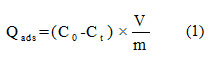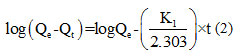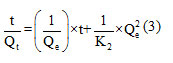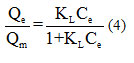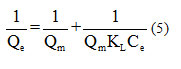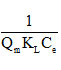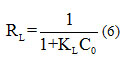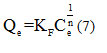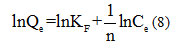Manuscript accepted on : 28-04-2021
Published online on: --
Plagiarism Check: Yes
Reviewed by: Dr. Mustafa Germeç
Second Review by: Dr. Bratin Sengupta
Final Approval by: Dr. rer. Nat. Hesham Ali El- Enshasy![]()

L.Kasmi1* , M. Soussi.El-begrani1
, M. Soussi.El-begrani1 , A. Ben Ali1
, A. Ben Ali1 , M.M.M. Hajaji1
, M.M.M. Hajaji1 , S. Tazi1
, S. Tazi1 and S. Haloui2
and S. Haloui2
1Materials and Interfacial Systems Laboratory; Abdelmalek Essadi University, Science Faculty, B.P. 2121, Mhannech II – 93002 – Tetouan – Morocco.
2Natural resources geosciences laboratory, Hydro-Informatics team, Faculty of Sciences. Ibn Tofail University- kenitra- Morocco.
Corresponding Author E-mail: La.kasmite@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2897
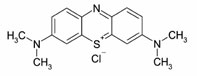
ABSTRACT: In the following study, we have tested Straw as a biomaterial for removing methylene blue (MB) by adsorption. The characterization of the adsorbent was carried out by scanning electron microscopy coupled with EDX (SEM), infra-red spectroscopy with Fourier transform and the point of zero charge (PZC). The studied variables are: the mass of adsorbent, the initial concentration of dye, the contact time, pH and temperature. Kinetic data were modeled by equations pseudo first order and pseudo-second order, and revealed that the adsorption of MB on the straw pseudo second order for initial dye concentration. The MB adsorption isotherms on straw were analyzed by models of Langmuir and Freundlich. The modeling of adsorption isotherms obtained good agreement with the model of Freundlich.
KEYWORDS: Adsorption; Isotherm; Kinetic; Modeling; Straw
Download this article as:| Copy the following to cite this article: Kasmi L, El-begrani M. S, Ali A. B, Hajaji M. M. M, Tazi S, Haloui S. The Use of Biomaterials as Adsorbents for Removing Colorants from Aqueous Solution Case of Straw with Respect to Methylene Blue. Biosci Biotech Res Asia 2021;18(1). |
| Copy the following to cite this URL: Kasmi L, El-begrani M. S, Ali A. B, Hajaji M. M. M, Tazi S, Haloui S. The Use of Biomaterials as Adsorbents for Removing Colorants from Aqueous Solution Case of Straw with Respect to Methylene Blue. Biosci Biotech Res Asia 2021;18(1). Available from: https://bit.ly/3gOmfrG |
Introduction
The presence of dyes in the effluents is of concerning because of its negative impact on many forms of life 1. It is estimated that there are more than 100,000 dyes available on the market with an annual production of 7 x 105 to 1 x 106 tonnes 2. Dyes are among other things that are used in many industries such as textiles, leather, paper and plastics. Furthermore, contamination by the dyes is a critical issue in the world, thus the issue needs to be taken care of and solved by looking for alternatives. The reduction or even elimination of these dyes is therefore necessary given the proven toxicity of some of them. Methods of operational treatments at the laboratory and at an industrial scale already exist, they include physicochemical methods (adsorption, membrane filtration, methods of solid-liquid separations: precipitation, coagulation, flocculation and settling), chemical methods (exchange resin ions, oxidation by oxygen, ozone …) and biological methods (anaerobic and aerobic treatment) 3, 4. These revelations could help industries to design a better wastewater treatment system which would help them to mitigate environmental pollution 5.
We will give an overview of the advantages and disadvantages of each process.
Flocculation and coagulation
Flocculation and coagulation is the technique in which a chemical is used to form colloid of the pollutants in wastewater and make them either settle down or float on top 6. This helps in easy removal of the contaminant. Flocculation is one of the most commonly used techniques in industrial wastewater treatment 7. this helps to easily remove the contaminant. In addition, large quantities of sludge are formed with this process: their regeneration or reuse remains the only way out but requires additional investments 8.
