Manuscript accepted on : 16 March 2017
Published online on: --
Plagiarism Check: Yes
Regina Eduardovna Dashko and Ivan Viktorovich Alekseev
Department of Hydrogeology and Engineering Geology, Mining University (University of Mines), St. Petersburg Russia.
Corresponding Author E-mail: alekseew.ivan@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2432
ABSTRACT: Role of biocorrosion in the early fracture of underground metal structures at the Yakovlev’s mine (Kursk Magnetic Anomaly) of high-grade iron ores (HGIO) is analysed based on integrated study including microbiological methods of investigation. Major natural and technogenic sources of microorganisms input into the iron ore body are specified. Underground microbiota of the Yakovlev’s mine along with factors promoting its intensification are studied. Particular attention is paid to favorable conditions existing down the mine that facilitate electrochemical and biochemical corrosion of ferrous metals. During the investigation a wide range of aerobic and anaerobic microorganisms including various species of bacteria, micromycetes and actinomycetes was encountered. Inoculated cultures were frequently characterized by abnormal microbial counts exceeding 106 cells per 1 g of dispersed iron ore. Special microbiological monitoring aimed to study in situ dynamics of deteriorating test metal coupons placed in the aggressive environment of moistened HGIO was conducted. Acquired results are suggesting the necessity of due account of microbial corrosion while assessing safety of mining operations and ensuring stability of the metal arch support.
KEYWORDS: biocorrosion; high-grade iron ores; mine workings; monitoring subsurface microbiota;
Download this article as:| Copy the following to cite this article: Dashko R. E, Alekseev I. V. Microbially-Induced Corrosion of Structural Materials in Underground Workings of the Yakovlev’s Mine (Kursk Magnetic Anomaly, Russia). Biosci Biotech Res Asia 2017;14(1). |
| Copy the following to cite this URL: Dashko R. E, Alekseev I. V. Microbially-Induced Corrosion of Structural Materials in Underground Workings of the Yakovlev’s Mine (Kursk Magnetic Anomaly, Russia). Biosci Biotech Res Asia 2017;14(1). Available from: https://www.biotech-asia.org/?p=22817 |
Introduction
The Yakovlev’s deposit of Kursk Magnetic Anomaly is considered to be unique by high-grade iron ore reserves and difficulty of engineering-geological conditions of mineral product excavation. Mining operations are conducted in the underground workings at depth about 650 m beneath the system of high-pressure aquifers, confined to clastic-carbonate formation of Upper Palaeozoic, Mesozoic and Cenozoic age. The drainage work is performed only in the iron ore body, which involves ore-crystalline aquifer drained by means of flowing horizontal and inclined wells. Iron ore body formation is related to chemical weathering processes of Archean-Proterozoic rocks in the crystalline basement that are presented by jaspilites and iron schists. Long continental stage of development in the Late Proterozoic – Middle Devonian time determined formation of massive weathering crust, accompanied by generation of HGIO with fine-grain structure and iron content up to 65%. Being water-saturated and influenced by hydrodynamic pressure these ores easily pass into the quick-sand state.
Thick chemical weathering crust formation is connected not only with chemical and physical-chemical processes of source rock transformation, but with activity of various microorganisms as well.1–5 The significance of biochemical processes in weathering crust formation was studied as far back as in XIX century, and over the course of the last century it was confirmed by numerous theoretical and experimental works of Russian and foreign scientists.1,5–9
Abundant microflora, attributed to chemical weathering crust and overlying sedimentary strata, continues actively developing in the massif of water-saturated rocks of the Yakovlev’s mine up to the present day. It was established, that it affects all components of underground space, including the weakest iron ores and rocks, leading to activation and development of hazardous engineering-geological processes in mining tunnels.10–12x
The purpose of current work is to study the role of subsurface microbiota in biocorrosion destruction of metal arch support at the Yakovlev’s mine. Primary objectives are following:
identify main sources of microbiota input into the underground workings;
consider the integrated use of different methods in microbiology and geology to assess activity of microorganisms in metals deterioration;
analyse the results of field and laboratory experiments aimed to evaluate driving rate of biocorrosion in the underground workings for the purpose of operating safety improvement.
