Net Rearrangements of 4-acetyl-3-(4-substituted phenylamino)-2-(5-nitropyridin-2-yl)isoxazol-5(2H)-ones to Imidazo [1, 2-a]pyridines by Flash-Vacuum-Pyrolysis (F.V.P)
Chalak Azimi* Farhad Sepehraddin and Helal Tahazade
Department of Chemistry Management, College of Chemistry, Mahabad Branch, Islamic Azad University, Mahabad, Iran
DOI : http://dx.doi.org/10.13005/ojc/290421
Article Received on :
Article Accepted on :
Article Published : 11 Jan 2014
4-acetyl-3-(4-substituted phenylamino)-2-(5-nitropyridin-2-yl) isoxazol-5(2H)-ones, rearranged under Flash-Vacuum-Pyrolysis (F.V.P) conditions accompanied by elimination of carbon dioxide to give imidazo[1, 2-a]pyridines in high to excellent yields (90-95%).
KEYWORDS:Isoxazolones;Imidazopyridines;Flash-Vacuum-Pyrolysis
Download this article as:| Copy the following to cite this article: Azimi C, Sepehraddin F, Tahazadeh H. Net Rearrangements of 4-acetyl-3-(4-substituted phenylamino)-2-(5-nitropyridin-2-yl)isoxazol-5(2H)-ones to Imidazo [1, 2-a]pyridines by Flash-Vacuum-Pyrolysis (F.V.P). Orient J Chem 2013;29(4) |
| Copy the following to cite this URL: Azimi C, Sepehraddin F, Tahazadeh H. Net Rearrangements of 4-acetyl-3-(4-substituted phenylamino)-2-(5-nitropyridin-2-yl)isoxazol-5(2H)-ones to Imidazo [1, 2-a]pyridines by Flash-Vacuum-Pyrolysis (F.V.P). Orient J Chem 2013;29(4). Available from: http://www.orientjchem.org/?p=1548 |
Introduction
The thermal or photochemical loss of nitrogen and carbon dioxide from triazoles and isoxazole-5-ones, respectively, have been reported.1 We have reported that the reaction of 4-acetyl-3-(4-substituted phenylamino)-2-(5-nitropyridin-2-yl) isoxazol-5(2H)-ones (1,X: Br, Me) 2 with triethylamine in ethanol, give imidazo annulated compounds (2, X: Br (84%), Me (75%) as net products, Scheme 1.
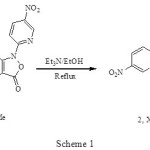 |
Scheme 1 Click here to View figure |
However, the reaction of 4-acetyl-3-(4-substituted phenylamino)-2-(5-nitropyridin-2-yl) isoxazol-5(2H)-ones (1, X: OMe) with triethylamine gives imidazo (2, X: OMe, 59%) and indole (3, 20%)2 annulated compounds respectively, Scheme 2.
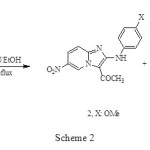 |
Scheme 2 Click here to View figure |
Here we describe the net rearrangement of 4-acetyl-3-(4-substituted phenylamino)-2-(5-nitropyridin-2-yl) isoxazol-5(2H)-ones (1, X: Br, Me and 3) to Imidazo[1, 2-a]pyridines (2, X: Br, Me, OMe) under Flash-Vacuum-Pyrolysis (F.V.P) conditions.
Results and Discussion
The required isoxazolones (1) were synthesized by reaction of 2-chloro-5-nitropyridine with 2H-isoxazolones (6) which in turn were made by modification of the procedure of Worrall.3, 4 Thus, the reaction of the sodium salt of ethylacetoacetate in ethanol with arylisothiocyanates (4) gave the thiocarbamates (5) in high yield (70-90%) and this converted to corresponding isoxazolone (6) by reaction with 2 equiv of hydroxylamine. N-arylation of isoxazolone (6) with 2-chloro-5-nitropyridine in solid phase condition gave the corresponding N-substituted isoxazolones (1) in fair yield (70-85%), Scheme 3.
