk predictor for cardiovascular disease.
Moss AJ, Goldstein RE, Marder VJ, Sparks CE, Oakes K, Greenberg H, et al. Thrombogenic factors and recurrent coronary events.
Corsetti JP, Zareba W, Moss AJ, Sparks CE. Serum glucose and triglyceride determine high-risk subgroups in non-diabetic postinfarction patients.
Corsetti JP, Zareba W, Moss AJ, Ridker PM, Marder VJ, Rainwater DL, et al. Metabolic syndrome best defines the multivariable distribution of blood variables in postinfarction patients.
Corsetti JP, Zareba W, Moss AJ, Sparks CE. Apolipoprotein B determines risk for recurrent coronary events in postinfarction patients with metabolic syndrome.
Rainwater DL, Moore PH, Shelledy WR, Dyer TD. Characterization of a composite gradient gel for the electrophoretic separation of lipoproteins.
Rainwater DL. Electrophoretic separation of LDL and HDL subclasses.
Brilakis ES, McConnell JP, Lennon RJ, Elesber AA, Meyer JG, Berger PB. Association of lipoprotein-associated phospholipase A2 levels with coronary artery disease risk factors, angiographic coronary artery disease, and major adverse events at follow-up.
Caslake MJ, Packard CJ, Suckling KE, Holmes SD, Chamberlain P, Macphee CH. Lipoprotein-associated phospholipase A2, platelet-activating factor acetylhydrolase: a potential new risk factor for coronary artery disease.
Blake GJ, Dada N, Fox JC, Manson JE, Ridker PM. A prospective evaluation of lipoprotein-associated phospholipase A2 levels and the risk of future cardiovascular events in women.
Ballantyne CM, Hoogeveen RC, Bang H, Coresh J, Folsom AR, Heiss G, et al. Lipoprotein-associated phospholipase A2, high-sensitivity C-reactive protein, and risk for incident coronary heart disease in middle-aged men and women in the Atherosclerosis Risk in Communities (ARIC) Study.
Koenig W, Khuseyinova N, Hannelore L, Trischler G, Meisinger C. Lipoprotein-associated phospholipase A2 adds to risk prediction of incident coronary events by C-reactive protein in apparently healthy middle-aged men from the general population.
Packard CJ, O’Reilly DSJ, Caslake MJ, McMahon AD, Ford I, Cooney J, et al. Lipoprotein-associated phospholipase A2 as an independent predictor of coronary heart disease.