Sources of Microorganisms
For a long time deep horizons of the Earth crust, characterized by extreme conditions, were considered to be sterile. However, since 1924 an abundant microflora was found in deep oil wells, black smokers of submarine trenches, geysers and others. Subsurface life was detected even at the depth of several thousand meters in bottom sediments of the Mariana Trench (10 898 m), where 2 barophilic strains of bacteria were identified.13 For continental conditions the maximum depth, where viable microorganisms have been retrieved, is 5278 m (granite massif in Sweden).14
Weathering crust formation obligatory involves participation of microbiotic activity. In the course of geological processes microorganisms are buried and fossilized.1,3,8,15,16 Thus, transformed jaspilites and iron schists of the Yakovlev’s deposit should be considered as a natural source of microorganisms, inherited through geological time and representing constantly evolving forms of archaic microflora. Their existence is controlled by autolytic fermentative processes, which lead to the dissipation of complex molecules and the destruction of toxic metabolism products.
According to our studies, among the sources of microbiota input into the iron ore body should be named the confined aquifer of Carboniferous age, which defines constant downward flow of groundwater containing hydrogen sulphide and a high content of aggressive CO2 (figure 1). In the central and bottom parts of this limestone bearing strata are traced some layers containing organic matter (brown coal, bituminous carboniferous clays and schists), that define formation of reductive conditions and development of anaerobic bacteria, including sulphate-reducing forms.
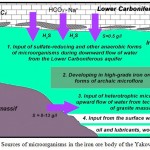 |
Figure 1: Sources of microorganisms in the iron ore body of the Yakovlev’s mine
|
At a later time another source of microorganisms input was found. It was attributed to the upward flow of sodium chloride water with mineralization 8–12 g/dm3 through the tectonic fractures of granite massif. Data acquired from hydrochemical, biochemical and gas-chromatography studies state that such water contains a lot of organics, actively recovered by heterotrophic forms of microorganisms..11 In the water using direct microbiological methods (bacterial inoculation) an exceptionally rich microbial association was established. Particularly, high numbers are typical of sulphate-reducing (107 cells/ml), iron-reducing, sulphur-oxidizing bacteria (106 cells/ml), and other species, including ammonifying bacteria. The possibility of microbiota input with mineralized water from intrusive bodies is confirmed by numerous research data of Sweden specialists in potential sites of radioactive waste disposal – igneous and metamorphic rocks of Fennoscandian shield. Based on these works natural content of microorganisms in groundwater of similar composition attains 103-107 cells per 1 ml. Notably species composition coincides to a large extent to that of the Yakovlev’s deposit.17,18,19
Apart from noted sources of microorganisms they can ingress into the underground workings with air flow from ventilation systems, and from the surface with mine personnel and different mechanisms.
Studying Features of Microbiota Development at the Yakovlevsky Mine
The intensity of microbial activity is defined by the presence of nutritious and energetic substrate, which comes from natural and technogenic sources. It is related as well to specifics of physical-mechanical, biochemical and temperature conditions of underground medium, effects of low radiation doses associated with radon exhalation from tectonic fractures (15–1220 Bq/cub. m), and to weak magnetic field (lower than1 mT), created by HGIO. Furthermore, ingress of diesel fuel, combustibles and lubricants, while operating mining machines and mechanisms, as well as secondary moistening of ores and rocks due to the water flow from confined aquifer lead to changes in physical-chemical, biochemical conditions and to intensification of microbial activity.