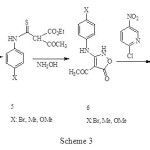 |
Scheme 3 Click here to View figure |
The rearrangement of (1) as shown in Scheme 4, proceeded in 90-95% yield under F.V.P conditions accompanied by elimination of carbon dioxide for 60 min. The reaction pathway leading to net Imidazo[1,2-a]pyridines which is consist the electronic requirement of the reaction as shown in Scheme 5 or with the alternative pathway suggested by Prager and Co-workers.5
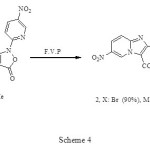 |
Scheme 4 Click here to View figure |
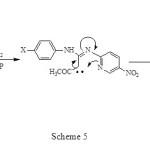 |
Scheme 5 Click here to View figure |
With a number of imidazopyridine structures in hand, the structures of all imidazopyridines were confirmed by 1H-NMR, 13C-NMR, FT-IR, MASS spectra or microanalyses. 4-methoxy derivative (1, Ar: MeOC6H4) reacts in refluxing ethanol with triethylamine to form a mixture of imidazopyridine (2, Ar: MeOC6H4) and indole (3) in a 2:1 ratio, respectively, but it only rearranges to imidazopyridine under F.V.P conditions. The exact mechanism of this synthetic method has been unclear so far. However, we think that the zwitterionoic (7) plays a role under refluxing ethanol with triethylamine, (scheme 6). This is consistent with electronic requirements of the reaction. The zwitterionoic (7) probably can be stabilised by ethanol as protic solvent, however under F.V.P conditions it can not be produced.
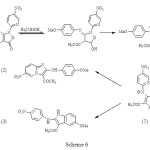 |
Scheme 6 Click here to View figure |
Conclusion
In conclusion we have shown that a variety of 4-acetyl-3-(4-substituted phenylamino)-2-(5-nitropyridin-2-yl) isoxazol-5(2H)-ones, rearranged under Flash-Vacuum-Pyrolysis (F.V.P) conditions to give imidazo [1,2-a] pyridines. These rearrangements, therefore, appear to be generally applicable to the synthesis of imidazoheterocycles which are suitable synthetic intermediates for a series of polycyclic heterocycles with possible pharmaceutical applications.
Experimental Section
General. Freshly distilled solvents were used throughout, and anhydrous solvents were dried according to Perrin and Amarego.6 1H-NMR and 13C-NMR spectra were recorded, in deuteriochloroform, unless otherwise stated, at 500 and 125 MHz respectively, with a Bruker DRX-500 Avance spectrometer. Tetramethylsilane was used as an internal standard and all signals due to amino protons were removed by exchange with D2O. Infrared spectra were recorded on a Unicam Matsson 1000 Fourier-Transform Spectrometer. Mass spectra were recorded on a Varian Matt 311 spectrometer and relative abundance of fragments are quoted in parentheses after the m/z values. Melting points were determined on a Philip Harris C4954718 apparatus and are uncorrected. Micronalyses were preformed on a Carlo–Erba Analyzer 1104.
4-acetyl-3-(4-bromo phenylamino) isoxazol-5(2H)-ones (6, X: Br).
To a solution of hydroxylamine hydrochloride (7.06 g, 102 mmol) in water (30 mL), sodium bicarbonate (10.17 g, 102 mmol) was added slowly. Ethanol (80 mL) was added and the resulting potassium chloride was filtered off. Ethyl-2-(4-bromophenyl) carbamothioyl)-3-oxobutanoate (5, X: Br) 12.71g, 34 mmol) was added to the filtrate and the mixture was stirred at room temperature for 24 hours. The reaction mixture was acidified with dilute HCl and the white precipitate was collected and recrystallized from acetone to give the title product (8.78 g, 79%) as colourless needles, m.p: 200-202 ºC (dec.);
1H-NMR (D6-DMSO)δ(ppm) 2.25(s, J=7.1Hz, 3H, CH3), 7.37(d, J=8.4Hz, 2H, Ar), 7.57(d, J=8.4Hz, 2H, Ar), 8.30 (bs, 1H, NH), 9.39 (bs, 1H, NH).
13C-NMR (D6-DMSO)δ(ppm) 15.31, 74.69, 118.02, 125.08, 132.94, 137.10, 163.53, 164.74, 167.39.