Membrane filtration
Membrane filtration at controlled hydraulic pressure is available in micro filtration, ultra filtration, nano filtration and reverse osmosis. Membrane technologies are environmentally friendly, but they are not applicable to all types of dyes and have limitations when used in the treatment of large volumes of water due to equipment costs and high energy consumption 9.
Oxidation
Oxidation is a method in which wastewater is treated through the use of oxidizing agents. Liakou and al used ozone for the degradation of ado dyes 10, while Malik and Saha used hydrogen peroxide for the oxidation of direct dyes 11. Oxidation methods are among the most widely used techniques for the decoloration of water, as they require small amounts of reagents and reaction times are relatively short But they have the disadvantage 12. of generating by-products of the oxidation reaction which can even be more toxic than the dye itself.
Advanced oxidation processes
They are techniques that involve more than one oxidation process. Advanced oxidation processes can be considered to be an alternative to the above-mentioned treatment procedure. In such processes hydroxyl radicals are generated which has the ability to degrade most of the organic pollutants present in the wastewater 13. Advanced oxidation has a very high degradation capacity and the process is very fast, it has been studied by other researchers; kumar and al (2016) in their work coupled carbon adsorption with advanced oxidation5. The Biotechnological methods are green technologies that ensure better aesthetics and a healthier environment while assisting the industries to minimize toxic compounds efficiency of these processes depends on many parameters such as the oxidant concentration, the intensity of UV light, pH, temperature .Thus cost and complication incurred during pH change would also be a big hindrance to commercial acceptance of such process 5.
Biological treatment
These processes are based on the presence of microorganisms in an oxygenated environment (aerobic), in the absence of oxygen (anaerobic) or on a combination of both 12 . Biotechnological methods are green technologies that ensure better aesthetics and a healthier environment while assisting the industries to minimize toxic compounds 14. The solution to this is the biological treatment that breaks down the organic loads of the wastewater into fundamental constituents forms a sludge which does not contain any chemicals like those used for chemical oxidation 15.
This makes it possible for the generated sludge to be used as fertilizer 16 Biological treatment methods seem to be an effective alternative treatment methods, but the prolong treatment time and high sensitivity of the biological degrading agents – bacteria and fungi poses a major threat to its commercial large scale implementation 5.
Adsorption
Adsorption is a surface phenomenon that involves the accumulation or concentration of substances on a surface or an interface 17. Adsorption is one of the most useful treatment processes of being cheaper, fast, efficient and eco-friendly 18. Moreover adsorption process edges over other processes as it is a sludge free process which provide efficient removal of toxic contaminants from their aqueous solutions 19. In addition, it is important to mention that, unlike the oxidation and electrochemical processes when treating water contaminated with dyes by adsorption, toxic by-products are not formed and in some cases the eliminated species can be recovered 20. In this study we chose the adsorption process as it is considered one of the most widely used techniques in treating water from metal ions and organic compounds. Adsorption process carried over the surface of activated carbon is considered as the most useful strategy among all the techniques owing to its several attributes such as being economical, faster, efficient and eco-benign 18. Several drawbacks limit its frequent use, of course the costs of treatment with this support are interesting and leading to the operational incumbencies during activated carbon regeneration, pore blocking and hygroscopic nature 21. Several studies have been conducted regarding removing of dyes with natural materials, they include the seaweeds 22 industrial waste: red mud 23, agricultural by-products 24 seafood waste: chitin 25. rice husk ash 26, Eichhorniacrassipes 19.
The objective of this study is to value the straw in the elimination of Methylene Blue (MB).
Material and methods
Adsorbat
The choice of MB dye meets the following criteria: their high solubility in water, simple and rapid analysis by UV spectrophotometry. Table1 represents the main physico-chemical properties of MB 27.
Table 1: Main physico-chemical characteristic of the MB.
Adsorbent
The straw used in this study was washed until all impurities were removed, then dried in the open air for 72 hours, then crushed and sifted to have particles of the same size (<125nm).
Material characterization
The morphology of the straw powder was observed using a scanning electron microscope coupled with EDX (SEM / EDX).Further, Fourier transform infrared spectrograph (FTIR) analysis of surface of raw straw and straw after adsorption of MB for the study of surface chemistry and identification of functional groups that exist. The spectra were collected from 400 cm−1to 4000 cm−1.