Study of microbiological activity at the Yakovlev’s mine was first conducted in 2003 by the department of hydrogeology and engineering geology of Saint-Petersburg Mining University under the supervision of Professor Dashko R.E. The investigation concerned damage source of metal pipes and cotton canvas bags with HGIO stored during 1–1.5 week. Conducted research of destroyed materials confirmed the presence of a numerous population of bacteria and micromycetes.10
Carried out down the mine microbiological studies included mycological analyses, microbial mass measurements in different sorts of HGIO with various moisture conditions and levels of contamination by fuels and lubricants. It was established that the secondary moistening of drained ores triggers increase in microbial mass on average by two. Studying size and composition of microbial population in the ore samples and groundwater allowed to draw a conclusion, that microbial activity is typical of the whole iron ore body and it is determined by development of both, aerobic and anaerobic forms of microbiota. There was identified more than 40 species of micromycetes, recorded active development of iron bacteria, magnetotactic, nitrifying, sulphate-reducing bacteria, clostridia and actinomycetes.11
In 2008 temporary mining work stoppage and diminution of ventilation in underground workings led to proliferation of anaerobic and facultative forms of microorganisms, and, as a consequence, ended up in profuse discharge of slimes on surface of underground tunnels and metal structures. At the same time on operating mining levels was detected development of macromycetes (figure 2).
 |
Figure 2: Various forms of macromycetes in the underground workings of the Yakovlev’s mine
|
Since 2010 we are studying role of microorganisms in development of hazardous engineering-geological processes in underground workings of the mine, including biocorrosion of structural materials. Repeated surveys of arch support on the operating levels of the mine were executed. Following special procedures detailed sampling was carried out. Investigation of microbial negative impact on metal structures and concrete of backfilling materials was conducted. It was established, that strength of cubic specimens, made of lightweight concrete and used down the mine for backfilling operations, after 3 months of being stored in a container with loose iron ores, saturated with water from the Ore-crystalline aquifer, decreased due to microbiological influence by 55-56%. During field tests of backfilling material strength using non-destructive inspection method were acquired comparable results. The most active development of microorganisms was observed in the zones of intense watering due to downward flow from the Lower Carboniferous aquifer.
Material and Methods
In practice, deterioration of structural materials is caused by a broad spectrum of microorganisms, which produce different gases, organic acids, enzymes, polysaccharides, and other exometabolites. Their destructive ability is stipulated by external environment. In this context while studying corrosion dynamics it is a matter of great importance to consider not only properties of materials and interacting with them components of underground medium (rocks, ores, groundwater), but hydrochemical, redox and acid-base conditions of environment and microbiota specifics as well.11,20,21
Metal Coupons Experiment
For a quantitative assessment of metal arch support corrosion a real experiment was conducted. It consisted in a long-term exposure of test metal coupons in aggressive anaerobic environment created by loose HGIO of different grain size composition with various water contents and levels of microbial contamination. This method, based on measurements of changing test samples mass, is widely used in Russia, as in other countries as well.22,23 For the experiments were used bright-finished stainless steel coupons (ST-3 steel grade) 5 centimetres-square, which were decontaminated, gauged on analytic balance and sterilized with flaming. Some samples were additionally covered by a thin layer of nutrient medium that would allow to accelerate microbiota absorption on the coupon surface and to facilitate growth of heterogenic organisms. In our experiments two types of culture medium were used: Czapek’s agar (for extracting micromycetes, actinomycetes and some bacteria), and meat-and-peptone agar (for a wide range of heterotrophic bacteria).
The coupons were placed on operating mine levels into the HGIO of micaceous hematite-martite (“blue ores”) and gothite-hydrohematite (“red ores”) composition within the areas of increased moistening by groundwater at the interface of arch support. After 2, 4 and 6 months corroded coupons were extracted, mechanically cleaned from corrosion products and weighed for an assessment of destruction rate. Corrosion products were then studied microbiologically to identify available microorganisms. For each test coupon after its retrieval from HGIO inoculation in different media was performed in order to reveal the whole microbial community.
