Manuscript accepted on : 19 July 2016
Published online on: --
R. S. Arvind Bharani and S. Karthick Raja Namasivayam
Department of Biotechnology, Sathyabama University, Chennai, Tamil Nadu, India. Corresponding Author E-mail: biologiask@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2308
ABSTRACT: Fungal biocontrol agents are now extensively used against a wide range of insect pests associated with economic important crops.Success of the fungal biocontrol agents is primarily depends on suitable formulation which enhances the post treatment persistence and efficacy.In the present study, effect of various oil formulation on the persistence,endophytic relationship with groundnut phyllosphere and compatibility with chemical synthetic pesticides under in vitro condition.Moreover, phytotoxicity of the formulations was also studied by determination of the seedling emergence and chlorophyll content. Among the various formulations,sunflower oil and groundnut oil revealed enhanced persistence and best compatibility with all the tested chemical synthetic pesticides.Seedling emergence and chlorophyll content was not affected in the above mentioned oil formulations.None of the formulation revealed endophytic association with the groundnut phyllopshere.Further study will helpful to exploit the formulation principles under field condition.
KEYWORDS: Metarhizium anisopliae; formulation; persistence; compatibility; endophyte
Download this article as:| Copy the following to cite this article: Bharani R. S. A, Namasivayam S. K. R. Evaluation of Persistence and Compatibility with Synthetic Chemical Pesticides of Formulated Entomopathogenic Fungi Metarhizium Anisopliae (M.) Sorokin. Biotech Res Asia 2016;13(3). |
| Copy the following to cite this URL: Bharani R. S. A, Namasivayam S. K. R. Evaluation of Persistence and Compatibility with Synthetic Chemical Pesticides of Formulated Entomopathogenic Fungi Metarhizium Anisopliae (M.) Sorokin. Biotech Res Asia 2016;13(3). Available from: https://www.biotech-asia.org/?p=16039 |
Introduction
Insect pathogenic fungi in the genera Beauveria, Conidiobolus, Metarhizium and Paecilomyces are all commonly found in the soil [1]. Many other fungal species have also been reported on diseased soil-inhabiting insects in various regions of the world and fungal epizootics in soil insect populations are also well documented [2].Metarhizium anisopliae Sorokin Sensu lato (Ascomycetres:Hypocreales) has been studied extensively for the biological control of a wide range of insect pests,fungi belonging to the genera Metarhizium have been found to be effective against several species of insects, including Lepidoptera. Metarhizium anisopliae is a widely distributed soil-inhabiting fungus.The spore of M. anisopliae can be formulated as dust and sprayable formulation. It isused to control termites, mosquitoes, leaf hopper,beetles etc. Metarhizium anisopliae, formerly known as Entomophthora anisopliae is a fungal organism that grows naturally in soils throughout the world and causes disease in various insect pests;it thus belongs to the entomopathogenic fungi. It is known to infect over 200 insect species, including termites ]3,4].
Formulation of biological control agent is an important criterion for sustainable agriculture[5]. Formulation can improve the product stability and viability may reduce in consistency of field performance of many potential biological control agents. Formulation of biocontrol products for use against diseases (biofungicides), weeds (bioherbicides) and insect pests [6]. (Gopalakrishnana and Mohan, 2000). Many of the biocontrol agents have been formulated with dried milk, powdered casein, gelatin, saponins, oils,soaps etc. In so far as microbial infecticides are concerned it is essential that the compound used should premature the growth or germination and that it should not inhibit the successful establishment of the pathogens [7].Formulating agents are also used as spreaders or wetting agents.These are added to the water diluent to ensure “wetting” of the surface to the sprayed. Many material have been used as wetting agents which including dried milk, powdered casein, gelatin, saponins, oils, soaps etc. In so far as microbial infecticides are concerned it is essential that the compound used should premature the growth or germination and that it should not inhibit the successful establishment of the pathogens [8,9].In the present study,different formulation of M.anisoliae on the post treatment persistence and their compatibility with the chemical synthetic pesticides.
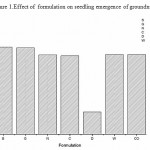 |
Figure 1: Effect of formulation on seedling emergence of groundnut
|
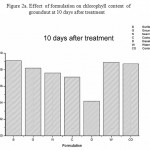 |
Figure 2a: Effect of formulation on chlorophyll content of groundnut at 10 days after treatment
|
Materials and Methods
Fungal strain
Laboratory stock culture of M.anisopliae previously isolated from the agriculture field soil was used in the present study [10]. Fungal strain was maintained on potato dextrose agar (PDA) slant.