Adsorption procedure
After each adsorption experiment, and agitation speed of 150 rpm, the adsorbent was eliminated by centrifugation. Then the obtained liquid was analyzed by UV-visible spectrophotometry watching the changes in absorbance at λmax (MB) = 663 nm. The quantity adsorbed is calculated using the following formula:
Qads: Quantity adsorbed at the moment t in mg/g.
V: volume of the solution in ml.
and : are the initial concentration and the concentration at the moment t of the dye respectively in mg/l.
m: mass of the adsorbent in g.
Results and Discussion
Characterization of the adsorbent
Scanning Electron Microscopy (SEM)
Figure 1 show the SEM of the biomaterial before contact between the dye and the biomaterial and after contact. The morphology illustrates the cellulose microfibrils present in a lignin and hemicelluloses matrix.
 |
Figure 1: SEM biomaterial before (a)and after (b) the contact time |
In figure 2 (a), we have the composition of the biomaterial, which shows the presence of carbon elements, oxygen, aluminum, sodium…. Figure 2 (b) shows the SEM of the biomaterial after the contact time. As is clearly shown, there is not much difference in composition; except for the presence of the element nitrogen and sulfur present in the dye. This is mainly because the components of the biomaterial and the dye are of the same nature, but there is a slight decrease in the percentages of carbon and oxygen, this difference being due to the possibility that they participate in the production of carbon dioxide during the absorption process.
 |
Figure 2: EDX biomaterial before (a) and after (b) the contact time |
Fourier Transforms Infrared Spectroscopy
Figures3(a) and 3(b) are showing respectively the infrared spectra (400-4000 cm-1) of the front straw powder and after adsorption by MB. In the figure 3(a), the spectrum has the peak (very broad) at 3443.16 cm-1 due to the valence vibration of the hydroxyl group (OH) (alcohol bound). The absorption peak at 2922.66 cm-1 could be attributed to the C-H valence vibration (aromatic). The adsorption peak at 1632.03 cm-1 confirms the presence of the carboxyle group C-O (aldehyde or ketone) and two peaks at 1399.51 cm-1-1349 cm-1 are due to the deformation vibrations of the aliphatic C-H grouping while the one at 1112.72 cm-1 corresponds to the valence vibration of the C-O, the band near 1034.18 cm-1 is due to stretching vibrations of the aliphatic C-H, finally the bands 672.32 cm-1 662.90 cm-1; 617.76 cm-1 correspond to the vibrations of mineral materials 29 .In figure 3(b) we observe the same spectra with the offset wave number corresponding to the variation of the functional energy groups. This indicates the existence of a binding process MB made on the surface of the straw powder, with the appearance of certain peak which are: two peak between 2397 cm-1 and 2249 cm-1 which indicates the presence of conjugated carbon or unsaturated di- or possibly the production of CO2 per decarboxylation of the biomass 29 between the bands 1554 cm-1– 1538 cm-1 which indicates the presence of amines primaries N-H, and finally the presence of peak at approximately 1035 cm-1 is due to CN vibration.
 |
Figure 3: Infrared biomaterial before the contact time (a). Infrared biomaterial after the contact time (b) |
Point Of Zero Charge (PZC)
Point of zero charge PZC, is defined as the pH value which is the total net charge (external and internal) the particles on the surface of the adsorbent material is neutral, that is, the number of positive and negative sites is equal 29. 50 ml of distilled water was taken in 100 ml of Erlenmeyers, adjusting the pH of each solution between 2.0 and 11, by adding the appropriate quantities of HCl 1N and of NaOH 1N.0.05g of the straw powder was added to these solutions and after 48 hours under agitation and at room temperature, the final pH value was measured. The PZC is the point where the final pH curve as a function of the initial pH cuts the diagonal. The straw PZC is 6.33, meaning that the adsorbent surface is positively charged at pH less than 6.33, and negatively at a pH greater than 6.33. Therefore, the determination of this parameter is very useful to establish favorable conditions in terms of pH value effectively removing a particular dye. In the case of straw, anionic dyes are expected to be retained at a pH of less than 6.33 and that the removal of cationic dyes is promoted at a pH greater than this PZC value 30.Figure 4.