Mycological Examination
It was targeting identification of structure and composition of microscopic fungi population, which are able to deteriorate metal constructions. Samples of HGIO from the locations of metal coupons placing were studied together with related corrosion products. For primary insulation, maintenance in the medium and micromycetes identification following culture media were used: Czapek’s agar, potato-glucose agar, the Sabouraud and wort agars. Isolation from dispersed ores was performed through its sieving on the culture medium, and from corrosion products – through surface washing, dilution of acquired suspension and its following sieving on the growth medium. Isolated cultures of micromycetes were incubated within thermostat during 2-3 weeks at 25°C until sporulation, and then were identified using light microscopy.
Bacteriological Analysis
For analysis were used washouts from metal coupons diluted in a certain volume of sterile distilled water. Microbes’ isolation required the use of agarized and liquid culture media. Cell-seeded plates were incubated in the dark at 30°C. Plate count and study of microbial colonies forming in aerobic conditions were made at day 3 and 7. Cultivation of anaerobic bacteria was achieved by using anaerobic jar, whereas colonies were analysed at day 7-25 starting from a sieving. Bacteriological count was performed using the dilution method. Microscopic study was realized according to standard methods. Based on collected data was drawn a preliminary conclusion as for affiliation of bacteria to certain groups. Dominant colonies morphotypes and unidentified bacterial species were selected for molecular study by means of DNA extraction followed by 16S rRNA gene sequencing using PCR.
Analytical Investigation
Simultaneously with large 5 centimetres-square metal coupons smaller test coupons of centimetre-square size were treated in a similar way to study character of metal surface deterioration using scanning electron microscopy (SEM). Coupons prepared for SEM (Jeol JCM-5000, Neoscope) were gold coated. Microscopy was conducted at the State University of Saint-Petersburg (Resource centre of molecular and cell technologies). The method provided a clear image of transformed surface layer of corroded material, gave an insight into its degree, destruction depth and character of corrosion processes.
Results and Discussion
Mycological Examination
In the iron ore samples from sites, where test metal coupons had been placed, a significant number of micromycetes with various species composition was found (Table 1). Developing on dispersed particles mostly in the form of biofilms, they produced extremely saturated populations with a high number of colony forming units (CFU) – up to 6700 CFU per gram. Totally in the samples from two operating mine levels 35 species of micromycetes were recognized. On the media designed for micromycetes frequently were developing bacterial species that is indicative of close links in microbial communities. Among others, the dominant position by number and occurrence frequency was held by Penicillium and Aspergillus species, which by virtue of their metabolism are able to significantly accelerate corrosion under conditions of periodical moistening. Therewith were locally found aggressive concrete destructors presented by Fusarium sp.
Table 1: Number and species composition of micromycetes in HGIO samples from sites of test metal coupons placing
| Spot No. and HGIO types | Placing sites of metal coupons | Species of micromycetes | CFU per 1 g |
| 1. “Blue ores” | Intersection of ventilation crosscut No.2 with filling gallery | Mucor hiemalis; Trichoderma viride; Fusarium oxysporum; Mucor plumbeus; Gliocladium penicillioides | 4500 |
| 2. “Red ores” | Technological tunnel No.3, +50,8 m from panel cross drift No.7 | Trichoderma viride; Doratomyces stemonotis; Penicillium purpurogenum; Penicillium herqueri; Fusarium solani; Penicillium oxalicum; Cladosporium cladosporioides | 6700 |
| 3. “Blue ores” | Prospecting drift No.8, drilling chamber on the 4th cross drift line | Doratomyces stemonitis; Fusarium solani; Trichoderma viride; Scopulariopsis acremonium | 3000 |
| 4. “Red ores” | Side drift No.3, drilling chamber on the 18th cross drift line | Trichoderma viride; Paecilomyces javanicus; Fusarium solani; Scopulariopsis acremonium | 900 |
Bacteriological Study
The highest bacterial count was found in the “red” type of ores (Table 2). Abnormal numbers of bacteria in “blue ores” are explained by excessive local moistening by groundwater. It was established that in the zone of metal coupons placing the greatest corrosion effect was exerted by sulphate-reducing (105 cells/g) and iron-reducing bacteria (104-106 cells/g). The first are acting through hydrogen sulfide generation, whereas the second are destroying structural materials owing to reduction of iron from electrically neutral form (Fe0) into ferrous iron, which behaves as soluble compound.