FT-IR νmax 3250, 2950, 2740, 1723, 1696, 1666, 1607, 1563, 1456, 1398, 1316, 1183, 1018, 818 cm-1.
4-acetyl-3-(4-methyl phenylamino) isoxazol-5(2H)-ones (6, X: Me).
This compound was prepared as described above using Ethyl-2-(4-methylphenyl) carbamothioyl)-3-oxobutanoate (5, X: Me) and Refluxing for 24 hours gave colourless crystals (85%), m.p: 164-166 ºC (dec.).
1H-NMR (D6-DMSO+CDCl3)δ(ppm) 2.30 (s, J=7.0Hz, 3H, CH3), 2.35 (s, 3H, Me), 6.78 (d, J=9.2Hz, 2H, Ar), 6.79 (bs, 1H, NH), 6.80(d, J=9.2Hz, 2H, Ar), 8.85 (bs, 1H, NH).
13C-NMR (D6-DMSO+CDCl3,400 MHz)δ(ppm) 14.52, 20.85, 74.69, 121.53, 130.13, 133.29, 135.64, 163.59, 165.51, 166.74.
FT-IR νmax 3669, 2979, 2746, 1705, 1669, 1615, 1331, 1208, 1115, 1023, 800 cm-1.
4-acetyl-3-(4-methoxy phenylamino) isoxazol-5(2H)-ones (6, X: OMe).
This compound was prepared as described above using Ethyl-2-(4-methoxyphenyl) carbamothioyl)-3-oxobutanoate (5, X: OMe) Refluxing for 24 hours gave the desired product (80%) which was recrystallized from ethanol/acetone (1:1) as a white solid, m.p: 206-207 ºC (dec.); 1H-NMR (D6-DMSO+CDCl3)δ(ppm) 2.3 (s, J= 7.0Hz, 3H, CH3), 3.35 (s, 3H, OMe), 6.38 (d, J=8.5Hz, 2H, Ar), 6.94 (d, J= 8.5Hz, 2H, Ar), 6.96 (bs,1H, NH), 7.70(bs,1H,NH).
13C-NMR(D6-DMSO+CDCl3)δ(ppm) 15.64, 55.55, 73.5 ,114.6, 118.6, 135.73, 153.7, 165.73, 168.14, 174.81.
FT-IR νmax 3407, 1708, 1615, 1554, 1248, 1077, 792 cm-1.
4-acetyl-3-(4-bromo phenylamino) -2-(5-nitropyridin-2-yl) isoxazol-5(2H)-ones (1, X: Br).
A mixture of 2-chloro-5-nitropyridine (48.5 mg, 0.30 mmol) and 4-acetyl-3-(4-bromo phenylamino) isoxazol-5(2H)-ones (6, X: Br), 100 mg, 0.30 mmol) was heated neat under nitrogen in an oil bath at 130 ºC for 2 hours. The residue was recrystallized from ethanol to give the desired isoxazolone as yellow crystals (112 mg, 82%), m.p: 218 ºC.
1H-NMR(D6-DMSO+CDCl3) δ(ppm) 2.25 (s, J=7.0Hz, 3H, CH3), 6.87 (d, J=8.5Hz, 2H,Ar), 7.18 (d, J=8.5Hz, 2H, Ar), 7.73 (d, J=9.1Hz,1H, Ar), 8.40 (dd, J1=9.1Hz, J2=2.3Hz, 1H, Ar), 8.75 (d, J=2.3Hz, 1H, Ar), 10.29 (bs, 1H, NH).
13C-NMR (D6-DMSO+CDCl3)δ(ppm) 14.41, 79.05, 114.63, 119.46, 124.11, 132.46, 135.08, 137.21, 141.66, 143.79, 153.92, 158.31, 161.38, 165.88.
FT-IR νmax 3140, 2965, 1773, 1683, 1591, 1531, 1324, 1188, 1114, 1010, 961, 832 cm-1.
MS m/z 419(M+, 27%), 417(M+, 30%), 406(74), 404(77), 279(100), 251(20), 184(35), 182(36), 157(29), 155(29), 102(22), 72(23), 44(59).
4-acetyl-3-(4-methyl phenylamino) -2-(5-nitropyridin-2-yl) isoxazol-5(2H)-ones (1, X: Me).