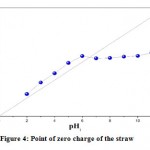 |
Figure 4: Point of zero charge of the straw |
Effect of different parameters on adsorption
Effect of Adsorbent Mass
The influence of the adsorbent mass was studied by shaking 50 ml of MB solution at 50mg/l, with different adsorbent masses (straw) ranging from 0.03 to 0.4g under constant agitation for 16 hours at room temperature and initial pH of the solution (pH free MB=4). The study of the influence of the mass of the MB on the adsorbed quantities of this pollutant is represented by the curve in Figure 5.
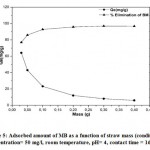 |
Figure 5: Adsorbed amount of MB as a function of straw mass (condition: concentration= 50 mg/l, room temperature, pH= 4, contact time = 16 h). |
We note that from the mass 0.1g, the adsorbed quantities of MB no longer evolve. This behavior can be due to the number of adsorption sites which increases with the amount of adsorbent up to a mass of 0.1g, from which the number of sites becomes stable 31, 32. On the other hand, some authors have shown that: the amount of adsorbent added to the dye solution is low, dye cations can easily access the adsorption sites; Adding adsorbent increases the numbers of adsorption sites but dye cations have more difficulty approaching these sites because of the clutter 33. In addition, a large amount of adsorbent creates the particles agglomerations, thus reducing the total adsorption area, therefore, a decrease in the amount of adsorbate per unit mass of adsorbent 33.
Effect of Initial Dye Concentration
To see the effect of MB concentration on adsorption capacity, the process was carried out with an initial dye concentration between 40 and 300 mg/l, the other parameters are constant (m=0.05g, t=16h, room temperature, pH=4). In figure 6, the elimination yield showed a downward trend when the initial MB concentration has been increased 34. At lower concentrations, all the MB present in the adsorption medium can interact with the bonding sites on the adsorbent surface; therefore higher adsorption yields were obtained. At higher concentrations, lower adsorption yields were observed due to saturation of adsorption sites 34.
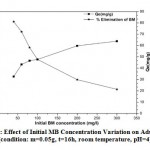 |
Figure 6: Effect of Initial MB Concentration Variation on Adsorption (condition: m=0.05g, t=16h, room temperature, pH=4) |
Effect of contact time
Adsorption of the MB on the straw is carried out at different contact times (0 to 180min) with an initial concentration of 70mg/l. The results are shown in Figure 7. They that the dye reduction by adsorption on the straw increases with the increase in contact time. This adsorption is fast and takes place in the first 15 to 20 minutes, beyond this time; the dye adsorption is constant at its maximum value. It can be deduced that the contact time or the appropriate equilibrium time is beyond 20min. For all other adsorption tests with an initial concentration of 70mg/l and to ensure the right choice of contact time, we opted for 60 minutes of agitation.
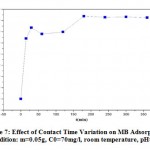 |
Figure 7: Effect of Contact Time Variation on MB Adsorption (condition: m=0.05g, C0=70mg/l, room temperature, pH=4) |
pH effect
The pH influence on MB removal rate on straw (Figure 8), was studied using a. VENGO 6230 brand pH meter. The experiments were carried out by mixing 0.05g of straw with 70mg/l concentration MB solution, and at room temperature. The pH of the solution has been adjusted to specific values of: 2; 4; 6; 8; 10 and 12 by adding a few drops of HCl (0.1M) and NaOH (0.1 M). The mixtures are subjected to constant agitation for 1h. After centrifugation, the liquid is recovered and analysed by UV-Visible. The initial pH for the adsorption is an important process parameter [35]. This parameter influences the distribution of adsorbent species as well as the ionization of functional groups on the surface of the adsorbent [36].Figure 8 shows the percentage of elimination of BM by the straw as a function of the pH of the medium (2, 4, 6, 8, 10 and 12). It can be seen that at pH = 2 the lowest percentage of elimination was obtained. This can be attributed to increased competition between hydronium ions and BM cations for adsorption sites on the surface of the biosorbent. In the pH range of 6 to 12, a large increase in the percentage of elimination.