Table 2: Bacteriological analysis results for HGIO samples from sites of test metal coupons placing
| Spot No. and HGIO types | Placing sites of metal coupons | Culture media and bacterial count per 1 gram of substrate | ||||||
| MPA | PW | MA | SAA | Thio | SR | IR | ||
| 1. “Blue ores” | Intersection of ventilation crosscut No.2 with filling gallery | 106 | 105 | 102 | 103 | 107 | >107 | 106 |
| 2. “Red ores” | Technological tunnel No.3, +50,8 m from panel cross drift No.7 | >107 | >107 | 102 | 103 | 105 | >107 | >107 |
| 3. “Blue ores” | Prospecting drift No.8, drilling chamber on the 4th cross drift line | 105 | 105 | 102 | 102 | 106 | 106 | 106 |
| 4. “Red ores” | Side drift No.3, drilling chamber on the 18th cross drift line | >107 | >107 | 102 | 104 | 106 | >107 | >107 |
Notes: MPA – meat-and-peptone agar for a wide range of heterotrophic bacteria; PW – agar peptone water for ammonifying bacteria; MA – Aleksandrov’s medium for silicate bacteria; SAA – starch-ammonia agar for actinomycetes; Thio – culture medium for thiobacteria; SR – sulfate-reducing bacteria medium; IR – iron-reducing bacteria medium.
Bacterial count of all species after each two months of experimental study increased by an order of magnitude that was followed by a gradual increase in rate of metal coupons corrosion. There are some tendencies and patterns of microbial development, established during the investigation that might exhibit dynamics of corrosion processes.
Heterotrophic bacterial count on the coupons with culture medium was higher, than in samples without it. This fact shows that presence of organic substances can significantly intensify heterotrophic microflora, which through various biochemical ways could affect metal. For instance, we recorded an enhanced capacity for acids production that is considered as one of the main causes of metal corrosion. Mass bacterial reproduction is leading to biofilms formation, which can be clearly observed in water seepage zones and in the areas of proliferating basidiomycetes mycelium. The latter accumulates a great amount of biomass, which serves as the primary nutrient source for aggressive heterotrophic microbiota.
The maximum bacterial counts were recorded at the closing stage of experiment for anaerobic species (sulphate-reducing and iron-reducing forms), that indicates their direct participation in metal corrosion. Sulphur and iron-oxidizing bacteria were characterized as well by a great number, but still were far less numerous than sulphate-reducing forms (by the order of magnitude of 1 or 2). In the course of laboratory study were noticed changes in bacterial cultures, related to transformation of redox conditions. Particularly in several cases in the liquid medium for thionic bacteria sulphur flocculation was observed at days 4 and 5 that could be explained by a complete exhaustion of dissolved oxygen, anaerobic conditions formation and the beginning of active development of sulphate-reducing microorganisms. Following sulphur disappearance and hydrotroilite production in the form of black residual matter clearly shows further reduction behaviour during decrease of redox potential value.
Altogether, data acquired point at extremely high development rate of aggressive microbiota in the iron ores, which both in aerobic and anaerobic conditions promotes failure of metal arch support. Moreover, it is well known that corrosion damage of structures under the stress proves to be significantly accelerated (stress-corrosion).