This compound was prepared as described for above using the corresponding isoxazolone (6, X: Me) and 2-chloro-5-nitropyridine to give the desired product after recrystalization from ethanol yellow needles (85%), m.p: 156-158 ºC, after recrystalization from ethanol.
1H-NMR (CDCl3)δ(ppm) 2.29 (s,J=7.05Hz, 3H, CH3), 2.35 (s, 3H, Me), 7.04 (d, J=8.5Hz, 2H, Ar), 7.07 (d, J=8.5Hz, 2H, Ar), 7.54 (d, J= 9.0Hz, 1H, Ar), 8.55 (dd, J1=9.0Hz, J2=2.5Hz, 1H, Ar), 8.91 (d, J=2.5Hz, 1H, Ar), 10.33 (s, 1H, NH).
13C-NMR (CDCl3)δ(ppm) 14.66, 21.35, 79.05, 115.42, 122.40, 130.29, 134.74, 135.36, 136.83, 141.89, 143.92, 154.28, 160.88, 163.62, 164.19.
FT-IR νmax 3177, 1762, 1700, 1600, 1515, 1338, 1208, 1123, 976, 838 cm-1.
MS m/z 355(M+, 13%), 354(100), 294(57), 269(16), 248(40), 230(16), 220(16), 158(39), 144(13), 118(21), 117(20), 107(16), 91(67), 78(16), 65(20), 44(33).
4-acetyl-3-(4-methoxy phenylamino) -2-(5-nitropyridin-2-yl) isoxazol-5(2H)-ones (1, X: OMe).
This compound was prepared as described for above using the corresponding isoxazolone (6, X: OMe) and 2-chloro-5-nitropyridine to give the desired product Yellow needles (80%), m.p: 186-188 ºC.
1H-NMR (CDCl3)δ(ppm) 2.30 (s, J=7.0Hz, 3H, CH3), 3.77 (s, 3H, OMe), 6.79 (d, J=8.7Hz, 2H,Ar), 7.10 (d, J=8.7Hz, 2H, Ar), 7.52 ( d, J=9.0Hz, 1H, Ar), 8.54 (dd, J1=9Hz, J2=2.1Hz, 1H, Ar), 8.93 (bd, J=2.1Hz, 1H, Ar), 10.26 (s,1H,NH).
13C-NMR (CDCl3)δ(ppm) 14.72, 55.89, 78.87, 114.85, 115.59, 124.34, 130.74, 134.68, 141.93, 143.95, 154.33, 158.40, 161.38, 163.69, 164.32.
FT-IR νmax 3823, 1785, 1700, 1592, 1345, 1207, 1115, 1030, 838 cm-1.
MS m/z 370 (M+, 10%), 356(100), 310(49), 295(43), 264(21), 249(13), 221(14), 193(12), 194(10), 174(21), 146(10), 134(34), 133(22), 123(17), 92(16), 77(29), 44(37).
1-(2-(4-bromo phenylamino) -6-nitroimidazo[1,2-a] pyridine-3-yl) ethanone (2, X: Br):
Pyrolysis (580°C, 0.01 mm Hg, sublimation flask 110°C, 60 min) of isoxazolone (1, X: Br) (100 mg, 0.23 mmol) gave pale cream needles (81.17 mg, 90%), m.p: 195-196 ºC.
1H-NMR(D6-DMSO)δ(ppm) 2.55 (s, J=7.0Hz, 3H, CH3), 7.49 (d, J=8.5Hz, Ar), 7.68 (d, J=9.7Hz, 1H, Ar), 7.74 (d, J=8.5Hz , 2H, Ar), 8.19 (dd, J1=9.7Hz, J2=1.6Hz, 1H, Ar), 8.87 (bd, J=1.6Hz, 1H, Ar), 9.88 (s,1H,NH).
13C-NMR (D6-DMSO)δ(ppm)15.25, 99.72, 114.62, 114.97, 121.76, 123.93, 127.61, 132.38, 137.66, 140.01, 147.17, 155.28, 160.87.
FT-IR νmax 3285, 2955, 1643, 1611, 1555, 1475, 1331, 1294, 1201, 1102, 1079, 1002, 820 cm-1.