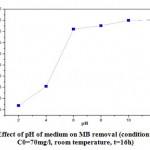 |
Figure 8: Effect of pH of medium on MB removal (condition: m=0.05g, C0=70mg/l, room temperature, t=16h). |
The change in pH of the sample affected the surface charge of the adsorbents and the adsorptive process. This might be due to the dissociation of functional groups present on the active sites of the adsorbent [37].The straw exhibited a pHpzc (6.33), therefore the greater increase in percent removal corresponds to a greater increase in the number of negatively charged sites. At pH values of the solution above the pHpzc, the number of negative charges predominates over the positive ones and, therefore, the feasibility of the BM adsorption process by ion exchange increases.
Effect of temperature
It can also affect the adsorption process, temperature factor. The adsorption of the MB of an aqueous solution at different temperatures was studied in a temperature range from 20 to 60°C.
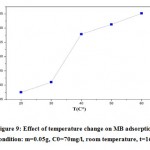 |
Figure 9: Effect of temperature change on MB adsorption (condition: m=0.05g, C0=70mg/l, room temperature, t=16h) |
As is clear in Figure 9, an increase in temperature from 20°C to 60°C is accompanied by an increase in the removal percentage of the MB dye from 53% to 93%. This phenomenon, suggests that the reaction is endothermic whose temperature increase favors the adsorption mechanism. This endothermic phenomenon of adsorption was also observed with other materials for the same dye studied 38, 39.
Kinetic Adsorption Models
The determination of the batch adsorption kinetic parameters is essential for the design of adsorption columns in pilot plants for further scale up 40. In this study, pseudo first order model, pseudo second order model were the key kinetic models that were investigated in the adsorption process by straw, their parameters and the MB correlation coefficients were calculated from figure (11) and listed in Table 2.
Pseudo-first order kinetic model (Lagergren model) 41 and Pseudo second order kinetics model: (Ho and Mckay)42
With: K1 (L.min-1): rate constant of adsorption of the kinetics of the pseudo first order.
With: K2 (g.mg-1.min): rate of adsorption constant of the pseudo second order kinetics.
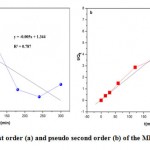 |
Figure 10: Pseudofirst order (a) and pseudo second order (b) of the MB adsorbed by straw. |
Figure10 (a) shows that for the first order kinetic model the coefficient of determination R2 is equal to 0.787 and the adsorption capacity is much lower than that obtained experimentally. On the other hand, the coefficient of determination for the second-order kinetic model shown in Figure10 (b) is 0.994 were closer to one, and the values obtained are comparable with the experimental values (Qexp=51.67mg/g). These observations lead us to say that the adsorption with methylene blue does not express a controlled diffusion process because it does not follow the pseudo-first equation; given by Lagergren the adsorption process therefore follows a second-order kinetic model, which considers the external mass transfer, the intra-particulate diffusion.
Table 2: Methylene blue adsorption kinetic parameters
| dye | K | Qe | R2 | |
| Pseudo first order | MB | 0.011515 | 22.08 | 0.787 |
| Pseudo second order | MB | 0.001402 | 55.56 | 0.994 |
Adsorption isotherm
The study of adsorption isotherms, allows us to better understand how adsorbed MB molecules interact with the adsorbent (straw), when the adsorption process approaches to a state of equilibrium. Adsorption isotherms provide many fundamental physico-chemical data to estimate the applicability of the adsorption process, to express surface properties and the adsorbent affinity which can also be used to find the maximum adsorption capacity of a mass [43]. Many isothermal models are available in the literature, Freundlich and Langmuir models are the most frequently used to describe the experimental adsorption isotherm data, due to their simplicity
Langmuir model
Langmuir isotherm model predicts the formation of a single layer of adsorbed molecules (molecular monolayer) on specific sites and without interaction between them with an adsorption heat independent of the surface. The Langmuir model is defined by the following equation 44:
With Ce (mg.l-1) is the concentration at equilibrium, Qe and Qm (mg.g-1)are the amount adsorbed to equilibrium and the maximum quantity adsorbed to the saturation of the monolayer, KL (l.mg-1) is the adsorption equilibrium constant depending on the temperature.
The linear transform of this model has as equation:
If Langmuir’s equation is checked, by carrying
![]() based on
based on
![]() , the slope line is
, the slope line is
![]() , which allows us to determine Qe and KL.
, which allows us to determine Qe and KL.
The graphical presentation of the Langmuir isotherm is shown in Figure11 (a).