Inoculum preparation
Conidial spore suspension derived from the fungal growth on PDA was used as the source of inoculum. The conidia were harvested from 15 day old surface cultures by flooding the plates with sterile 0.1% Tween 20 in water and lightly scrapping the fungal culture surface with a sterile glass rod. The suspensions were vortexed for five minutes to dissociate conidial clumps and filtered through one layer of cheese cloth to remove remaining clumps and mycelial debris. The concentration of each suspension was adjusted to 1×108 conidia/ml determined by a hemocytometer.
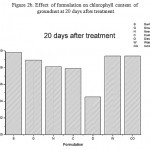 |
Figure 2b: Effect of formulation on chlorophyll content of groundnut at 20 days after treatment
|
Preparation of fungal oil formulation
Fungal oil formulation was prepared with sunflower oil, groundnut oil, neem oil, castor seed oil, diesel and water. Respective fungal oil formulation was prepared by mixing 25 ml of fungal spore suspension (1×108 conidia/ml) with 2.5 ml of respective agent, kept under magnetic stirrer for two hours.
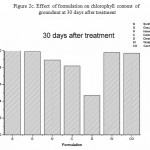 |
Figure 2c: Effect of formulation on chlorophyll content of groundnut at 30 days after treatment
|
Pot assay
Effect of oil formulation on M.anisopliae was studied by pot assay. Respective fungal formulation was sprayed using ultra volume sprayer on the groundnut plants (25 days after seedlings emergence) grown in pots.Five replications including untreated control was maintained in each treatment.
Persistence study
Effect of formulation on the post treatment persistence of fungi was studied by microbiological examination of leaves collected from the groundnut plants grown in pots treated with respective fungal formulation after ten days after treatment till 30 days post application.Leaves were collected from top, bottom and middle of the plant,kept in sterile polythene bag and brought to the laboratory for the microbiological examination. 5 gram leaves of respected pots was suspended in 100 ml of sterile distilled water with 0.01% Tween-80 for 1 hour on a rotatory shaker at 100 rpm. The suspension was serially diluted. 0.1 ml of aliquot was spread plated on potato dextrose agar (PDA). After the incubation at 260 C in darkness, the fungal growth was assessed to quantify the number of colony forming units (CFU).
Table 1: Effect of formulation on M.anisopliae colonies
| S.No | Formulation | CFU/ml days after treatment
|
||
| 10 | 20 | 30 | ||
| 1 | Sunflower oil | 12.0 X 10 8 | 33.2 X 10 8 | 44.1X10 8 |
| 2 | Groundnut oil | 9.0 X 10 8 | 38.0 X 10 8 | 41.0 X 10 8 |
| 3 | Neem oil | 27.3X10 7 | 32.4X10 7 | 39.0X10 7 |
| 4 | Castor oil | 23.0 X10 7 | 29.0 X10 7 | 32.2 X10 7 |
| 5 | Diesel | 19.2X10 3 a | 20.1X10 3 a | 21.2 X10 3 a |
| 6 | Water | 24.0X10 3 a | 25.0X10 3 a | 26.0 X10 3 a |
| 7 | Control | 29.0X10 3 a | 32.0X10 3 a | 33.0 X10 3 a |
In column, the mean followed by the alphabet is statistically significant (P<0.05) by DMRT
Evaluation of endophytic relationship on the groundnut phyllosphere
Assessment of endophytic relationship of the respective fungal formulation on the groundnut phyllosphere was also studied. Leaves from the respective formulation treated pots were collected at the respective time period. The collected leaves were cut into 1mm square surface using sterile blade and then rinsed.in sterile distilled water. The cut sections were then consecutively washed in sterile distilled water containing 0.1%HgCl2. Then the cut sections were transferred consecutively to petriplate containing only sterile distilled water retaining in each for one minute. Then the sections were carefully taken one by one with sterile forceps, the excess water was blotted on to a sterile tissue paper and then transferred into the Potato Dextrose Agar medium(PDA). Each petriplate containing the PDA medium would hold a maximum of 4 to 5 leaves sections. The plates were then incubated at 28ºC for 10 days. Daily observation was made to record the fungal growth.