MS m/z 375(M+, 62%), 374(M+,64%), 360(5), 358(6), 279(100), 251(16), 233(14), 205(12), 184(11), 182(12), 157(12), 155(12), 102(14), 78(13), 77(11).
1-(2-(4-methyl phenylamino) -6-nitroimidazo[1,2-a] pyridine-3-yl) ethanone (2, X:Me):
Pyrolysis (580°C, 0.01 mm Hg, sublimation flask 110°C, 60 min) of isoxazolone (1, X: Me) (100 mg, 0.26 mmol) gave the desired imidazole as pale cream needles (82.34 mg, 93%), m.p: 187-188 ºC.
1H-NMR (D6-DMSO)δ(ppm) 2.35 (s, J=6.9Hz, 3H, CH3), 2.55 (s, 3H, Me), 7.19 (d, J=8.2Hz, 2H, Ar), 7.51 (d, J=9.7Hz, 1H, Ar), 7.59 (bd, J=8.2Hz, 2H, Ar), 8.15 (bd, J=6.7Hz, 1H, Ar), 8.85 (bs, 1H, NH), 9.84 (bs, 1H, Ar).
13C-NMR (CDCl3)δ(ppm): 15.03, 61.44, 98.99, 114.30, 119.33, 122.80, 127.26, 130.07, 132.93, 137.11, 147.34, 157.76, 161,15 .
FT-IR νmax 3455, 1662, 1608, 1555, 1308, 1208, 1015, 822 cm-1.
MS m/z 310 (M+, 100%), 294(48), 248(27), 220(9), 144(10), 118(13), 91(20), 78(6), 65(6).
1-(2-(4-methoxy phenyl amino) -6-nitroimidazo[1,2-a] pyridine-3-yl) ethanone(2, X: OMe).
Pyrolysis (580°C, 0.01 mm Hg, sublimation flask 110°C, 60 min) of isoxazolone (1, X: OMe) (100 mg, 0.25 mmol) gave the title compound as a red solid (84.55 mg, 95%), m.p: 160-161 ºC;
1H-NMR (CDCl3)δ(ppm) 2.52 (s, J=7.1Hz, 3H, CH3), 3.73 (s, 3H, OMe), 6.86 (dd, J1=8.7Hz, J2= 2.5Hz, 1H, Ar), 6.92 (d, J=9.1Hz, 1H, Ar), 7.32 (d, J=8.7Hz, 1H, Ar), 7.45 (bs, 1H, Ar), 8.42 (dd, J1=9.1Hz, J2=2.6Hz, 1H, Ar), 9.26 (d, J=2.6Hz, 1H, Ar), 10.72 (bs, 1H, NH).
13C-NMR (CDCl3)δ(ppm) 15.02, 50.43, 89.99, 103.86, 111.00, 111.69, 112.14, 125.67, 126.89, 133.76, 138.65, 145.26, 145.59, 156.41, 156.58.
FT-IR νmax 3345, 1642, 1615, 1500, 1331, 1215, 1117, 1035, 838 cm-1.
MS m/z 326(M+, 70%), 310(100), 295(27), 264(32), 249(13), 221(29), 193(12), 194(10), 150(13), 78(6), 77(5).
Acknowledgements
The authors gratefully appreciated the financial support of this, work by the research council of Islamic Azad University branch of Mahabad.
References
- Ang, K. A.; Prager, R. H.; Smith, J. A.; Weber, B.; Williams, C. M, Tetrahedron Letters, 1996, 37(5), 675.
- Khalafy, J.; Setamdideh, D.; Akbari Dilmaghani, K, Molecules, 2002, 2, 907.
- Worrall, D. E, J. Am. Chem. Soc, 1923, 45, 3092.
- Worrall, D. E, J. Am. Chem. Soc, 1918, 40, 415.
- Nishiwaki, N.; Nakanishi, M.; Hida, T.; Miya, Y.; Tamura, M.; Hori, K.; Ariga, M, J.Org .Chem, 2001, 66, 7535.
- Perrin, D. D.; Amarego, W. L. F, In Purification of Laboratory Chemicals, Pergamon Press: Oxford., U. K., 1988.

This work is licensed under a Creative Commons Attribution 4.0 International License.