The adimensional parameter of Hall RL can verify the favorability of the Langmuir isotherm in the following form 45:
With C0 the initial concentration in mg L-1. If RL<1 Isothermal favourable, RL>1 Isothermal unfavourable and if RL=1 Isothermal linear.
Freundlich model
The Freundlich isotherm model assumes heterogeneity of the adsorption surface with sites of different adsorption energies, as well as the possibility of multi-layer formation of adsorbed molecules with interactions between them [46]. The Freundlich model is described by the following equation:
Where Qe (mg.g-1) is the amount adsorbed to equilibrium, KF and n are Freundlich constants characteristic of the efficiency of a given adsorbent towards of a given solute, this (mg .L-1) is the solute concentration at equilibrium.
The logarithmic scaling of this equation makes it possible to verify its linear transformation:
By tracing lnQe based on lnCe, slope line 1/n is obtained and ordered originally lnKF.
The graphical presentation of the Freundlich isotherm is shown in Figure 11 (b)
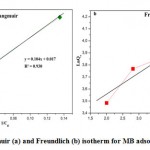 |
Figure 11: Langmuir (a) and Freundlich (b) isotherm for MB adsorption on straw |
Table (3) shows the values of the Langmuir and Freundlich constants, extrapolated from the lines of these two models.
Table 3: Langmuir and Freundlich constant
| Langmuir model | Freundlich model | |||||
| Qm | KL | RL | R2 | KF | n | R2 |
| 58.8235 | 0.163461 | 0.08037 | 0.930 | 23.9507 | 5.49 | 0.961 |
Based on coefficients of determination, presented in table (3), it can be said that the Freundlich model (Figure11 (b)). Better describes the adsorption isotherm of the MB on the straw, with a correlation coefficient R2=0.961, and like n>1, then it is a physical adsorption.
Conclusion
The results of the adsorption of methylene blue on the straw showed that retention is fast. The temperature has a favorable effect on the percentage dye removal which suggests that the adsorption of MB on the straw was an endothermic process. The MB maximum adsorption occurred at pH> PZC meaning that the electrostatic forces influence on the dye binding power. The modeling of adsorption results showed that the model of pseudo-second-order offers better correlation of kinetic data and the Freundlich’s model better describes the adsorption phenomenon of methylene blue on the straw. The powder of the straw is a biomaterial having interesting adsorption capacities and can be an alternative to other commercial supports.
Acknowledgement
The authors would like to thank the stuff in charge of the laboratory “Materials and Interfacial Systems”; Abdelmalek Essadi University, Science Faculty, Tetouan, Morocco, for their assistance in making this study a success.
Conflict of Interest
All the authors acknowledge that they have no conflicts of interest.
Funding Source
There is no funding source
References
- Hammed B.H. Evaluation of papaya seeds as a novel non-conventional low-cost adsorbent for removal of methylene blue. Journal ofHazardous Materials. 2009; 162:939-944.
CrossRef - Ravi K., Deebika B., Balu K. Decolourization of aqueous dye solutions by a novel adsorbent: application of statistical designs and surface plots for the optimization and regression analysis. Journal of Hazardous Materials. 2005; 122:75-83.
CrossRef - Pokhel D., Viraraghavan T. Treatment of pulp and paper mill wastewater: a review. Science of the Total Environement.2004; 333:37-58.
CrossRef - Robinson T., McMullan G., Marchant R., Nigam P. Remediation of dyes in textiles effluent: a critical review on current treatment technologies with a proposed alternative. 2001; 77:247-255.
CrossRef - Kumar A., Sengupta B., Kannaujiya M.C, Priyadarshinee R., Singha S.,Dasguptamandal D., Mandal T. Treatment of coke oven using ozone with hydrogen peroxide and activated carbon. Desalin Water Treat. 2017; 69:352–365.
CrossRef - Hargeaves A.J., Vale P., Whelan J., Alibardi L., Constantino C., Dotro G., Cartmell E., Campo P. Coagulation-floculation process with metal salts, synthetic polymers and biopolymers for the removal of trace metals (Cu, Pb, Ni, Zn) from municipal .Wasterwater Clean Environ . 2018; 20(2) : 393-402.
CrossRef - Nair A.T., Ahammed M.M. The reuse of water treatment sludge as acoagulant for post-treatment of UASB reactor treating urban wasterwater. J Clean Prod .2015; 96:272-281.