Exposing test Metal Coupons in HGIO
Conducted experiment showed that the most active destruction was typical of metal coupons placed into the “red ores”, which exhibited total surface corrosion in the form of rust and pitting (figure 3). In the “blue ores” corrosion damage was developing at a slower rate, being noticeable only in local zones. Under conditions of supplementary moistening we could observe accelerated material deterioration. The dominant role in corrosion was attributed to microbial communities that are mostly formed by sulphate-reducing and iron-reducing bacteria.
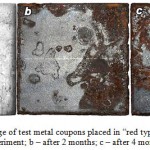 |
Figure 3: Corrosion damage of test metal coupons placed in “red type” of HGIO: a – before the experiment; b – after 2 months; c – after 4 months |
 |
Figure 4: Surface of metal coupon after 4 months of exposure in the “red” type of ores. Loose areas with deep microcavities are observed
|
 |
Figure 5: Clear boundary between destroyed and intact zones of metal coupon surface after 4 months of experiment |
Weighing test metal coupons revealed that their relative mass loss during first 2 months of experimental exposing was 0,25-2%, whereas in the course of following periods it increased nonlinearly. The results indicate a very high rate of corrosion processes featured by different forms of microorganisms that are developing under various redox conditions.
Conclusions
Investigations of metal structures biocorrosion carried out at the Yakovlev’s mine showed that under favourable temperature and moisture conditions in contact with HGIO with various compositions, rate of structural materials corrosion might be very high. Mycological study of HGIO samples from sites of test metal coupons placing detected that the greatest number of micromycetes is typical of zones with wooden backfilling, which is the main nutritious substrate for such microorganisms. Bacteriological analysis of iron ores displayed the presence of a wide variety of bacteria, the count of which was defined by moisture level. It was established that metal samples deterioration is significantly influenced by sulphate-reducing and iron-reducing bacteria. The highest bacterial counts were obtained in case of “red ores” where the most intense corrosion occurred. Weighing test coupons showed that the rate of their mass loss was approximately 1.1% per month and it was going up. In several cases deep pit corrosion was observed. The results of mass measurements and visual analysis fully correlate with data of microscopic study (figures 4, 5).
It becomes quiet clear that if operating time of metal arch support does not exceed 3–4 months biocorrosion can be not taken into consideration, as it would be insignificant. However, operational safety in case of a more extended service largely depends on the intensity of biocorrosion, as it accelerates loss of structures strength and bearing capacity. This was frequently evidenced in practice of the Yakovlev’s mine operation.
Acknowledgments
This research was financially supported by the Saint-Petersburg Mining University within a framework of economic agreement with LLC “Metal Group”, the Yakovlev’s mine. We thank our colleagues from the Saint-Petersburg State University who provided insight and expertise in the area of microbiology and greatly assisted the research. We would like to express our particular gratitude to Dr. Dmitriy Yu. Vlasov, Professor of the Department of Phytopathology and Entomology, Head of Mycological laboratory, Saint-Petersburg State University for invaluable help in microbial studies and his professional assistance in field experiments. We thank as well the Resource centre of molecular and cell technologies at the State University of Saint-Petersburg for assistance with microscopic investigations.
Conflict of Interest
R.E. Dashko and I.V. Alekseev state that there are no conflicts of interest.
References
- Bugelskiy Yu. Yu. Role of microorganisms in the formation of ultrabasites chemical weathering crust. Weathering crust. Moscow: Science. 1978;16:9–18.
- Messineva M. A. Geological activity of bacteria and its influence on geochemical processes. In: Proceedings of Microbiology Institute. Geological activity of microorganisms. Moscow: Academy of Sciences of USSR. 1961;9:12–22.
- Nikitina A. P., Yu. C. G., Aristovskaya T. A. Microbial role in formation of lateritic weathering crust and bauxites. Weathering crust. Moscow: Science. 1991;20:179–190.
- Yachontova L. K., Nesterovich L. G., Lubarskaya G. A. Silicates destruction with the aid of bacteria. J. Mineral. 1983; 5(2):28–38.
- Vologdin A. G. Geological activity of microorganisms. Academy of Sciences of USSR Proceedings. 1947;3:19–38.