Table 2: Effect of formulation on compatibility of pesticides
| S.No | Formulation | CFU/ml days after treatment
|
||
| Agent plus-Lambda yhalothr
0.2% 0.4 % 0.6% |
Ekalux-Quinakphos
0.2% 0.4 % 0.6 |
Hostathion-Triazophos 0.2% 0.4 % 0.6% | ||
| 1 | Sunflower oil | 31.0X10 8 29.5X10 8 29.0X10 8 | 26.5X108 24X108 24.0X108 | 25.0X108 22.4X108 22.0X108 |
| 2 | Groundnut oil | 28.0X10 8 26.0X10 8 26.0X10 8 | 25.4X108 23.0X108 22.0X108 | 21.0X108 19.0X108 16.0X108 |
| 3 | Neem oil | 27.3X10 7 26.03X10 7 12.0X07 | 17.3X10 7 16.03X10 7 12.0X07 | 12.3X10 7 9.0X10 7 11.0X07 |
| 4 | Castor oil | 22.3X10 7 20.03X10 7 20.0X07 | 17.3X10 7 16.03X10 7 12.0X07 | 12.3X10 7 9.0X10 7 11.0X07 |
| 5 | Diesel | 12.1X103a 9.0X103a 3.0X103 a | 18.1X103a 9.0X103a 4.0X103a | 10.1X103a 7.0X103a 4.0X103a |
| 6 | Water | 24.0X103 27.0X10 3 28.1 X103 | 31.0X103 28.0X103 23X103 | 23.0X103 19.0X103 8.0X103 |
| 7 | Control | 20.0X10 3 17.0X10 3 11.1 X10 3 | 21.0X103 18.0X103 23X103 | 920.0X103 19.0X103 9.0X103 |
In column, the mean followed by the alphabet is statistically significant (P<0.05) by DMRT
Compatibility with chemical pesticides
Effect of formulation on compatibility of pesticides with M. anisopliae. The three pesticides included Agent plus (lambda cyhalothrin), Ekalux (quinolphos) and Hostathion (triazophos). Pesticides were prepared with different concentrations as 0.02, 0.04 and 0.06 % and mixed with the respective fungal formulation
Evaluation of phytotoxicity
Phytotoxicity of formulation was evaluated by the seed germination technique. The germination index and chlorophyll determination has been extensively used as an indicator of phytotoxicity. Seed germination was studied using groundnut seeds. Healthy and mature seeds of groundnut was collected from Agricore department,Chennai in sterile polythene bags and all seeds were cleaned thrice in sterile distilled water and immersed in respective formulation for 1 hr. After the treatment, the treated seeds were transferred to the petriplate lined with moistened tissue paper,plates were incubated at 25 C. Control and replications were maintained in each treatment.Germinated seeds from the respective treatment were transferred to the pots of 14 cm diameter and 12 cm in height were filled with the fertile loam soil. After 20 days after seedlings emergence (DASE),respective fungal formulation was sprayed using ultra low volume sprayer. The amount of chlorophyll present in the leaves was estimated by standard methods.
Result and Discussion
Entomopathogenic fungi belong to 750 species of fungi under ascomycetes, deuteromycetes and entomophthorales are pathogenic to insects as biological control agents of insect pests has been increasing during the last few decade [11].Among the different species of fungi,Metarhizium anisopliae Sorokin Sensu lato (Ascomycetres: Hypocreales) has been studied extensively for the biological control of a wide range of insect pests.Formulation of the fungal biocontrol agents determine the success in pest management [12]. In the present study,effect of formulation on the post treatment persistence of Metarhizium anisopliae has been carried out.Among the formulation, sunflower oil formulation recorded maximum number of fungal colonies in all the tested time period which revealed 12.0 X 10 8,43.2 X 10 8 and 44.1X10 8 CFU/ml fungal count at 10,20 and 30 days after post treatment (Table1). Similar fungal count has been reported in groundnut oil formulation. Significant reduction of fungal colonies was observed in diesel and water formulation (P>0.05). Moreover, fungal count was recorded at 10 and 20 days of post treatment. Complete absence of fungi was observed during 30 th day of post treatment. Neem oil and castor seed oil showed moderate reduction. Improved efficacy of oil based formulation might be due to the fact that oil formulation prevents the desiccation of the conidia and helps in longer survival period and better penetration of peg into the integument These results are in agreement with Sahayaraj and Karthick Raja Namasivayam [13] who reported that higher mortality of aphids noticed in oil formulations of entomopathogenic fungi than aqueous formulation. Effect of formulation on establishment of endophytic relationship study revealed none of the formulation supported endophytic association in the tested groundnut phyllosphere.