CrossRef - Errais E .Réactivité de surface d’argiles naturelles étude de l’adsorption de colorants anioniques. Thèse Université de Strasbourg, France.2011 p75-86.
- Kumar K.V., Porkodi K. Mass transfer, kinetics and equilibrium studies for the biosorption of methylene blue using Paspalum notatum. Journal of Hazardous Materials. 2007; 146: 214-226.
CrossRef - Liakou S., Pavlou S., Lyberatos G. Ozonation of azo dyes. Water Science and Technology.1997; 35: 279-286.
CrossRef - Malik P.K., Saha S.K. Oxidation of direct dyes with hydrogen peroxide using ferrous ion as catalyst. Separation and Purification Technology. 2003; 31: 241-250.
CrossRef - Gupta V.K., Carrott P.J., Ribeiro M.M., Suhas. Low-cost adsorbents: growing approach to wastewater treatment. Critical Reviews in Environmental Science and Technology. 2009; 39: 783-842.
CrossRef - Akpotu S.O., Oseghe E.O., Ayanda O.S., Skelton A.A., Msagati T.A., Ofomaja A.E. Photocatalysis and biodegradation of pharmaceuticals in wasterwater. Effect of abiotic and biotic factors. Clean Technol environ. 2019
CrossRef - Kumar A., Priyadarshinee R., Singha S., Sengupt B., roy A., Dasgupta D., Mandal T.Biodegradation of alkali lignin by Bacillus flexus RMWWII: analyzing performance for abatement of rice mill wastewater. Waterscience and Tecnology 2019; 80(9): 1623-1632.
CrossRef - Bokare A.D., Choi W. Review of iron-free Fenton-like systems for activating H2O2 in advanced oxidation processes. J Hazard Mater. 2014; 275:121–135.
CrossRef - Cartes J., Neumann P., Hospido A., Vidal G. Life cycle assessment of management alternatives for sludge from sewage treatment plants in Chile: does advanced anaerobic digestion improve environmental performance compared to current practices?J Mater Cycles Waste.2018; 20:1530–1540.
CrossRef - Cooney D. Adsorption design for wastewater treatment. Lewis Publishers. 1999 p. 30.
- Pizarro J., Castillo X., Jara S., Ortiz C., Navarro P., Cid H., Rioseco H., Barros D., Barros N. Adsorption of Cu2+on coal fly ash modified withfunctionalized mesoporous silica. Fuel.2015; 156: 96–102.
CrossRef - Kumar A., Jash A., priyadarshinee R., Sengupta B., Dasgupta D., Halder G., Mandal T. Removal of catechol from aqueous solutions by adsorption using low cost activated carbon prepared from Eichhornia crassipes.Desalin Water Treat.2017; 73: 389-398
CrossRef - Rafatullah M., Sulaiman O., Hashim R., Ahmad A. Adsorption of methylene blue on low-cost adsorbents. Journal of Hazardous Materials. 2010; 177: 70-80.
CrossRef - Mohan D., Pittman C. U., Steele P. H. Journal Colloid interface Sci. 2006; 297:489-504.
CrossRef - Ranam A., Rao J.R., Nair B.U. Adsorption of phenol onto activated carbon from seaweed, determination of the optimal experimental parameters using factorial design. Taiwan Inst. Chem. Eng. 2011; 142:952-956.
CrossRef - Bhatnagar A., Vilar V. J. P., Botelho C. M. S., Boaventura R. A. R. A review of the use of red mud as adsorbent for the removal of toxic pollutants from water and wastewater. Technol. 2011; 32:231-249.
CrossRef - Djilani C., Zaghdoudi R., Modarressi A., Rogalski M., DjaziF.,Lallam Elimination of organic micropollutants by adsorption on activated carbon prepared from agricultural waste. Chem. Eng 2012; 189-190, 203-212.
CrossRef - Liu Y., Zheng Y., Wang A. Enhanced adsorption of methylene blue from aqueous solution by chitosan-g-poly, acrylic acid./vermiculite hydrogel composites. J. Environ. Sci. 2010; 22:486-493.