- Akai J., Akai K., Ito M. Biologically induced iron ore at Gunma iron mine, Japan. American Mineralogist. 1999;84: 171–182.
CrossRef - Kuznetsov S. I., Ivanov M. V., Lyalikova N. N. Introduction in Geomicrobiology. Moscow: Academy of Sciences of USSR, Microbiology Institute. 1962;240.
- Yu R. A. Fossil bacteria and new insights in the sedimentation processes. Sorosov Educational Journal. Earth Sciences. 1999;10:63–67.
- Yachontova L. K., Zvereva V. P., Sheka S. A. Principles of hypergenesis mineralogy (Cheka S. A., ed.). Vladivostok: Dalnauka, Russian Academy of Science. 2000;331.
- Dashko R. E., Volkova A. V.,Yu V.D. Microbial activity in the underground workings and its influence on properties of high-grade iron ores and structural materials. Proceedings of Mining Institute. 2006;168:165–174. Saint-Petersburg.
- Dashko R. E., Alekseev I. V. Significance of microbiological processes in the context of geotechnical and engineering-geological assurance of underground workings stability at the Yakovlev’s mine (Kursk Magnetic Anomaly). Geotechnics J. 2013;3:36–47.
- Dashko R. E., Alekseev I. V. Features of mining-and-geological processes at the Yakovlev’s mine of high-grade iron ore in case of microbial activity intensification. Proceedings of higher education establishments. Mining Journal. 2013;5:115–122.
- Ch K., Li L., Yu N. Extremely Barophilic Bacteria Isolated from the Mariana Trench, Challenger Deep at a Depth of 11,000 Meters. Environ. Microbiol. 1998;64(4):1510–1513.
- Szewzyk U., Szewzyk R., Stenstrom T. A. Thermophilic, anaerobic bacteria isolated from a deep borehole in granite in Sweden. Natl. Acad. Sci. USA. 1994;91(5):1810–1813.
CrossRef - Fru C., Ivarsson M., Kilias S. P. Fossilized iron bacteria reveal a pathway to the biological origin of banded iron formation. Nature Communications. 2013;4. Article number 2050.
- Aristovskaya T. V., Zykina L.V. Biological factors of Aluminum migration and accumulation in soils and weathering crust. In: Soil formation issues. Leningrad: Nauka. 1978;11–19.
- Nyyssönen M., Bomberg M., Kapanen A. Methanogenic and sulphate-reducing microbial communities in deep groundwater of crystalline rock fractures in Olkiluoto. Finland. J. 2012;29:863–878.
- Nyyssönen M., Hultman J., Ahonen L. Taxonomically and functionally diverse microbial communities in deep crystalline rocks of the Fennoscandian shield. ISME J. 2013;8:126–138.
CrossRef - Pedersen K., Arlinger J., Eriksson S. Numbers, biomass and cultivable diversity of microbial populations relate to depth and borehole specific conditions in groundwater from depths of 4–450 m in Olkiluoto, Finland. ISME J. 2008;2:760–775.
CrossRef - Libert M., Schütz M. K., Esnault L. Impact of microbial activity on the radioactive waste disposal long term prediction of biocorrosion processes. Bioelectrochemistry. 2014;97:162-168.
CrossRef - Videla H. A. Microbially induced corrosion: an updated overview. International Biodeterioration & Biodegradation J. 2001;48:176-201.
CrossRef - Andrejuk E. I., Bilay V. I., Koval E. Z. Microbial corrosion and its agents (Smirnov V. V., ed.). Academy of Science of Ukranian SSR. Institute of microbiology and virology named after Zabolotniy D.K. Kiev: Naukova dumka. 1980;287.
- Else T. A., Amy P. S., Curtis R. P. Boundaries for Biofilm Formation: Humidity and Temperature. Applied and Environmental Microbiology. 2003;69(8):5006–5010.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