Effect of formulation on the compatibility of M. anisopliae with the synthetic chemical pesticides was studied. In this study, M. anisopliae showed compatibility with all the three tested synthetic pesticides- lambda cyhalothrin, quinolphos and triazophos (Table 2).Among the different formulation, groundnut and sunflower oil formulation showed best computability with all the tested pesticides .Fungal count was not affected in all the concentration used. In contrast to the above mentioned formulation, dissed formulation revealed less computability and the fungal count was found to be reduced in all the tested pesticides. Phytotoxicity was studied by seed germination technique and chlorophyll estimation. The germination index has been extensively used as an indicator of phytotoxicity [5].From the figure 1 it is clear that all the formulation supported seed germination except diesel which showed only 25.0 % germination. Effect on chlorophyll content was carried out by Weber method. Results presented in figure 2a-c revealed chlorophyll content was not reduced in all the treatment except diesel formulation. Further study will helpful to exploit the principles of formulation techniques in biocontrol program,
References
- Nicolai V. Meyling and Jørgen Eilenberg 2007.Ecology of the entomopathogenic fungiBeauveria bassiana and Metarhizium anisopliae in temperate agroecosystems: Potential for conservation biological control. Biological Control, 43:145–155
- Bing, D.S. and Xing, Z. L. 2008. Occurrence and diversity of insect-associated fungi in natural soils in China. Applied Soil Ecology, 39:100–108.Biotechnology, 10(6): 933-936
- Padmaja, V. 2005. Role of entomopathogenic fungi in insect pest management. In, Ignachimuthu,S.J and Jayaraj, S. (eds.) Green pesticides for integrated management. Narosa publishing house, New Delhi 324.
- Karthick Raja Namasivayam, S. and Vinoth Kumar,P. 2009. Influence of media on the pathogenicity of Metarhizium anisopliae (M.) Sorokin against Chilo partellus (Swinhoe). Journal of Biopesticides, 2(1): 92-93.
- Sharma, 2004. Biocontrol management of pest in organic forming Agrobios Newsletter,2: 12-15
- Gopalakrishnan, C. and Mohan, S.C. 2000. A Simple and cost effective In vitro method for the mass production of Conidia of Nomuraea rileyi. Insect Environ. 6 : 52-53.
- Tincilley, A., Easwaramoorthy, G. and Santhanalakshmi,G. 2000. Attempts on mass production of Nomuraea rileyi on various agricultural products and byproducts. J. Biol. Contr. 18 (1) : 33-40
- Vimala Devi, P.S., Chowdray, A. and Prasad, Y.G. 2000.Cost effective multiplication of the entomogenous fungi Nomuraea rileyi (F.) Samson. Mycopathologia. 15 : 35-39.
- Tiquia, M.S. and Tam, N.F.Y. 1998. Elimination of phytotoxicity during co-composting of spent pig-manure sawdust litter and pig sludge. Bioresource Technology, 65: 43–49.
- Karthick Raja Namasivayam, S,Aarthi, R. and Anbazhahan, P.2015. Studies on factors influencing the viability of entomopathogenic fungi Metarhizium anisopliae in soil adapting culture dependent method. Journal of Biopesticides, 8(1):23-27
- Sahayaraj, K., Karthick Raja Namasivayam, S. and Martin Rathi, J. 2011. Compatibility of entomopathogenic fungi with extracts and commercial botanicals. African Journal of Biotechnology, 10(6): 933-936.
- Karthick Raja Namasivayam, S. Abinaya vidyasankar and Manoj santhosh,T.2014. Biocompatible formulation of potential fungal biopesticide Nomuraea rileyi (f.) Samson for the Improved post treatment persistence and Biocontrol potential. Asian Jr. of Microbiol. Biotech. Env. Sc, 16 (4) 895-899
- Sahayaraj, K. and Karthick Raja Namasivayam, S. 2007.Bioefficacy of entomopathogenic fungi against Aphis craccivora in groundnut. Indian Journal of Plant Protection. 35 : 352-353.

This work is licensed under a Creative Commons Attribution 4.0 International License.