CrossRef - Kumar A., Singha S., Sengupta B., Dasgupta D., Datta S., Mandal T. Intensive insight into the enhanced utilization of rice hisk ash: abatement of rice mill wastewater and recovery of silica as avalue added product. Eco Eng. 2016 ; 91: 270-281
CrossRef - Bouchemal N., Merzougui Z., Addoun F. Adsorption en milieux aqueux de deux colorants sur charbons actifs à base de noyaux de dattes.Journal de la société Algérienne de chimie. 2011; 21:1-14
- Albis A., López A., Romero M. Removal of methylene blue from aqueous solutions using cassava peel (Manihot esculenta) modified with phosphoric acid. 2017; 15:60-73.
CrossRef - Franks G. V., Meagher L. The isoelectric points of sapphire crystals and alpha-alumina powder. Colloids and Surfaces A:Physicochemical and Engineering Aspects. 2003; 214(1-3): 99-110.
CrossRef - Villa F., Anaguano A. Detemination of the point of zero change andisoelectric point of two agricultural wastes and their application in the removal of colorants. Revista de investigacionAgraria y ambiental. 2013; 4:27-36.
- Gupta V.K., Mittal A., Gajbe V. Adsorption and desorption studies of a water soluble dye, Quinoline Yellow, using waste materials .Colloid and Interface Science. 2005; 284(1) : 89-98
CrossRef - Tsai W.T., Hsu H.C., SuT.Y., Lin K.Y., Lin C.M., Dai T.H. The adsorption of cationic dye from aqueous solution onto acid-activated andesite. Hazard Mater. 2007; 147(3):1056‑1062.
CrossRef - Bennani K. A., Mounir B., Hachkar M., Bakasse M., Yaacoubi A. Removal of basic dye Methylene Blue in aqueous solution by Safi clay .Sci. Eau.2010; 23(4) :375-388
CrossRef - Ozer A., DursunG.Removal of methylene blue from aqueous solution by dehydrated wheat bran carbon. J. Hazard. 2007; 146 : 262-269
CrossRef - Kushwaha J.P., Srivastava V.C., Mall I.D. Treatment of dairy wastewater bycommercial activated carbon and bagasse fly ash parametric, kinetic andequilibrium modelling, disposal studies. Bioresour. Technol.2010; 101: 3474–3483).
CrossRef - Rosas C.J.M Aplicacion de residues agricolas para el tratamiento de agua contaminada con colorants. Theses Universidad Autónoma de Nuevo León, México.2012p4
- Totlani K., Mehta R., Mandavgane S.A. 2012. Comparative study of adsorption of Ni (II) on RHA and carbon embedded silica obtained from RHA. Chem. Eng. 2012; 181: 376–386.).
CrossRef - Somasekhara R. M. C., Sivaramakrishna L., Varada R. A. The use of an agricultural waste material, Jujuba seeds for the removal of anionicdye (Congo red) from aqueous medium. Journal of Hazardous Materials 2012; 203-204:118– 127.
CrossRef - Venkat S ., Vijay B. P. V.Kinetic and equilibrium studies on the removal of Congo red from aqueous solution using Eucalyptus wood (Eucalyptus globulus) saw dust. Journal of the Taiwan Institute of Chemical Engineers 2013; 44:81-88.
CrossRef - Chatterjee S., Kumar A., Basu S., Dutta S. Application of response surfacemethodology for methylene blue dye removal from aqueous solution usinglow cost adsorbent. Chem. Eng.2012; 181: 289–299.).
CrossRef - Lagergren S.Kungliga Svenka Vetenspsakademiens. Handlingar. 1898; 24:1-39
- Ho Y. S., McKay G. The Kinetics of Sorption of Divalent Metal Ions onto Sphagnum Moss Peat. Water Research2000; 34: 735-742.
CrossRef - Senturk H. B., ozdes D., Duran C. Biosorption of Rhodamine 6G from aqueous solutions onto almond shell (Prunus dulcis) as a low cost biosorbent. Desalination. 2010; 252: 81-87.
CrossRef - Langmuir I. The Constituction and fundamental properties of solids and liquids. Part I. Solids. Journal of the American Chemical Soc.1916; 38: 2221-2295.
CrossRef - Hall K. R., Eagleton L. C., Acrivos A., Vermeulen T., Industrial and Engineering Chemistry Fundamentals. 1966; 5(2): 212-223.
CrossRef - Freundlich H.M.F. Uber Die Adsorption in Losungen. Industrial and Engineering Chemistry Fundamentals. 1906; 57: 385- 470.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.